Examining Participation Disparities in Cancer Clinical Trials
Objectives: To examine factors that account for disparities in cancer clinical trial participation.
Sample & Setting: Pooled data from Behavioral Risk Factor Surveillance System surveys between 2010 and 2017.
Methods & Variables: Univariate and binary logistic regression analyses were used to examine the associations between participation in clinical trials and demographic and health characteristics, using SAS® procedures to account for complex sample features.
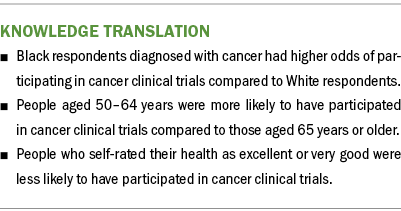
Results: Univariate analyses showed that age, race, income, and self-rated health status were significantly associated with the likelihood of participating in cancer clinical trials. Binary logistic analyses showed that Black respondents who were ever diagnosed with cancer were more likely to participate in cancer clinical trials relative to White counterparts. Respondents aged 50–64 years were more likely to have participated in cancer clinical trials compared to those aged 65 years or older. However, respondents who self-rated their health as excellent or very good were less likely to participate in cancer clinical trials.
Implications for Nursing: Involving properly trained nurses and nurse practitioners from diverse backgrounds in cancer clinical trials to inform people with cancer about trials and ways to reduce personal barriers will increase participation from all people, regardless of socioeconomic and demographic characteristics.
Jump to a section
Health disparities, commonly defined as differences in the burden of disease, injury, violence, or opportunities to obtain optimal health, are preventable but have been obstinate (Centers for Disease Control and Prevention [CDC], 2020). Evidence suggests that there are disparities in cancer clinical trial participation (American Cancer Society Cancer Action Network, 2018; Colon-Otero et al., 2008; Loree et al., 2019; Murthy et al., 2004; Nipp et al., 2019; Unger et al., 2016, 2020; Winkfield et al., 2018; Wong et al., 2016). Multiple factors contribute to the disparities in clinical trial participation. Lower socioeconomic status creates financial burden on people with cancer (Nipp et al., 2019; Winkfield et al., 2018; Wong et al., 2016), which has been linked to low medication adherence, poor quality of life, and increased mortality rates (Chino et al., 2017; Nipp et al., 2019; Ramsey et al., 2013, 2016). There is a large disparity in cancer clinical trials based on age. Less than 3% of adults aged 20 years or older and less than 1% of those aged 70 years or older participate in cancer clinical trials; however, 50% of all children with cancer take part in clinical trials (Colon-Otero et al., 2008; Sedrak et al., 2021). Evidence indicates an association between cancer clinical trial participation and population mortality or survival (Unger et al., 2016). Consistent reduction in mortality rates has been recorded among children aged younger than 15 years with an increase in clinical trial participation (Bond & Pritchard, 2006; Hunger et al., 2012). Of note, clinical trial participation by children aged younger than 15 years has always been higher than that of their adult counterparts (Bond & Pritchard, 2006; Markham et al., 2020; Unger et al., 2016).
Structural and clinical barriers to cancer clinical trial participation have also been reported. Clinical trials are not available to all people with cancer (Go et al., 2006; Unger et al., 2021). This is the case for more than half of individuals with cancer (Green et al., 2012). In addition, structural factors, such as transportation, travel costs, access to insurance, and availability of child care, have all been noted as challenges for people with cancer to readily participate in clinical trials (Asare et al., 2017; Nazha et al., 2019; Rivers et al., 2013). A proportion of people with cancer (17%) who qualify for clinical trials are later disqualified because of narrow inclusion criteria (Hunter et al., 1987; Klabunde et al., 1999; Rivers et al., 2013; Unger et al., 2016). The inherent uncertainty and inability of clinicians to appropriately gauge the risk–benefit ratio of these trials for people with cancer also contributes to disparity in participation (Green et al., 2012; Nipp et al., 2019). It is estimated that clinical and structural barriers hamper the chances of participation in about two-thirds of people with cancer at larger academic cancer centers, and in about three-fourths of individuals with cancer in smaller community treatment centers (American Cancer Society Cancer Action Network, 2018).
Other barriers, such as those related to care providers or institutions, are also contributing factors. A physician’s decision or preference for a specific treatment is a barrier for people with cancer who are eligible for clinical trials (Begg et al., 1983; Benson et al., 1991; Hunter et al., 1987; Stewart & Stewart, 2022; Unger et al., 2021). Although some physicians consider clinical trials time-consuming and find it burdensome to obtain informed consent (Benson et al., 1991; Melisko et al., 2005), others are concerned about the interference of clinical trial participation in the physician–patient relationship (Javid et al., 2012; Ross et al., 1999; Taylor et al., 1984). In addition, providers may not have the appropriate support, resources, and incentives to recruit participants for cancer clinical trials (Minasian & Unger, 2020; Ross et al., 1999; Somkin et al., 2005).
Patient-related factors are also reported as barriers. For example, people with cancer may be apprehensive about the research procedure or feel that clinical trials are invasive (Clark et al., 2019; Cox & Avis, 1996; Coyne et al., 2004; Ellis et al., 2002; Kemeny et al., 2003). Concerns about side effects from clinical trials deter people with cancer from participating in trials (Meropol et al., 2007; Unger et al., 2021). They may lack family support or experience an increased level of anxiety (Cox & Avis, 1996; Fleissig et al., 2001; Granda-Cameron et al., 2022; Meropol et al., 2007). Several studies have reported feelings of uncertainty among people with cancer (Coyne et al., 2004; Javid et al., 2012; Kemeny et al., 2003; Slevin et al., 1995; Solomon et al., 2003) and fear of a reduced quality of life (Brown et al., 2000; Jenkins et al., 1999; Solomon et al., 2003).
Although the burden of cancer is greater among racially and ethnically underrepresented groups, particularly for Black patients, cancer clinical trial participation rates are lower among people of color (Colon-Otero et al., 2008; Hamel et al., 2016; Murthy et al., 2004). Several factors may explain the racial and ethnic disparities in cancer clinical trials. Physicians with implicit or unconscious bias are less likely to recommend cancer clinical trials to underrepresented participants because they believe that these patients will be nonadherent (Colon-Otero et al., 2008; Sabin et al., 2008) or are not good study candidates (Joseph & Dohan, 2009a; Unger et al., 2021). Some physicians may be apprehensive of recruiting people in underrepresented groups because they believe that these patients mistrust healthcare systems and clinical trials (Pinto et al., 2000; Stewart & Stewart, 2022). In addition, some researchers reported that the racially and ethnically segregated American healthcare system—wherein one class of facilities, care providers, and financing mostly meets the needs of a specific group—makes it difficult for underrepresented groups to participate in clinical trials (Joseph & Dohan, 2009b; Wendler et al., 2005; Witte et al., 2004). Consequently, White individuals are more likely to be recruited for clinical trials compared to people of color (Hamel et al., 2016; Howerton et al., 2007; Joseph & Dohan, 2009b).
All these factors show that disparities in cancer clinical trial participation are complex and challenging (Smith et al., 2021). For increased representation in clinical trial participants, the federal government, through the National Institutes of Health Revitalization Act of 1993, mandated that women and underrepresented groups must be included in any National Institutes of Health–funded clinical research. Studies have reported that although there has been an increase in the total number of participants in cancer clinical trials, participation rates among underrepresented racial and ethnic groups remain significantly low relative to the respective proportions in those populations (Chen et al., 2014; Ibraheem & Polite, 2017). The present study used data from the Behavioral Risk Factor Surveillance System (BRFSS), pooled from 2010 to 2017, to determine the following: (a) factors associated with cancer clinical trial participation and (b) whether underrepresented groups, particularly Black respondents, are less likely to participate in cancer clinical trials. Univariate and binary logistic analyses were used.
Data and Methods
First administered in 1984, BRFSS is a coordinated system of telephone surveys sponsored by the CDC (2018) aimed at producing state-level estimates of the noninstitutionalized U.S. population aged 18 years or older regarding health risk behaviors, chronic diseases and conditions, and healthcare access and use. Local health departments in each state, the District of Columbia, Guam, and Puerto Rico oversee their own field operations following protocols and technical guidance provided by the CDC, then transmit data to the CDC for streamlined editing, processing, weighting, and public release. In addition to a universally administered core questionnaire component, states are given the option to include standardized special-topic modules or customized questions to capture information on other public health topics of interest.
Data were collected from the BRFSS core component and from the cancer survivorship special-topic module, which asked individuals to indicate a prior cancer diagnosis and whether they participated in a clinical trial as part of their cancer treatment. To increase the scope and statistical power of the dataset for analysis, the authors pooled data from the following five independent BRFSS administrations: 2010, 2012, 2014, 2016, and 2017. Data from 2011, 2013, and 2015 were excluded because the cancer survivorship module was not used during those administrations. In total, the final dataset consisted of 31,978 respondents indicating a prior cancer diagnosis.
Health Disparities Framework
This study used an adapted version of the three phases of the health disparities framework by Kilbourne et al. (2006) to examine factors that account for disparities in cancer clinical trial participation. This framework allows for a basic detection of disparities to understand factors that explain the disparities to develop policy interventions that may reduce or eliminate these disparities (Kilbourne et al., 2006). There are three phases to the health disparities framework. Phase 1 (detecting) explores the following: (a) Disparities in cancer clinical trial participation is the defined health disparities problem; (b) individuals with cancer represent the vulnerable population of interest; (c) demographic factors were used to measure disparities in this vulnerable population; (d) to reduce confounding effects of the demographic variables on participation in clinical trials among people with cancer, participants’ self-reported health statuses were added. For phase 2 (understanding), this study identified the determinants of disparities in cancer clinical trials at individual levels by focusing on demographic variables that have been reported in the literature. Phase 3 (reducing) led this study to make recommendations for possible interventions and policy changes based on findings to reduce disparities in cancer clinical trial participation.
Data Analyses
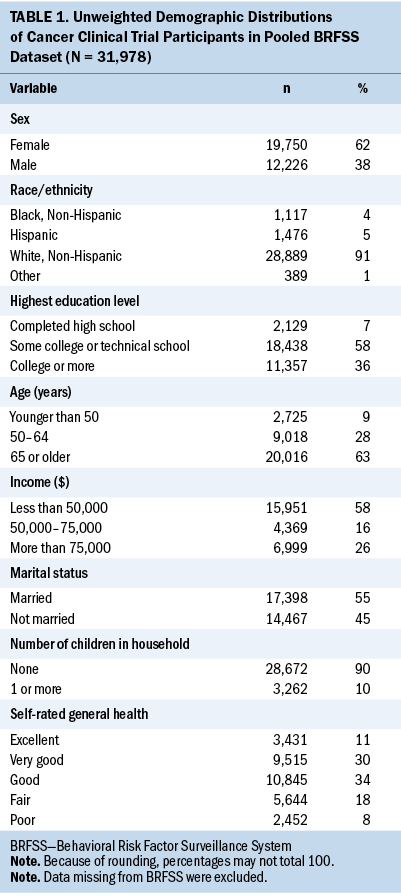
As detailed by the CDC (2018), the BRFSS involves three complex sample features as follows: (a) Telephone numbers of participants are stratified by state (variable _STSTR); (b) respondents are clustered at the household level (variable _PSU); and (c) respondents are assigned weights to compensate for unequal selection probabilities and nonresponse (variable _LLCPWT). Ianchan et al. (2016) includes more details on current BRFSS weighting procedures. All subsequent analyses and inferences reported here account for these features via the family of SAS/STAT® survey procedures (Lewis, 2016). Researchers were interested in the likelihood of clinical trial participation among people with cancer. The dependent variable asked whether survey respondents participated in a clinical trial as part of cancer treatment. The answer choices were 1 (yes) and 2 (no). A set of independent variables related to clinical trial participation derived from the literature included the following: age, income, sex, education, race, marital status, number of children in household, and self-rating of health. Distributions of these variables are shown in Table 1. Item nonresponse rates were trivial on these independent variables, so missing values were removed from the analysis.
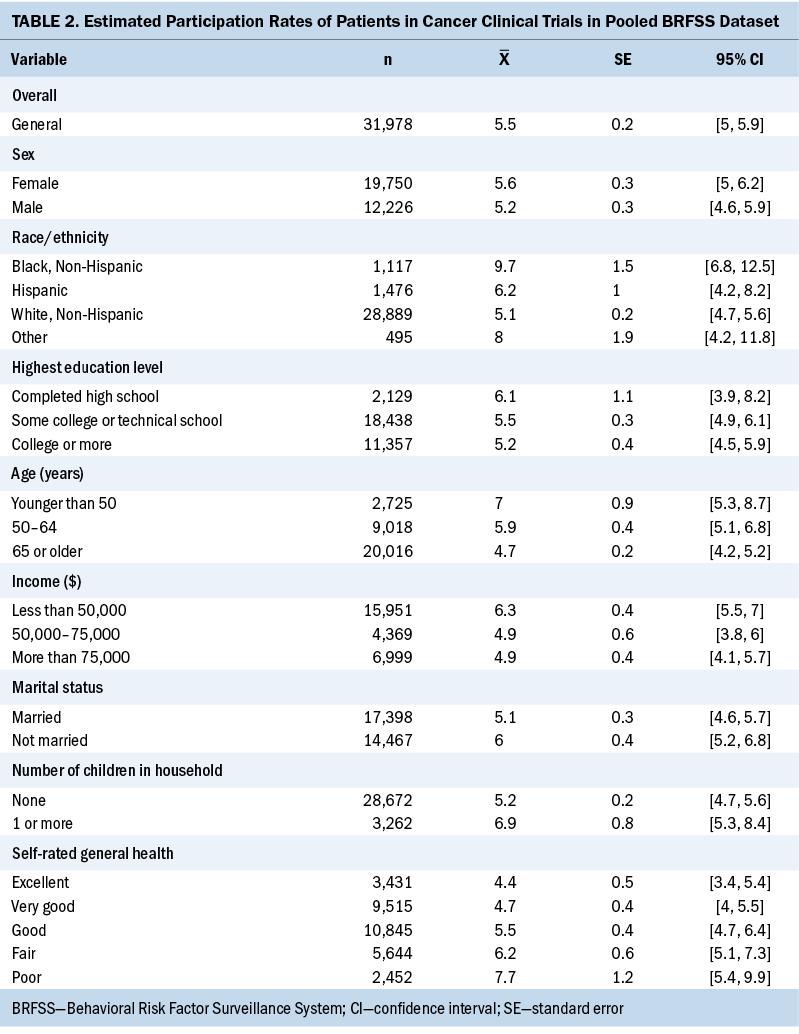
Two sets of analyses were undertaken. The first was to examine clinical trial participation rates among the various levels of the independent variables presented in Table 2. Table 3 presents adjusted odds ratios accounting for these independent variables simultaneously via a binary logistic regression model. When fitting the model, certain independent variable categories needed to be collapsed because they were leading to sparse cross-tabulations with the dependent variable and unstable logistic regression model parameter estimates.
Results
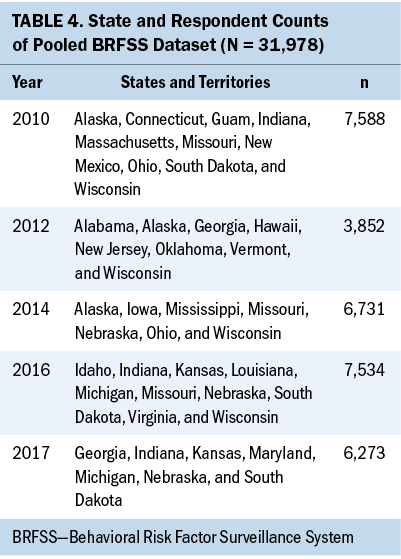
A few states included the cancer survivorship module in the administrations of the BRFSS (see Table 4). Although 10 states included the cancer survivorship module in 2010 and 2016, only 7 states included this module in 2014 and 2017. In addition, there is a variation in the frequency of inclusion of the module. For example, Connecticut included the cancer survivorship module only once (2010), Michigan included the module twice (2016 and 2017), Indiana included it three times (2010, 2016, and 2017), and Wisconsin included it four times (2010, 2012, 2014, and 2016).
Respondents were more likely to be a non-Hispanic White woman, aged 65 years or older, with an annual income of less than $50,000. They were also more likely to have at least some college or technical education, be married, have no children in the household at the time of survey, and self-rate their health status as good, very good, or excellent.
Overall, 5.5% of respondents participated in a clinical trial. Only modest differences in clinical trial participation were observed between men (5.2%) and women (5.6%) and between respondents who were currently married (6%) and those who were not married (5.1%). With respect to race and ethnicity, White respondents were less likely to have participated in clinical trials than all other racial and ethnic groups, with the largest difference being between White (5.1%) and Black (9.7%) respondents. In addition, clinical trial participation rates were higher for younger people, those with a high school education at most, and those with an annual income of less than $50,000. Rates were also higher for those reporting lower self-rated general health. For example, people with a self-rating of poor health were more likely to have participated in a clinical trial than those with a self-rating of excellent health (7.7% versus 4.4%, respectively).
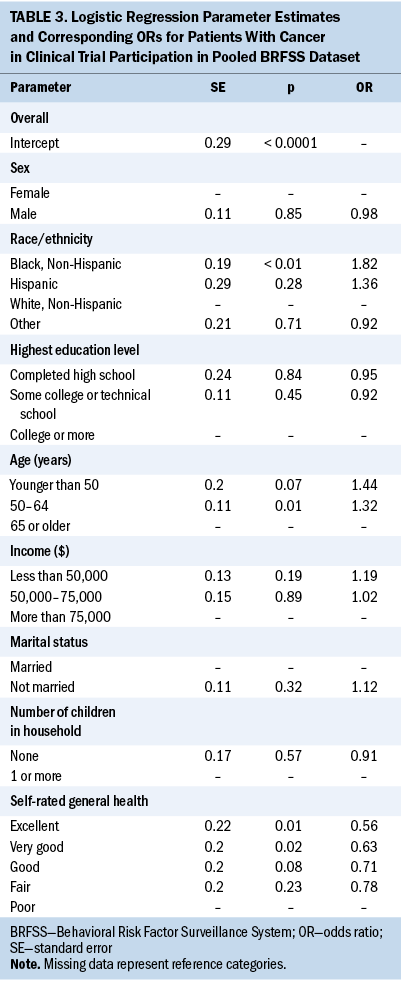
A binary logistic regression model was fitted to the pooled dataset to obtain estimates of the likelihood of clinical trial participation, controlling for all the independent variables to answer the second research question: namely, whether underrepresented groups, in particular Black individuals, are less likely to participate in clinical trials. The three significant effects in the model were race and ethnicity, age, and general health self-rating. In particular, Black respondents ever diagnosed with cancer had 82% higher odds of participating in cancer clinical trials relative to White counterparts, controlling for sociodemographic and health factors. Respondents aged 50–64 years had 32% higher odds of having participated in cancer clinical trials compared to those aged older than 65 years. However, compared to those who considered themselves to be in poor health, respondents who self-rated their health as excellent and very good had 44% and 37% lower odds of participating in cancer clinical trials, respectively. All the other variables, such as income and marital status, that were significant in the univariate analysis were no longer significant when controlling for all the demographic and health status variables.
Discussion
This study examined factors that determine participation in clinical trials as part of cancer treatment among Americans diagnosed with cancer. Similar to other studies that have reported low rates (2%–8%) of clinical trial participation among adults with cancer over many years (Institute of Medicine, 2010; Murthy et al., 2004; Unger et al., 2019), the participation rate was only 5.5% among this study population. This study also found that unmarried people with cancer participated more in clinical trials relative to their married counterparts, but the association becomes insignificant when controlled for the other demographic and health variables. In their study of barriers and facilitators that influence people’s decisions whether to enroll in early-phase clinical trials, Zonderman et al. (2014) reported that married people would not participate in clinical trials as much as their unmarried counterparts.
This study also found a modest sex advantage in favor of women, but this difference disappeared when other variables were controlled in the analysis. However, it is important to note that the U.S. Food and Drug Administration’s (2020) reports on drug trials show that although the sex difference between men and women who took part in clinical trials is negligible, the participation rates of women of color were substantially low. Participation ranges from 10% for Asian women, to 8% for Black women, to 1% for American Indian or Alaska Native women, compared to 76% for White women. In addition, this study found higher clinical trial participation among younger people, participants with a high school education at most, and those with an annual income of less than $50,000. Although all these associations were nonsignificant once other demographic and health variables were introduced, Clark et al. (2019) reported that higher levels of education (beyond high school) and an understanding of clinical trials are correlates of a greater willingness to participate in clinical trials.
The binary logistic regression analysis showed that cancer clinical trial participation was higher among underrepresented populations, in particular among Black respondents compared to White counterparts. This is surprising because numerous studies have indicated racial and ethnic disparities in cancer clinical trial participation (Colon-Otero et al., 2008; Eskander et al., 2022; Hamel et al., 2016; Murthy et al., 2004; Nazha et al., 2019). Several factors could explain these findings. First, because cancer-related mortality is higher among underrepresented groups, in particular Black groups (Smith et al., 2021), it could be that Black respondents who took part in the survey were mostly cancer survivors who had participated in cancer clinical trials as part of their cancer treatment. Second, because the cancer survivorship module was not used by all 50 states as standardized special-topic modules or customized questions to capture information on other public health topics of interest, the states that used this module may be proactive in their endeavor to increase cancer clinical trial participation among underrepresented groups. Third, the question neither specified the type or stage of the cancers that respondents had nor the phase of the clinical trials. Nevertheless, few studies have also reported that Black and Hispanic patients are as equally likely to willingly participate in cancer clinical trials as their White counterparts, and factors such as mistrust and lack of knowledge of clinical trials are barriers to participation (Byrne et al., 2014; Wendler et al., 2005). Unger et al. (2021) also reported that Black, Hispanic, and Asian patients are as likely to participate in cancer clinical trials as White patients when asked to participate.
Although the findings in this article may point to a positive direction with cancer clinical trial participation from underrepresented groups, it is important to recognize that government-funded clinical trials tend to have better representation. In fact, a study by Unger et al. (2020) reported poor representation of Black participants in pharmaceutical company–sponsored clinical trials in which participation of Black individuals was only 2.9%. Although significant barriers prevent participation in cancer clinical trials, efforts to reduce participation barriers have shown improved participation rates. Some emerging studies have demonstrated that strategic efforts, such as embedding cancer prevention programs and research in the community, can lead to increases in the rates of underrepresented groups who participate in cancer clinical trials (Kim et al., 2020; Wallington et al., 2016). In addition, increasing knowledge about clinical trials in underrepresented communities could increase willingness to participate (Echeverri et al., 2018; Simon et al., 2019, 2021).
This study also found that age was a significant factor in participating in cancer clinical trials. People aged 50–64 years were more likely to have participated in clinical trials compared to those aged 65 years or older. This finding confirms the fact that few cancer clinical trials target people aged 65 years or older (Markham et al., 2020; Sedrak et al., 2021). In addition, people with cancer who rated their health as excellent or very good were less likely to have participated in clinical trials compared to those who rated their health as poor. Because disparities in cancer clinical trial participation are complex and multifactorial, efforts to combat these disparities must be intentional and targeted to combat barriers for specific population groups.
Limitations
The study used secondary data, and some pertinent questions that could have clarified some of the findings were missing in the dataset. For example, participants’ types and stages of cancer were unknown. Information was also missing about the phases of the clinical trials, as one of the challenges of recruiting individuals with cancer for clinical trials is to select people with cancer who will survive throughout the trials (Mahipal & Nguyen, 2014). Underrepresented people are less likely to enroll in phase 1 of a cancer clinical trial relative to phases 2 and 3 (Perni et al., 2021). In addition, some states participated more in the BRFSS cancer survivorship module and others participated less. Another limitation is that the BRFSS does not have data on rates of cancer clinical trial participation by each state over the years to assess general trends. Although some states, such as Connecticut, included the cancer survivorship module only once in 2010, other states, such as Wisconsin, included the module four times, but others did not participate at all. Although the general procedure for assimilating module-specific data outlined by the CDC (2018) was adhered to, a nonrandom subset of states was represented in the cancer survivorship module. Future studies should examine clinical trial participation focusing on specific cancers, specific phases of the trial, states, and modes of recruitment of individuals with cancer.
Implications for Practice
Healthcare providers are at the forefront of recruiting individuals with cancer for participation in clinical trials. A study by Granda-Cameron et al. (2022) found that, in terms of perception of clinical trial participation, Black cancer survivors and caregivers felt more comfortable with nonphysician healthcare providers, such as nurses and nurse practitioners. Therefore, nurses from diverse racial and ethnic backgrounds should be trained and included in clinical trials. This will enable recruitment of people from diverse population groups. Nurses are usually not only the first-line healthcare providers who interact with people with cancer, but they also provide physical, emotional, and social support to individuals with cancer and their families. Disparities in cancer clinical trials are complex and challenging (Smith et al., 2021). Involving nurses to devise sound and strategic methods to recruit individuals with cancer for clinical trials may reduce some of these disparities.
Conclusion
Although the results of this study show that cancer clinical trial participation was higher among underrepresented populations, in particular among Black populations compared to White populations, continued efforts are needed to recruit people for cancer clinical trials representative of the population as a whole. Healthcare providers, including nurses, must continue to use novel strategies to recruit underrepresented individuals for cancer clinical trials. Some emerging studies show that strategic efforts, such as embedding cancer prevention programs and research in the community, can increase the participation rates of underrepresented groups in cancer clinical trials (Kim et al., 2020; Wallington et al., 2016).
About the Authors
Ami R. Moore, PhD, MPH, CPH, is an associate professor and director of the public health undergraduate program in the College of Health and Public Service at the University of North Texas in Denton; Taylor Hudson Lewis, PhD, is a senior research statistician at Research Triangle Institute International in Washington, DC; Theresa Abah, PhD, is an assistant professor in the Department of Gerontology at California State University in Sacramento; Mehmet Celebi, PhD, is an assistant professor of sociology in the department of social sciences at Nicholls State University in Thibodaux, LA; and Foster K. Amey, PhD, is a professor emeritus in the Department of Sociology and Anthropology at Middle Tennessee State University in Mufreesboro. No financial relationships to disclose. Moore contributed to the conceptualization and design. Celebi completed the data collection. Moore, Lewis, Celebi, and Amey provided statistical support. Moore, Hudson Lewis, and Amey provided the analysis. Moore, Hudson Lewis, Abah, and Amey contributed to the manuscript preparation. Moore can be reached at ami.moore@unt.edu, with copy to ONFEditor@ons.org. (Submitted November 2021. Accepted July 2, 2022.)
References
American Cancer Society Cancer Action Network. (2018). Barriers to patient enrollment in therapeutic clinical trials for cancer. https://www.fightcancer.org/policy-resources/clinical-trial-barriers
Asare, M., Flannery, M., & Kamen, C. (2017). Social determinants of health: A framework for studying cancer health disparities and minority participation in research. Oncology Nursing Forum, 44(1), 20–23. https://doi.org/10.1188/17.ONF.20-23
Begg, C.B., Zelen, M., Carbone, P.P., McFadden, E.T., Brodovsky, H., Engstrom, P., . . . Stolbach, L. (1983). Cooperative groups and community hospitals. Measurement of impact in the community hospitals. Cancer, 52(9), 1760–1767.
Benson, A.B., III, Pregler, J.P., Bean, J.A., Rademaker, A.W., Eshler, B., & Anderson, K. (1991). Oncologists’ reluctance to accrue patients onto clinical trials: An Illinois Cancer Center study. Journal of Clinical Oncology, 9(11), 2067–2075. https://doi.org/10.1200/JCO.1991.9.11.2067
Bond, M.C., & Pritchard, S. (2006). Understanding clinical trials in childhood cancer. Paediatrics and Child Health, 11(3), 148–150.
Brown, D.R., Fouad, M.N., Basen-Engquist, K., & Tortolero-Luna, G. (2000). Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Annals of Epidemiology, 10(8, Suppl.), S13–S21. https://doi.org/10.1016/s1047-2797(00)00197-6
Byrne, M.M., Tannenbaum, S.L., Glück, S., Hurley, J., & Antoni, M. (2014). Participation in cancer clinical trials: Why are patients not participating? Medical Decision Making, 34(1), 116–126. https://doi.org/10.1177/0272989X13497264
Centers for Disease Control and Prevention. (2018). The Behavioral Risk Factor Surveillance System: Complex sampling weights and preparing 2017 BRFSS module data for analysis. https://www.cdc.gov/brfss/annual_data/2017/pdf/Complex-Smple-Weights-Pr…
Centers for Disease Control and Prevention. (2020). Health disparities. https://www.cdc.gov/healthyyouth/disparities/index.htm
Chen, M.S., Jr., Lara, P.N., Dang, J.H.T., Paterniti, D.A., & Kelly, K. (2014). Twenty years post-NIH Revitalization Act: Enhancing minority participation in clinical trials (EMPaCT): Laying the groundwork for improving minority clinical trial accrual. Cancer, 120(Suppl. 7), 1091–1096. https://doi.org/10.1002/cncr.28575
Chino, F., Peppercorn, J.M., Rushing, C., Kamal, A.H., Altomare, I., Samsa, G., & Zafar, S.Y. (2017). Out-of-pocket costs, financial distress, and underinsurance in cancer care. JAMA Oncology, 3(11), 1582–1584. https://doi.org/10.1001/jamaoncol.2017.2148
Clark, L.T., Watkins, L., Piña, I.L., Elmer, M., Akinboboye, O., Gorham, M., . . . Regnante, J.M. (2019). Increasing diversity in clinical trials: Overcoming critical barriers. Current Problems in Cardiology, 44(5), 148–172. https://doi.org/10.1016/j.cpcardiol.2018.11.002
Colon-Otero, G., Smallridge, R.C., Solberg, L.A., Jr., Keith, T.D., Woodward, T.A., Willis, F.B., & Dunn, A.N. (2008). Disparities in participation in cancer clinical trials in the United States: A symptom of a healthcare system in crisis. Cancer, 112(3), 447–454. https://doi.org/10.1002/cncr.23201
Cox, K., & Avis, M. (1996). Psychosocial aspects of participation in early anticancer drug trials: Report of a pilot study. Cancer Nursing, 19(3), 177–186. https://doi.org/10.1097/00002820-199606000-00004
Coyne, C.A., Demian-Popescu, C., & Brown, P. (2004). Rural cancer patients’ perspectives on clinical trials: A qualitative study. Journal of Cancer Education, 19(3), 165–169. https://doi.org/10.1207/s15430154jce1903_11
Echeverri, M., Anderson, D., Nápoles, A.M., Haas, J.M., Johnson, M.E., & Serrano, F.S.A. (2018). Cancer health literacy and willingness to participate in cancer research and donate bio-specimens. International Journal of Environmental Research and Public Health, 15(10), 2091. https://doi.org/10.3390/ijerph15102091
Ellis, P.M., Butow, P.N., & Tattersall, M.H.N. (2002). Informing breast cancer patients about clinical trials: A randomized clinical trial of an educational booklet. Annals of Oncology, 13(9), 1414–1423. https://doi.org/10.1093/annonc/mdf255
Eskander, M.F., Gil, L., Beal, E.W., Li, Y., Hamad, A., Oppong, B., . . . Tsung, A. (2022). Access denied: Inequities in clinical trial enrollment for pancreatic cancer. Annals of Surgical Oncology, 29(2), 1271–1277. https://doi.org/10.1245/s10434-021-10868-4
Fleissig, A., Jenkins, V., & Fallowfield, L. (2001). Results of an intervention study to improve communication about randomised clinical trials of cancer therapy. European Journal of Cancer, 37(3), 322–331. https://doi.org/10.1016/s0959-8049(00)00415-9
Go, R.S., Frisb, K.A., Lee, J.A., Mathiason, M.A., Meyer, C.M., Ostern, J.L., . . . Umberger, K.E. (2006). Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer, 106(2), 426–433. https://doi.org/10.1002/cncr.21597
Granda-Cameron, C., Florence, Y.M., Whitfield-Harris, L., Kates, J., & Lenzo, J.M. (2022). Perceptions of clinical trial participation in African American cancer survivors and caregivers. Oncology Nursing Forum, 49(2), 113–124. https://doi.org/10.1188/22.onf.113-124
Green, S., Benedetti, J., Smith, A., & Crowley, J. (2012). Clinical trials in oncology (Vol. 28). CRC Press.
Hamel, L.M., Penner, L.A., Albrecht, T.L., Heath, E., Gwede, C.K., & Eggly, S. (2016). Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control, 23(4), 327–337. https://doi.org/10.1177/107327481602300404
Howerton, M.W., Gibbons, M.C., Baffi, C.R., Gary, T.L., Lai, G.Y., Bolen, S., . . . Ford, J.G. (2007). Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer, 109(3), 465–476. https://doi.org/10.1002/cncr.22436
Hunger, S.P., Lu, X., Devidas, M., Camitta, B.M., Gaynon, P.S., Winick, N.J., . . . Carroll, W.L. (2012). Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children’s Oncology Group. Journal of Clinical Oncology, 30(14), 1663–1669. https://doi.org/10.1200/jco.2011.37.8018
Hunter, C.P., Frelick, R.W., Feldman, A.R., Bavier, A.R., Dunlap, W.H., Ford, L., . . . Yates, J.W. (1987). Selection factors in clinical trials: Results from the Community Clinical Oncology Program Physician’s Patient Log. Cancer Treatment Reports, 71(6), 559–565.
Ianchan, R., Pierannunzi, C., Healy, K., Greenlund, K.J., & Town, M. (2016). National weighting of data from the Behavioral Risk Factor Surveillance System (BRFSS). BMC Medical Research Methodology, 16. https://doi.org/ 10.1186/s12874-016-0255-7
Ibraheem, A., & Polite, B. (2017). Improving the accrual of racial and ethnic minority patients in clinical trials: Time to raise the stakes. Cancer, 123(24), 4752–4756. https://doi.org/10.1002/cncr.31073
Institute of Medicine. (2010). Redesigning continuing education in the health professions. National Academies Press.
Javid, S.H., Unger, J.M., Gralow, J.R., Moinpour, C.M., Wozniak, A.J., Goodwin, J.W., . . . Albain, K.S. (2012). A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). Oncologist, 17(9), 1180–1190. https://doi.org/10.1634/theoncologist.2011-0384
Jenkins, V.A., Fallowfield, L.J., Souhami, A., & Sawtell, M. (1999). How do doctors explain randomised clinical trials to their patients? European Journal of Cancer, 35(8), 1187–1193. https://doi.org/10.1016/s0959-8049(99)00116-1
Joseph, G., & Dohan, D. (2009a). Diversity of participants in clinical trials in an academic medical center: The role of the ‘Good Study Patient?’ Cancer, 115(3), 608–615. https://doi.org/10.1002/cncr.24028
Joseph, G., & Dohan, D. (2009b). Recruiting minorities where they receive care: Institutional barriers to cancer clinical trials recruitment in a safety-net hospital. Contemporary Clinical Trials, 30(6), 552–559. https://doi.org/10.1016/j.cct.2009.06.009
Kemeny, M.M., Peterson, B.L., Kornblith, A.B., Muss, H.B., Wheeler, J., Levine, E., . . . Cohen, H.J. (2003). Barriers to clinical trial participation by older women with breast cancer. Journal of Clinical Oncology, 21(12), 2268–2275. https://doi.org/10.1200/JCO.2003.09.124
Kilbourne, A.M., Switzer, G., Hyman, K., Crowley-Matoka, M. & Fine, M.J. (2006). Advancing health disparities research within the health care system: A conceptual framework. American Journal of Public Health, 96(12), 2113–2121. https://doi.org/10.2105/ajph.2005.077628
Kim, D.J., Otap, D., Ruel, N., Gupta, N., Khan, N., & Dorff, T. (2020). NCI-clinical trial accrual in a community network affiliated with a designated cancer center. Journal of Clinical Medicine, 9(6), 1970. https://doi.org/10.3390/jcm9061970
Klabunde, C.N., Springer, B.C., Butler, B., White, M.S., & Atkins, J. (1999). Factors influencing enrollment in clinical trials for cancer treatment. Southern Medical Journal, 92(12), 1189–1193. https://doi.org/10.1097/00007611-199912000-00011
Lewis, T. (2016). Complex survey data analysis with SAS®. Chapman and Hall/CRC Press.
Loree, J.M., Anand, S., Dasari, A., Unger, J.M., Gothwal, A., Ellis, L.M., . . . Raghav, K. (2019). Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008–2018. JAMA Oncology, 5(10), e191870. https://doi.org/10.1001/jamaoncol.2019.1870
Mahipal, A., & Nguyen, D. (2014). Risks and benefits of phase 1 clinical trial participation. Cancer Control, 21(3), 193–199. https://doi.org/10.1177/107327481402100303
Markham, M.J., Wachter, K., Agarwal, N., Bertagnolli, M.M., Chang, S.M., Dale, W., . . . Westin, S.N. (2020). Clinical cancer advances 2020: Annual report on progress against cancer from the American Society of Clinical Oncology. Journal of Clinical Oncology, 38(10), 1081. https://doi.org/10.1200/jco.19.03141
Melisko, M.E., Hassin, F., Metzroth, L., Moore, D.H., Brown, B., Patel, K., . . . Tripathy, D. (2005). Patient and physician attitudes toward breast cancer clinical trials: Developing interventions based on understanding barriers. Clinical Breast Cancer, 6(1), 45–54. https://doi.org/10.3816/CBC.2005.n.008
Meropol, N.J., Buzaglo, J.S., Millard, J., Damjanov, N., Miller, S.M., Ridgway, C., . . . Watts, P. (2007). Barriers to clinical trial participation as perceived by oncologists and patients. Journal of the National Comprehensive Cancer Network, 5(8), 655–664. https://doi.org/10.6004/jnccn.2007.0067
Minasian, L.M., & Unger, J.M. (2020). What keeps patients out of clinical trials? JCO Oncology Practice, 16(3), 125–127. https://doi.org/10.1200/jop.19.00735
Murthy, V.H., Krumholz, H.M., & Gross, C.P. (2004). Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA, 291(22), 2720–2726. https://doi.org/10.1001/jama.291.22.2720
National Institutes of Health Revitalization Act of 1993. (1993). S.1—National Institutes of Health Revitalization Act of 1993. https://www.congress.gov/bill/103rd-congress/senate-bill/1
Nazha, B., Mishra, M., Pentz, R., & Owonikoko, T.K. (2019). Enrollment of racial minorities in clinical trials: Old problem assumes new urgency in the age of immunotherapy. American Society of Clinical Oncology Educational Book, 39, 3–10. https://doi.org/10.1200/edbk_100021
Nipp, R.D., Hong, K., & Paskett, E.D. (2019). Overcoming barriers to clinical trial enrollment. American Society of Clinical Oncology Educational Book, 39, 105–114. https://doi.org/10.1200/edbk_243729
Perni, S., Moy, B., & Nipp, R.D. (2021). Disparities in phase 1 cancer clinical trial enrollment. Cancer, 127(23), 4464–4469. https://doi.org/10.1002/cncr.33853
Pinto, H.A., McCaskill-Stevens, W., Wolfe, P., & Marcus, A.C. (2000). Physician perspectives on increasing minorities in cancer clinical trials: An Eastern Cooperative Oncology Group (ECOG) initiative. Annals of Epidemiology, 10(8, Suppl.), S78–S84. https://doi.org/10.1016/s1047-2797(00)00191-5
Ramsey, S., Blough, D., Kirchhoff, A., Kreizenbeck, K., Fedorenko, C., Snell, K., . . . Overstreet, K. (2013). Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Affairs, 32(6), 1143–1152. https://doi.org/10.1377/hlthaff.2012.1263
Ramsey, S.D., Bansal, A., Fedorenko, C.R., Blough, D.K., Overstreet, K.A., Shankaran, V., & Newcomb, P. (2016). Financial insolvency as a risk factor for early mortality among patients with cancer. Journal of Clinical Oncology, 34(9), 980–986. https://doi.org/10.1200/jco.2015.64.6620
Rivers, D., August, E.M., Sehovic, I., Green, B.L., & Quinn, G.P. (2013). A systematic review of the factors influencing African Americans’ participation in cancer clinical trials. Contemporary Clinical Trials, 35(2), 13–32. https://doi.org/10.1016/j.cct.2013.03.007
Ross, S., Grant, A., Counsel, C., Gillespie, W., Russell, I., & Prescott, R. (1999). Barriers to participation in randomised controlled trials: A systematic review. Journal of Clinical Epidemiology, 52(12), 1143–1156. https://doi.org/10.1016/s0895-4356(99)00141-9
Sabin, J.A., Rivara, F.P., & Greenwald, A.G. (2008). Physician implicit attitudes and stereotypes about race and quality of medical care. Medical Care, 46(7), 678–685. https://doi.org/10.1097/mlr.0b013e3181653d58
Sedrak, M.S., Freedman, R.A., Cohen, H.J., Muss, H.B., Jatoi, A., Klepin, H.D., . . . Dale, W. (2021). Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA: A Cancer Journal for Clinicians, 71(1), 78–92. https://doi.org/10.3322/caac.21638
Simon, M.A., Haring, R., Rodriguez, E.M., González, E., Kaur, J.S., Kirschner, M., . . . Katz, M.L. (2019). Improving research literacy in diverse minority populations with a novel communication tool. Journal of Cancer Education, 34(6), 1120–1129. https://doi.org/10.1007/s13187-018-1418-5
Simon, M.A., O’Brian, C.A., Tom, L., Wafford, Q.E., Mack, S., Mendez, S.R., . . . Holmes, K.L. (2021). Development of a web tool to increase research literacy in underserved populations through public library partnerships. PLOS ONE, 16(2), e0246098. https://doi.org/10.1371/journal.pone.0246098
Slevin, M., Mossman, J., Bowling, A., Leonard, R., Steward, W., Harper, P., . . . Thatcher, N. (1995). Volunteers or victims: Patients’ views of randomised cancer clinical trials. British Journal of Cancer, 71(6), 1270–1274. https://doi.org/10.1038/bjc.1995.245
Smith, S.M., Wachter, K., Burris, H.A., III, Schisky, R.L., George, D.J., Peterson, D.E., . . . Uzzo, R. (2021). Clinical cancer advances 2021: ASCO’s report on progress against cancer. Journal of Clinical Oncology, 39(10), 1165–1184. https://doi.org/10.1200/jco.20.03420
Solomon, M.J., Pager, C.K., Young, J.M., Roberts, R., & Butow, P. (2003). Patient entry into randomized controlled trials of colorectal cancer treatment: Factors influencing participation. Surgery, 133(6), 608–613. https://doi.org/10.1067/msy.2003.119
Somkin, C.P., Altschuler, A., Ackerson, L., Geiger, A.M., Greene, S.M., Mouchawar, J., . . . Wagner, E. (2005). Organizational barriers to physician participation. American Journal of Managed Care, 11(7), 413–421.
Stewart, B.A., & Stewart, J.H., IV. (2022). Disparities in clinical trial participation: Multilevel opportunities for improvement. Surgical Oncology Clinics of North America, 31(1), 55–64. https://doi.org/10.1016/j.soc.2021.07.007
Taylor, K.M., Margolese, R.G., & Soskolne, C.L. (1984). Physicians’ reasons for not entering eligible patients in a randomized clinical trial of surgery for breast cancer. New England Journal of Medicine, 310(21), 1363–1367. https://doi.org/10.1056/NEJM198405243102106
Unger, J.M., Cook, E., Tai, E., & Bleyer, A. (2016). The role of clinical trial participation in cancer research: Barriers, evidence, and strategies. American Society of Clinical Oncology Educational Book, 36, 185–198. https://doi.org/10.1200/edbk_156686
Unger, J.M., Hershman, D.L., Osarogiagbon, R.U., Gothwal, A., Anand, S., Dasari, A., . . . Raghav, K. (2020). Representativeness of Black patients in cancer clinical trials sponsored by the National Cancer Institute compared with pharmaceutical companies. JNCI Cancer Spectrum, 4(4), pkaa034. https://doi.org/10.1093/jncics/pkaa034
Unger, J.M., Hershman, D.L., Till, C., Minasian, L.M., Osarogiagbon, R.U., Fleury, M.E., & Vaidya, R. (2021). “When offered to participate”: A systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. Journal of the National Cancer Institute, 113(3), 244–257. https://doi.org/10.1093/jnci/djaa155
Unger, J.M., Vaidya, R., Hershman, D.L., Minasian, L.M., & Fleury, M.E. (2019). Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. Journal of the National Cancer Institute, 113(3), 245–255. https://doi.org/10.1093/jnci/djy221
U.S. Food and Drug Administration. (2020). 2015–2019 drug trials snapshots summary report. https://www.fda.gov/media/143592/download
Wallington, S.F., Dash, C., Sheppard, V.B., Goode, T.D., Oppong, B.A., Dodson, E.E., . . . Adams-Campbell, L.L. (2016). Enrolling minority and underserved populations in cancer clinical research. American Journal of Preventive Medicine, 50(1), 111–117. https://doi.org/10.1016/j.amepre.2015.07.036
Wendler, D., Kington, R., Madans, J., Van Wye, G., Christ-Schmidt, H., Pratt, L.A., . . . Emanuel, E. (2005). Are racial and ethnic minorities less willing to participate in health research? PLOS Medicine, 3(2), e19. https://doi.org/10.1371/journal.pmed.0030019
Winkfield, K.M., Phillips, J.K., Joffe, S., Halpern, M.T., Wollins, D.S., & Moy, B. (2018). Addressing financial barriers to patient participation in clinical trials: ASCO policy statement. Journal of Clinical Oncology, 36(33), 3331–3339. https://doi.org/10.1200/JCO.18.01132
Witte, S.S., El-Bassel, N., Gilbert, L., Wu, E., Chang, M., & Steinglass, P. (2004). Recruitment of minority women and their main sexual partners in an HIV/STI prevention trial. Journal of Women’s Health, 13(10), 1137–1147. https://doi.org/10.1089/jwh.2004.13.1137
Wong, Y.-N., Schluchter, M.D., Albrecht, T.L., Benson, A.B., III, Buzaglo, J., Collins, M., . . . Meropol, N.J. (2016). Financial concerns about participation in clinical trials among patients with cancer. Journal of Clinical Oncology, 34(5), 479–487. https://doi.org/10.1200/JCO.2015.63.2463
Zonderman, A.B., Ejiogu, N., Norbeck, J., & Evans, M.K. (2014). The influence of health disparities on targeting cancer prevention efforts. American Journal of Preventive Medicine, 46(3, Suppl. 1), S87–S97. https://doi.org/10.1016/j.amepre.2013.10.026


