Associations of Demographic and Social Factors on Health-Related Quality-of-Life Changes Among Older Women With Breast or Gynecologic Cancer
Objectives: To examine associations of sociodemographic factors and social limitations with health-related quality of life (HRQOL) from pre- to postdiagnosis in older female cancer survivors.
Sample & Setting: 9,807 women aged 65 years or older with breast or gynecologic cancer from the Surveillance, Epidemiology, and End Results–Medicare Health Outcomes Survey.
Methods & Variables: Physical and mental HRQOL were assessed using the physical component summary (PCS) and mental component summary (MCS) of the Veterans RAND 12-Item Health Survey. Descriptive statistics and mixed-effects models for repeated measures were used.
Results: Social limitations were the only significant factor associated with changes in MCS scores. Race and ethnicity, rurality, and social interference were associated with significant decreases in PCS scores.
Implications for Nursing: Nurses can assess mental and physical HRQOL after diagnosis and advocate for appropriate referrals. Oncology care should be tailored to cultural considerations, including race and ethnicity, rurality, and social support.
Jump to a section
With the aging U.S. population and advances in the prevention, screening, and treatment of cancer continuing to accumulate, quality and length of life in cancer survivorship has become a pressing issue. Of particular interest are older cancer survivors (aged 65 years or older), who are a rapidly growing segment of the cancer survivor population (Bluethmann et al., 2016). In 2022, an estimated 67% of the more than 18 million cancer survivors living in the United States were aged 65 years or older. By 2040, these figures are projected to increase to 26.1 million cancer survivors, of whom an estimated 73% will be aged 65 years or older. In addition, there is a growing population of older breast and gynecologic cancer survivors, accounting for about 43% of new cancer diagnoses in U.S. women (Siegel et al., 2024). In addition to the hallmarks of aging, which include declines in functional capacity and a greater prevalence of preexisting comorbidities, older cancer survivors also contend with social vulnerabilities (i.e., social influences on health) that may jeopardize their cancer survivorship experience (Rowland & Bellizzi, 2014; Williams et al., 2016).
Health-related quality of life (HRQOL) is an individual’s perceived physical and mental health, which can be influenced by an individual’s clinical status and demographic background (Reeve et al., 2009). HRQOL has been associated with a myriad of longitudinal outcomes among older adults, including overall cancer risk and survival (Klapheke et al., 2020b; Mukand et al., 2022; Park et al., 2021; Smith et al., 2008). Higher HRQOL is also associated with more frequent social and emotional support received by adult cancer survivors (Gudina et al., 2021). In addition, older cancer survivors have considered HRQOL as more important than survival gains when making decisions about cancer treatment (Bhandari et al., 2020; Dos Santos Hughes & Malone, 2023; Kent et al., 2015). Tracking changes in HRQOL could offer potential opportunities for early identification of and appropriate interventions for patients at risk for poor outcomes (Bhandari et al., 2020).
Despite the need for longitudinal exploration, a majority of research on HRQOL includes single assessments of post–cancer diagnosis HRQOL and makes comparisons to disease-free older adults. The small number of studies that have focused on pre- and postdiagnosis HRQOL found significant changes in HRQOL over time and lower HRQOL among older cancer survivors compared to the general older adult population (Klapheke et al., 2020b; Stover et al., 2014). In addition, there was considerable variability in HRQOL trajectories among cancer survivors (Durá-Ferrandis et al., 2017; Jones et al., 2015) in part because of sociodemographic factors. An unexplored area in this population is how self-reported sociodemographic characteristics (e.g., race and ethnicity, rurality) and social limitations (i.e., health interfering with social activities) are associated with changes in HRQOL. Previous cross-sectional research found that members of racial and ethnic minority groups and those living in rural areas have lower HRQOL compared to their non-Hispanic White and/or urban-dwelling peers (Moss et al., 2021; Pinheiro et al., 2015; Rao et al., 2008). In addition, older cancer survivors who did not have a partner and experienced lower social support and greater health impacts on social functioning reported lower HRQOL (Han et al., 2021; Leung et al., 2016; Zandbergen et al., 2019). Expanding on prior analyses of women with a breast or gynecologic cancer diagnosis, this study examines the associations of sociodemographic factors and social limitations with changes in HRQOL from pre- to postdiagnosis among older women with breast or gynecologic cancer.
Methods
Dataset and Study Population
This study was approved by the Ohio State University Cancer Institutional Review Board (#2020C0068). The data for this study were derived from the Surveillance, Epidemiology, and End Results–Medicare Health Outcomes Survey (SEER-MHOS) combined dataset (Ambs et al., 2008). In brief, SEER-MHOS provides detailed cancer registry data (e.g., cancer type[s], stage, histology, diagnosis date) from selected U.S. regions, as well as self-administered, longitudinal survey data assessing physical and mental functioning, in addition to functional status, smoking status, and chronic medical conditions. Medicare beneficiaries were included in this analysis if they were self-reported female, were aged 65 years or older, had exactly one breast or gynecologic (e.g., ovarian, endometrial, cervical, vulvar, vaginal) cancer, had at least one complete MHOS datapoint between 1998 and 2017 and within five years of their cancer diagnosis, and had completed at least one survey before diagnosis and one after.
HRQOL Outcomes
Physical and mental health statuses were assessed using the Veterans RAND 12-Item Health Survey (VR-12), a brief, generic, multiuse, self-administered health survey consisting of 12 items related to 8 physical and mental health domains: general health perceptions, physical functioning, role limitations because of physical problems, role limitations because of emotional problems, body pain, energy–fatigue, social functioning, and mental health (Selim et al., 2009). Physical component summary (PCS) and mental component summary (MCS) scores were then calculated and used as outcomes in this study. Scores range from 0 to 100, with higher scores indicating better HRQOL. Previous research has established a three-point minimal clinically important difference in VR-12 scores (Hays et al., 2005; Pandit et al., 2022; Revicki et al., 2008). Studies have demonstrated good internal consistency and reliability of the VR-12, with a Cronbach’s alpha of 0.09 for the PCS and MCS subscales (Kazis et al., 2006; Kent et al., 2015).
Independent Variables
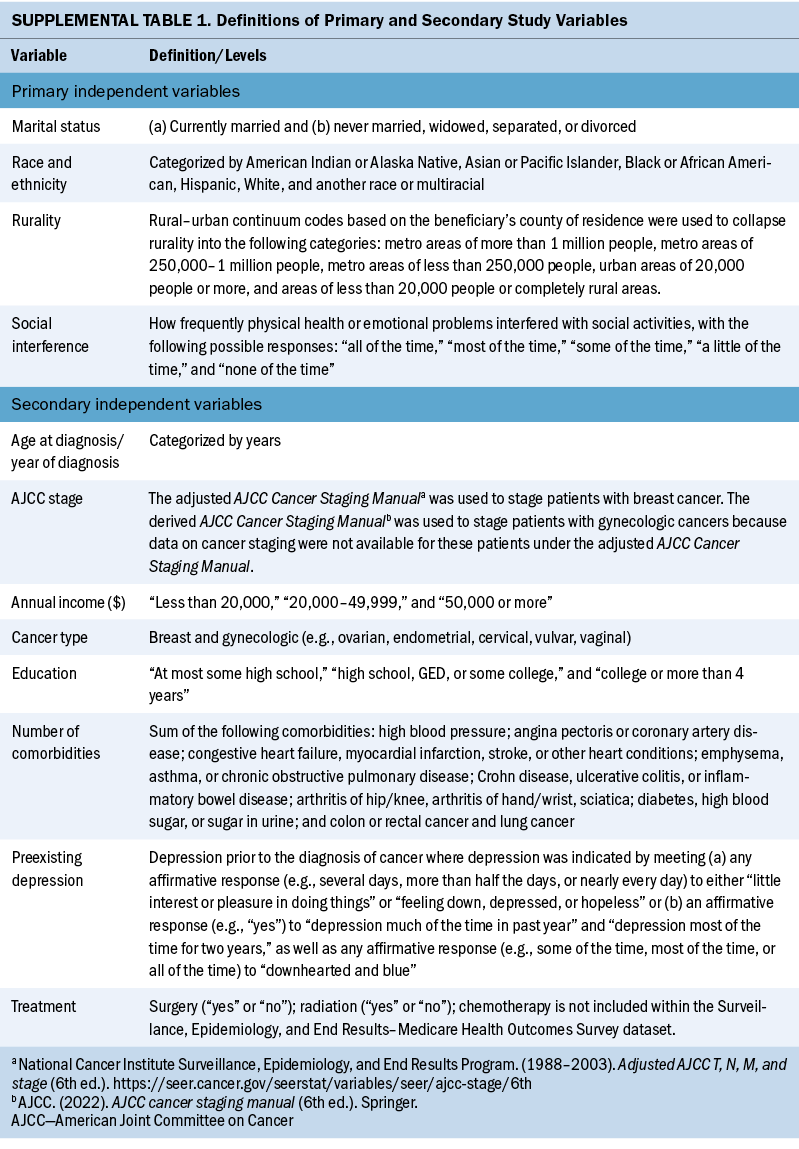
This study had four main independent variables of interest: social interference, rurality, race and ethnicity, and marital status. For social interference, beneficiaries were specifically asked how frequently physical health or emotional problems interfered with social activities, with the following possible responses: “all of the time,” “most of the time,” “some of the time,” “a little of the time,” and “none of the time.” To assess rurality, rural–urban continuum codes based on the beneficiary’s county of residence were used (Economic Research Service, 2023; Moss et al., 2021). Other variables in this study included the following: cancer treatment (e.g., radiation therapy, surgery), preexisting depressive symptoms as outlined by Klapheke et al. (2020a), educational attainment, annual income, comorbidity burden, and American Joint Committee on Cancer stage. See Supplemental Table 1 online for definitions of the variables included in this study.
Statistical Analysis
Descriptive statistics of patient demographic and clinical characteristics included medians and interquartile ranges (IQRs) for continuous variables and frequencies and percentages for categorical variables. A repeated-measures mixed-effects model was used to assess mean MCS and PCS scores over time. An unstructured covariance matrix parameterized through its Cholesky root was used to model the covariance matrix for each participant, and the marginal likelihood was approximated using Laplace’s method. Adjusted mean MCS and PCS scores were assessed pre- and post–cancer diagnoses for the primary independent variables, and the significance of the change in pre- and postdiagnosis estimates was evaluated at the 0.05 level. In addition, adjusted mean MCS and PCS scores were evaluated at each month using locally estimated scatter plot smoothing, with a smoothing parameter of 0.6. All analyses were performed using SAS, version 9.4.
Results
A total of 9,807 older women with breast or gynecologic cancer were included in this study (see Table 1). More than 80% (n = 8,000) of the sample had breast cancer, 66% (n = 6,482) had less than $50,000 in annual income, 60% (n = 5,894) had at least completed high school, and the median age at diagnosis was 74 years (IQR = 70–79). The median number of comorbidities was two (IQR = 1–3), and 5% (n = 451) of participants had preexisting depressive symptoms. More than half (n = 5,400) of participants had stage I cancer, 92% (n = 9,041) of participants received surgical treatment for their cancer, and 45% (n = 4,387) received radiation therapy treatment. Although most demographic and clinical characteristics were similar between older women with breast and gynecologic cancer, the median survey follow-up time for those with breast cancer was two years longer than for those with gynecologic cancer (median follow-up = 7.6 years and 5.6 years, respectively). At the first survey, median MCS and PCS scores were 53 (IQR = 43–60) and 39 (IQR = 29–49), respectively, and did not differ by cancer type (see Table 2). Most participants were White (n = 6,987, 71%), lived in a metro area with a population of one million or greater (n = 5,765, 59%), or indicated on their first survey that physical health or emotional problems never interfered with social activities (n = 4,988, 51%).
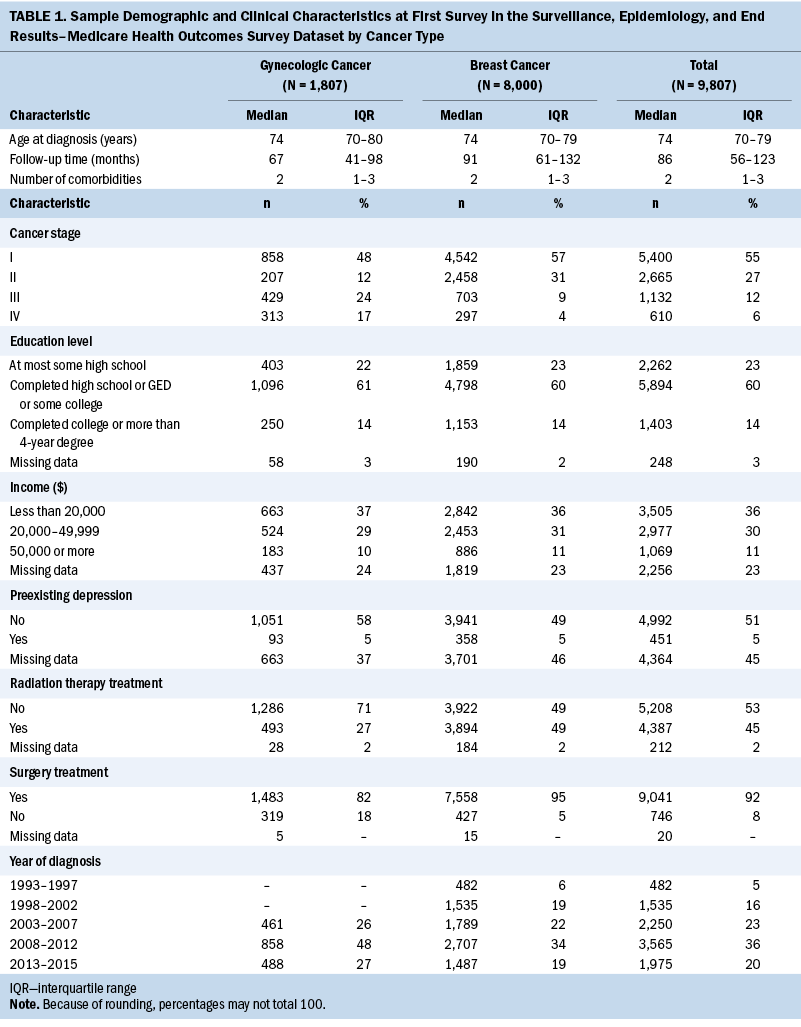
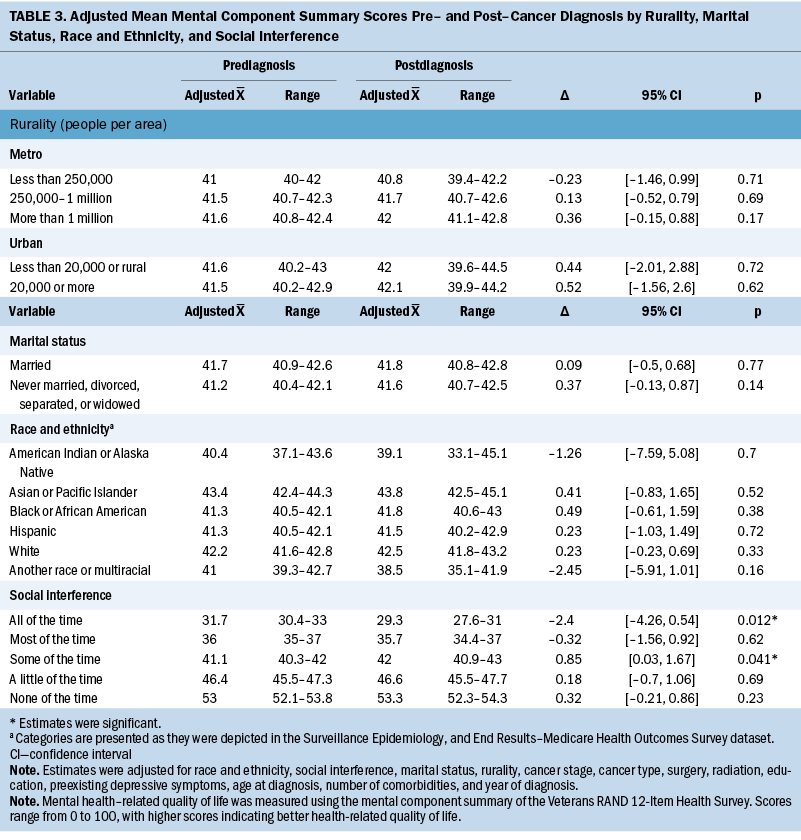
Overall, no change in MCS scores was observed from before and after cancer diagnosis (Δ = 0.27, 95% confidence interval [CI] [–0.14, 0.67], p = 0.2) (see Table 3). Associations between patient factors and changes in MCS revealed no significant relationships with rurality, social interference, race and ethnicity, or marital status. In addition, no trends were observed across rurality and social interference; however, the most severe category of social interference (i.e., “all of the time”) was associated with a significant decrease in MCS scores. Specifically, among participants who reported having social interference “all of the time” (i.e., interference of physical or emotional health on social activities), MCS scores decreased 2.4 points on average (95% CI [–4.26, –0.54], p = 0.012).
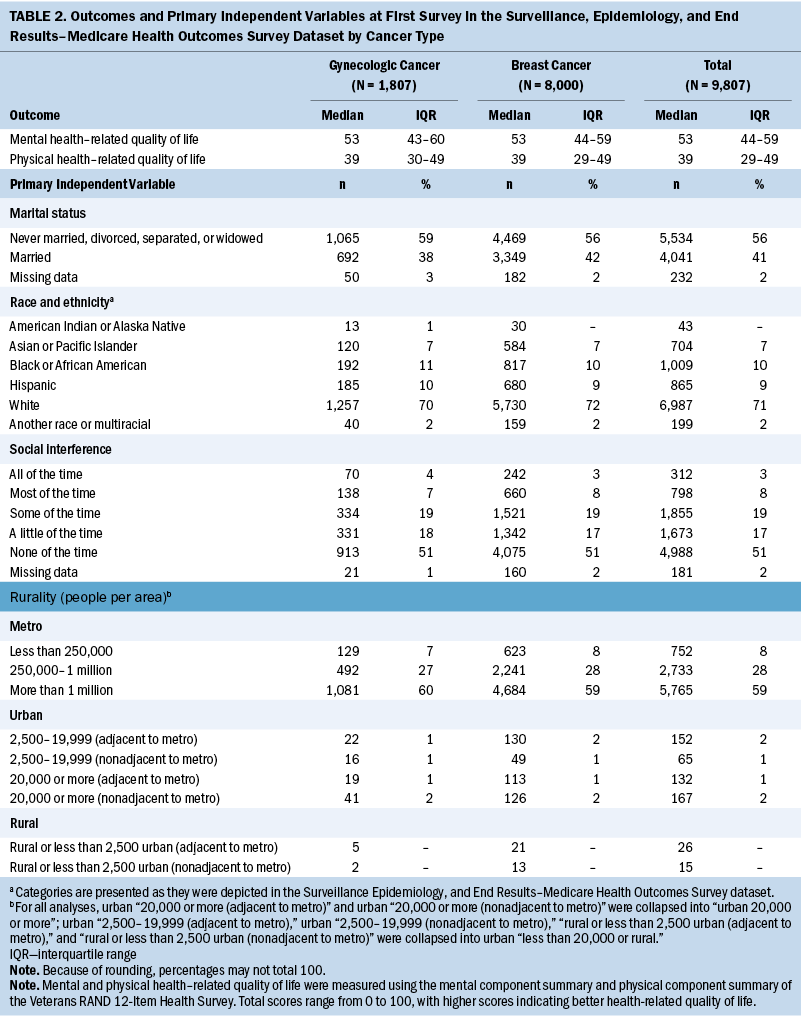
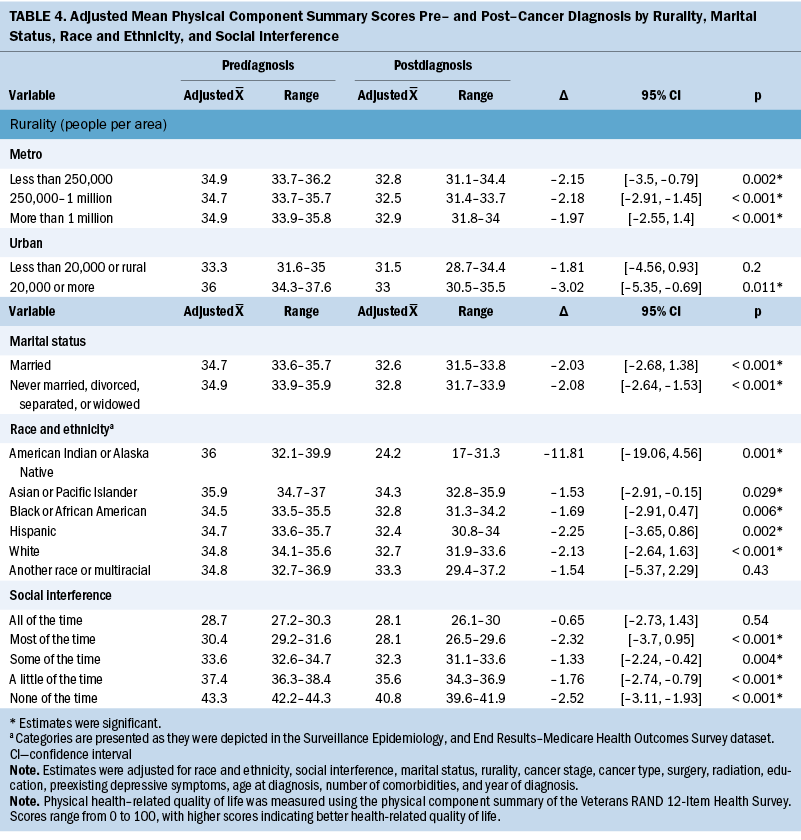
Adjusted PCS scores decreased significantly overall from 34.8 to 32.7 (Δ = –2.06, 95% CI [–2.51, –1.61], p < 0.001) (see Table 4). In addition, variability in the changes of adjusted mean PCS scores was noted for all primary independent variables. In particular, changes in PCS scores ranged from –3.02 for participants in areas of 20,000 people or more (95% CI [–5.35, –0.69], p = 0.011) to –1.81 in rural or urban areas of less than 20,000 people (95% CI [–4.56, 0.93], p = 0.2). For social interference, significant decreases in PCS scores ranged from –1.33 to –2.52, where those who experienced social interference “none of the time” had the largest reduction in PCS scores (95% CI [–3.11, –1.93], p < 0.001), and participants who experienced social interference “some of the time” (i.e., interference of physical or emotional health on social activities) had the smallest significant change in scores (95% CI [–2.24, –0.42], p = 0.004). Of note, participants who reported experiencing social interference “all of the time” did not have a significant decrease in mean PCS score (Δ = –0.65, 95% CI [–2.73, 1.43], p = 0.54). Regarding race and ethnicity, significant decreases in PCS scores ranged from –1.53 to –11.81, where those who identified as American Indian or Alaska Native (AIAN) had the largest reduction in PCS scores (95% CI [–19.06, –4.56], p = 0.001), and those who identified as Asian or Pacific Islander had the smallest decrease in PCS scores (95% CI [–2.91, –0.15], p = 0.029). Participants who identified as another race or multiracial did not have a significant decrease in mean PCS scores (Δ = –1.54, 95% CI [–5.37, 2.29], p = 0.43). Both marital status groups had significant decreases in PCS scores before and after cancer diagnosis; however, the change was slightly larger among those who were unmarried (Δ = –2.08, 95% CI [–2.64, –1.53], p < 0.001) compared to those who were married (Δ = –2.03, 95% CI [–2.68, –1.38], p < 0.001).
Figures 1 and 2 show the changes in mean PCS and MCS scores over time. Briefly, except for social interference, stratifications demonstrated varied temporal changes in PCS and MCS around the time of cancer onset. For example, patients who were married had relatively stable PCS more than two years before and two years after cancer diagnosis; however, there was a substantial drop around the time of cancer diagnosis. Conversely, married patients had a decrease in MCS leading up to the diagnosis of cancer and then, during the following five years, increased to near-baseline levels.
Discussion
This study examined factors associated with changes in HRQOL from pre- to postdiagnosis in a cohort of older women diagnosed with breast or gynecologic cancer. Results indicated that HRQOL changed over time. In particular, physical HRQOL decreased over time, which is supported by the literature (Karlsen et al., 2016; Leung et al., 2014). Previous longitudinal studies have shown that changes in mental HRQOL after cancer diagnosis generally followed a U-shaped trajectory, demonstrating a return to precancer levels of HRQOL (Sauer et al., 2019; Stover et al., 2014). This return may demonstrate the psychological resilience of older cancer survivors in the face of potentially life-threatening illnesses (Seiler & Jenewein, 2019). However, of note, physical, psychological, and social difficulties are reported by older breast and gynecologic cancer survivors years after diagnosis (Bernardo et al., 2023; Krok-Schoen et al., 2018).
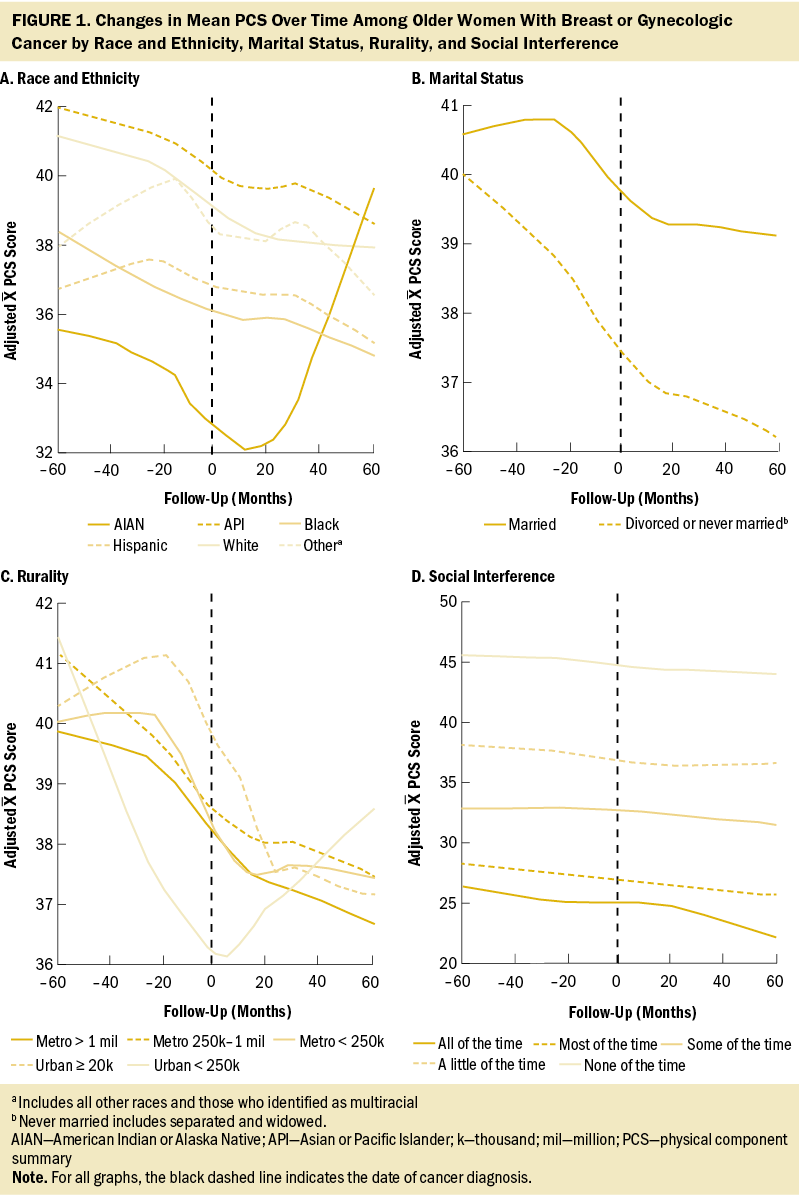
Social interference was the only significant factor associated with changes in mental HRQOL. Older women reporting the most severe category of social interference (i.e., interference of physical or emotional health on social activities “all of the time”) had the lowest mental HRQOL scores and experienced significant decreases in their scores. Older women who experienced social interference “some of the time” also had significant decreases in their mental HRQOL scores. Older women who experienced social interference “most of the time” through “none of the time” also experienced significant decreases in their physical HRQOL scores. However, among women with social interference “all of the time,” there was no significant change. This finding is counterintuitive and may be because of a floor effect, meaning that at prediagnosis, these women had poor physical health. Additional research with larger sample sizes is warranted to explore this finding.
Interference of physical health or emotional problems on social activities has been shown to reduce HRQOL among older adults and cancer survivors (Hawton et al., 2011). Social activity interference can also negatively affect other outcomes among older cancer survivors. For example, Trevino et al. (2020) found that social activity interference was associated with a greater likelihood of receipt of mental health services among older cancer survivors. Additional research (e.g., focus groups, semistructured interviews) is needed to further examine how cancer and aging can affect social activity among older cancer survivors, as well as methods (e.g., technology, informal groups) to increase social engagement despite poorer health status.
There were several significant determinants of change in physical HRQOL from pre- to postdiagnosis among older women diagnosed with breast or gynecologic cancer. The most drastic drop of 11.8 points occurred among older women who self-identified as AIAN, which was well above the three-point minimal clinically important difference in VR-12 scores (Hays et al., 2005; Pandit et al., 2022; Revicki et al., 2008). The prediagnosis median score of 36 is similar to the previously reported mean VR-12 PCS score of 36.2 among older AIAN people. A potential reason for this finding may be the poor health status of older AIAN adults compared to their White counterparts (Du & Xu, 2016; Shippee et al., 2020; Yoo et al., 2014). AIAN communities experience significant inequity in health care and poorer health status, including lower life expectancy and a disproportionate disease burden compared to other U.S. populations (Kruse et al., 2022; Sequist, 2021). The Indian Health Service, which provides health care to AIAN people, is consistently underfunded, understaffed with limited technology, and often located at lengthy distances from AIAN people, which further exacerbates these health disparities (Cromer et al., 2019; Kruse et al., 2022). Specific to this study’s population, Kim et al. (2022) found that older AIAN adults had the greatest number of chronic conditions and the highest prevalence of difficulties with activities of daily living compared to older adults from all other racial and ethnic groups. This result may also be attributed to the small sample size of this group within the cohort and outliers affecting the mean PCS score.
Older women from the other racial and ethnic groups (White, Black, Hispanic, Asian), apart from those identifying as multiracial or another race, experienced significant reductions in physical HRQOL pre– and post–cancer diagnosis compared to older AIAN women. The current study’s findings are consistent with those of other studies of cancer survivors, indicating that racial or ethnic differences in HRQOL at follow-up are negligible and clinically insignificant (i.e., less than three points) (Pinheiro et al., 2015; Rao et al., 2008). Despite a lack of differences in PCS scores between racial and ethnic groups, older adults of racial and ethnic minority groups may have unique needs, including diverse health beliefs or attitudes, and may report or perceive their health status differently than their non-Hispanic White counterparts (Ng et al., 2014). Therefore, it is imperative for researchers and clinicians to communicate with older cancer survivors to understand and, ideally, address their comprehensive health needs, health and treatment priorities, experiences with current and historic racism, and barriers to improve their physical HRQOL.
Physical HRQOL was higher among metro-dwelling older women compared to rural-dwelling older women. In addition, older women who lived in areas with populations of 20,000–250,000 people had the highest physical HRQOL before cancer diagnosis, then had a clinically meaningful decrease (more than three points) in physical HRQOL postdiagnosis. Similarly, previous studies (Moss et al., 2021; Weaver et al., 2013) have found HRQOL scores were higher among older cancer survivors in urban areas than in rural areas. Lower HRQOL among rural populations may be attributed to rural–urban disparities in access to healthcare providers, including supportive care, and may give rise to differences in treatment options and, subsequently, HRQOL. However, the high level of social connectedness in rural communities may provide some buffer to reduced HRQOL among older cancer survivors in these areas (Garrard et al., 2017; Rivera et al., 2023). Additional information about barriers to supportive care experienced by rural- and urban-dwelling older cancer survivors, including perceptions and opinions of supportive care, availability and use of telehealth, distance traveled to appointments, built environment, the role of social networks, and unmet needs, would provide some context to these observed differences.
In this sample, significant decreases in mean PCS scores were observed for married and unmarried older women, which deviates from a study that found that HRQOL differed by marital status among older cancer survivors (Karlsen et al., 2016). Measuring the quality of multiple social relationships (e.g., family, friends, significant other) in addition to marital status could help to discern this finding (Durá-Ferrandis et al., 2017). A potential explanation for this observation and the overall declines in physical HRQOL observed may be the age of the sample itself. This study included HRQOL measures in a 10-year span, during which physical health can decline among older adults. Previous research has demonstrated the additive effects of cancer treatments on physical deficits among older cancer survivors compared to cancer-free controls (Kenzik et al., 2015; Williams et al., 2020).
Strengths and Limitations
The large sample size and relative representativeness of the population were strengths of the study. However, the results of this study should be considered in light of several limitations. First, survivor bias is inherent in the SEER-MHOS dataset, which can result in a healthier sample size reporting higher physical and mental HRQOL. Sample sizes of older women identifying themselves as AIAN and multiple races or other race were small as were the sample sizes of rural and smaller urban populations, limiting generalizability. Last, physical or mental health interference in social activities was limited to a single item, which may not recapitulate a validated scale. However, the VR-12 is a commonly used measure and has been validated in previous studies of older cancer survivors within the SEER-MHOS (Doll et al., 2017; Kent et al., 2015). Qualitative research is warranted to understand the context of HRQOL among older women with and without cancer. Future research should explore the associations of sociodemographic variables and HRQOL before and after cancer diagnosis with greater representation of members of vulnerable populations (e.g., low-income individuals, immigrants, sexual and gender minority groups).
Implications for Nursing
The results of this study can be used to inform nurses and other clinicians’ discussions with older women with cancer about changes in HRQOL. Clinical interventions to increase physical and mental HRQOL as part of survivorship care can promote healthy aging in women with cancer. Previous research has denoted that greater social support is associated with higher HRQOL (Kadambi et al., 2020; Teo et al., 2019; Utley et al., 2022). In addition, social support interventions led by nurses and other clinicians have been effective in improving HRQOL (Clark et al., 2013; Kornblith et al., 2006; Porter et al., 2011; Teo et al., 2019). For example, Kornblith et al. (2006) found that older women who received nurse-led monthly telephone social support in addition to educational materials reported significantly higher HRQOL and lower levels of anxiety and depression compared to the education-only group. Results of this study also pointed to the importance of screening for depressive symptoms even prior to diagnosis because they can be indicative of continued distress and poor health (Kornblith et al., 2006). In addition, for those with significant physical or mental health disabilities after cancer, nurses can assess to what extent the survivor’s social activities and connections are affected, putting them at higher risk for poor health outcomes. Nurses should be alert to significant decreases in physical HRQOL after cancer diagnosis in all groups of older women with cancer. To develop a comprehensive understanding of the needs of older cancer survivors, nurses can advocate for comprehensive geriatric assessments for survivors (Overcash et al., 2019). A geriatric assessment tool can inform clinicians of their patients’ supportive care needs and help generate subsequent referrals and follow-up services. Finally, the current study’s results highlight the importance of tailoring nursing and survivorship care to cultural considerations, including race, ethnicity, rurality, and the patient’s support system.
Conclusion
This study provides insight into associations between patient characteristics (marital status, race and ethnicity, rurality, and health interference on social activities) and physical and mental HRQOL among older women pre- and postdiagnosis. Results indicated that physical and mental HRQOL changed over time, with mental HRQOL returning to prediagnosis levels while physical HRQOL continued to decrease. Sociodemographic factors of race and ethnicity, rurality, marital status, and social interference were associated with changes in physical HRQOL, whereas social interference was associated with mental HRQOL changes. These pre- and postdiagnosis data are pertinent for clinicians, such as primary care physicians and oncologists, and researchers who can identify and support older female cancer survivors who have reduced physical and mental HRQOL.
The authors gratefully acknowledge the efforts of the National Cancer Institute, the Centers for Medicare and Medicaid Services, Information Management Services, Inc., and the Surveillance, Epidemiology, and End Results tumor registries in the creation of the Surveillance, Epidemiology, and End Results–Medicare Health Outcomes Survey.
About the Authors
Elizabeth K. Arthur, PhD, APRN-CNP, AOCNP®, is a nurse scientist in the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, Melica Nikahd, MS, is a biostatistician I in the Center for Biostatistics in the College of Medicine, and J. Madison Hyer, MS, is the principal biostatistician in the Center for Biostatistics in the College of Medicine, all at the Ohio State University in Columbus; Emily Ridgway-Limle, MPH, MS, APRN-CNP, is a nurse practitioner at the Columbus Public Health Ben Franklin Tuberculosis Program; Timiya S. Nolan, PhD, APRN-CNP, ANP-BC, is an associate professor in the Department of Medicine in the Marnix E. Heersink School of Medicine in the Division of Preventive Medicine, and the associate director of community outreach and engagement at the O’Neal Comprehensive Cancer Center, both at the University of Alabama at Birmingham; Ashley Felix, PhD, MPH, is an associate professor in the Division of Epidemiology in the College of Public Health, Menglin Xu, PhD, is a research statistician in the Department of Internal Medicine in the College of Medicine, and Allison Quick, MD, is a radiation oncologist in the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, all at the Ohio State University; and Camille Paoletta is an undergraduate research student and Jessica L. Krok-Schoen, PhD, MA, is an assistant professor, both in the School of Health and Rehabilitation Sciences in the College of Medicine at the Ohio State University. This research was funded, in part, by the Ohio State University Comprehensive Cancer Center 2020 Intramural Supportive Care Pilot Research Award (GR122413). Arthur, Hyer, Ridgway-Limle, Nolan, Felix, and Krok-Schoen contributed to the conceptualization and design. Krok-Schoen completed the data collection. Nikahd, Hyer, Xu, and Krok-Schoen provided statistical support. Arthur, Nikahd, and Hyer provided the analysis. Arthur, Nikahd, Hyer, Ridgway-Limle, Nolan, Felix, Quick, Paoletta, and Krok-Schoen contributed to the manuscript preparation. Arthur can be reached at liz.arthur@osumc.edu, with copy to ONFEditor@ons.org. (Submitted September 2023. Accepted December 9, 2023.)
References
Ambs, A., Warren, J.L., Bellizzi, K.M., Topor, M., Haffer, S.C., & Clauser, S.B. (2008). Overview of the SEER–Medicare Health Outcomes Survey linked dataset. Health Care Financing Review, 29(4), 5–21.
Bernardo, B.M., Pennell, M.L., Naughton, M.J., Brodin, N.P., Neuhouser, M.L., Chlebowski, R.T., & Paskett, E.D. (2023). Self-reported symptoms among cancer survivors in the Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) cohort. Journal of Cancer Survivorship: Research and Practice, 17(5), 1427–1434. https://doi.org/10.1007/s11764-022-01200-4
Bhandari, N.R., Ounpraseuth, S.T., Kamel, M.H., Kent, E.E., McAdam-Marx, C., Tilford, J.M., & Payakachat, N. (2020). Changes in health-related quality of life outcomes in older patients with kidney cancer: A longitudinal cohort analysis with matched controls. Urologic Oncology, 38(11), 852.e11–852.e20. https://doi.org/10.1016/j.urolonc.2020.08.015
Bluethmann, S.M., Mariotto, A.B., & Rowland, J.H. (2016). Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiology, Biomarkers and Prevention, 25(7), 1029–1036. https://doi.org/10.1158/1055-9965.EPI-16-0133
Clark, M.M., Rummans, T.A., Atherton, P.J., Cheville, A.L., Johnson, M.E., Frost, M.H., . . . Brown, P.D. (2013). Randomized controlled trial of maintaining quality of life during radiotherapy for advanced cancer. Cancer, 119(4), 880–887. https://doi.org/10.1002/cncr.27776
Cromer, K.J., Wofford, L., & Wyant, D.K. (2019). Barriers to healthcare access facing American Indian and Alaska Natives in rural America. Journal of Community Health Nursing, 36(4), 165–187. https://doi.org/10.1080/07370016.2019.1665320
Doll, K.M., Pinheiro, L.C., & Reeve, B.B. (2017). Pre-diagnosis health-related quality of life, surgery, and survival in women with advanced epithelial ovarian cancer: A SEER-MHOS study. Gynecologic Oncology, 144(2), 348–353. https://doi.org/10.1016/j.ygyno.2016.12.005
Dos Santos Hughes, S.F., & Malone, M.L. (2023). “What matters most?” for older patients who have cancer. Journal of the American Geriatrics Society, 71(1), 8–10. https://doi.org/10.1111/jgs.18096
Du, Y., & Xu, Q. (2016). Health disparities and delayed health care among older adults in California: A perspective from race, ethnicity, and immigration. Public Health Nursing, 33(5), 383–394. https://doi.org/10.1111/phn.12260
Durá-Ferrandis, E., Mandelblatt, J.S., Clapp, J., Luta, G., Faul, L., Kimmick, G., . . . Hurria, A. (2017). Personality, coping, and social support as predictors of long-term quality-of-life trajectories in older breast cancer survivors: CALGB protocol 369901 (Alliance). Psycho-Oncology, 26(11), 1914–1921. https://doi.org/10.1002/pon.4404
Economic Research Service. (2023). Rural–urban continuum codes. U.S. Department of Agriculture. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes
Garrard, E.D., Fennell, K.M., & Wilson, C. (2017). ‘We’re completely back to normal, but I’d say it’s a new normal’: A qualitative exploration of adaptive functioning in rural families following a parental cancer diagnosis. Supportive Care in Cancer, 25(11), 3561–3568. https://doi.org/10.1007/s00520-017-3785-6
Gudina, A.T., Cheruvu, V.K., Gilmore, N.J., Kleckner, A.S., Arana-Chicas, E., Kehoe, L.A., . . . Cupertino, A.P. (2021). Health related quality of life in adult cancer survivors: Importance of social and emotional support. Cancer Epidemiology, 74, 101996. https://doi.org/10.1016/j.canep.2021.101996
Han, X., Robinson, L.A., Jensen, R.E., Smith, T.G., & Yabroff, K.R. (2021). Factors associated with health-related quality of life among cancer survivors in the United States. JNCI Cancer Spectrum, 5(1), pkaa123. https://doi.org/10.1093/jncics/pkaa123
Hawton, A., Green, C., Dickens, A.P., Richards, S.H., Taylor, R.S., Edwards, R., . . . Campbell, J.L. (2011). The impact of social isolation on the health status and health-related quality of life of older people. Quality of Life Research, 20(1), 57–67. https://doi.org/10.1007/s11136-010-9717-2
Hays, R.D., Farivar, S.S., & Liu, H. (2005). Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD, 2(1), 63–67. https://doi.org/10.1081/copd-200050663
Jones, S.M.W., LaCroix, A.Z., Li, W., Zaslavsky, O., Wassertheil-Smoller, S., Weitlauf, J., . . . Danhauer, S.C. (2015). Depression and quality of life before and after breast cancer diagnosis in older women from the women’s health initiative. Journal of Cancer Survivorship, 9(4), 620–629. https://doi.org/10.1007/s11764-015-0438-y
Kadambi, S., Soto-Perez-de-Celis, E., Garg, T., Loh, K.P., Krok-Schoen, J.L., Battisti, N.M.L., . . . Hsu, T. (2020). Social support for older adults with cancer: Young International Society of Geriatric Oncology review paper. Journal of Geriatric Oncology, 11(2), 217–224. https://doi.org/10.1016/j.jgo.2019.09.005
Karlsen, R.V., Frederiksen, K., Larsen, M.B., von Heymann-Horan, A.B., Appel, C.W., Christensen, J., . . . Bidstrup, P.E. (2016). The impact of a breast cancer diagnosis on health-related quality of life. A prospective comparison among middle-aged to elderly women with and without breast cancer. Acta Oncologica, 55(6), 720–727. https://doi.org/10.3109/0284186x.2015.1127415
Kazis, L.E., Selim, A., Rogers, W., Ren, X.S., Lee, A., & Miller, D.R. (2006). Dissemination of methods and results from the veterans health study: Final comments and implications for future monitoring strategies within and outside the veterans healthcare system. Journal of Ambulatory Care Management, 29(4), 310–319. https://doi.org/10.1097/00004479-200610000-00007
Kent, E.E., Ambs, A., Mitchell, S.A., Clauser, S.B., Smith, A.W., & Hays, R.D. (2015). Health-related quality of life in older adult survivors of selected cancers: Data from the SEER-MHOS linkage. Cancer, 121(5), 758–765. https://doi.org/10.1002/cncr.29119
Kenzik, K.M., Morey, M.C., Cohen, H.J., Sloane, R., & Demark-Wahnefried, W. (2015). Symptoms, weight loss, and physical function in a lifestyle intervention study of older cancer survivors. Journal of Geriatric Oncology, 6(6), 424–432. https://doi.org/10.1016/j.jgo.2015.08.004
Kim, T., White, K., & DuGoff, E. (2022). Racial/ethnic variations in social determinants of mental health among Medicare advantage beneficiaries. Journal of Applied Gerontology, 41(3), 690–698. https://doi.org/10.1177/07334648211039311
Klapheke, A.K., Keegan, T.H.M., Ruskin, R., & Cress, R.D. (2020a). Depressive symptoms and health-related quality of life in older women with gynecologic cancers. Journal of Geriatric Oncology, 11(5), 820–827. https://doi.org/10.1016/j.jgo.2019.10.001
Klapheke, A.K., Keegan, T.H.M., Ruskin, R., & Cress, R.D. (2020b). Pre-diagnosis health-related quality of life and survival in older women with endometrial cancer. Supportive Care in Cancer, 28(10), 4901–4909. https://doi.org/10.1007/s00520-020-05324-0
Kornblith, A.B., Dowell, J.M., Herndon, J.E., II, Engelman, B.J., Bauer-Wu, S., Small, E.J., . . . Holland, J.C. (2006). Telephone monitoring of distress in patients aged 65 years or older with advanced stage cancer: A cancer and leukemia group B study. Cancer, 107(11), 2706–2714. https://doi.org/10.1002/cncr.22296
Krok-Schoen, J.L., Naughton, M.J., Bernardo, B.M., Young, G.S., & Paskett, E.D. (2018). Fear of recurrence among older breast, ovarian, endometrial, and colorectal cancer survivors: Findings from the WHI LILAC study. Psycho-Oncology, 27(7), 1810–1815. https://doi.org/10.1002/pon.4731
Kruse, G., Lopez-Carmen, V.A., Jensen, A., Hardie, L., & Sequist, T.D. (2022). The Indian health service and American Indian/Alaska Native health outcomes. Annual Review of Public Health, 43, 559–576. https://doi.org/10.1146/annurev-publhealth-052620-103633
Leung, J., Pachana, N.A., & McLaughlin, D. (2014). Social support and health-related quality of life in women with breast cancer: A longitudinal study. Psycho-Oncology, 23(9), 1014–1020. https://doi.org/10.1002/pon.3523
Leung, J., Smith, M.D., & McLaughlin, D. (2016). Inequalities in long term health-related quality of life between partnered and not partnered breast cancer survivors through the mediation effect of social support. Psycho-Oncology, 25(10), 1222–1228. https://doi.org/10.1002/pon.4131
Moss, J.L., Pinto, C.N., Mama, S.K., Rincon, M., Kent, E.E., Yu, M., & Cronin, K.A. (2021). Rural–urban differences in health-related quality of life: Patterns for cancer survivors compared to other older adults. Quality of Life Research, 30(4), 1131–1143. https://doi.org/10.1007/s11136-020-02683-3
Mukand, N.H., Ko, N.Y., Nabulsi, N.A., Hubbard, C.C., Chiu, B.C.-H., Hoskins, K.F., & Calip, G.S. (2022). The association between physical health-related quality of life, physical functioning, and risk of contralateral breast cancer among older women. Breast Cancer, 29(2), 287–295. https://doi.org/10.1007/s12282-02101309-x
Ng, J.H., Bierman, A.S., Elliott, M.N., Wilson, R.L., Xia, C., & Scholle, S.H. (2014). Beyond black and white: Race/ethnicity and health status among older adults. American Journal of Managed Care, 20(3), 239–248.
Overcash, J., Ford, N., Kress, E., Ubbing, C., & Williams, N. (2019). Comprehensive geriatric assessment as a versatile tool to enhance the care of the older person diagnosed with cancer. Geriatrics, 4(2), 39. https://doi.org/10.3390/geriatrics4020039
Pandit, A.A., Bhandari, N.R., Khalil, M.I., Kamel, M.H., Davis, R., & Payakachat, N. (2022). Evaluating health-related quality of life as a prognostic tool for overall survival in routine cancer care for older patients with bladder cancer: A US based study. Urologic Oncology, 40(1), 11.e1–11.e8. https://doi.org/10.1016/j.urolonc.2021.09.012
Park, J., Rodriguez, J.L., O’Brien, K.M., Nichols, H.B., Hodgson, M.E., Weinberg, C.R., & Sandler, D.P. (2021). Health-related quality of life outcomes among breast cancer survivors. Cancer, 127(7), 1114–1125. https://doi.org/10.1002/cncr.33348
Pinheiro, L.C., Wheeler, S.B., Chen, R.C., Mayer, D.K., Lyons, J.C., & Reeve, B.B. (2015). The effects of cancer and racial disparities in health-related quality of life among older Americans: A case-control, population-based study. Cancer, 121(8), 1312–1320. https://doi.org/10.1002/cncr.29205
Porter, L.S., Keefe, F.J., Garst, J., Baucom, D.H., McBride, C.M., McKee, D.C., . . . Scipio, C. (2011). Caregiver-assisted coping skills training for lung cancer: Results of a randomized clinical trial. Journal of Pain and Symptom Management, 41(1), 1–13. https://doi.org/10.1016/j.jpainsymman.2010.04.014
Rao, D., Debb, S., Blitz, D., Choi, S.W., & Cella, D. (2008). Racial/ethnic differences in the health-related quality of life of cancer patients. Journal of Pain and Symptom Management, 36(5), 488–496. https://doi.org/10.1016/j.jpainsymman.2007.11.012
Reeve, B.B., Potosky, A.L., Smith, A.W., Han, P.K., Hays, R.D., Davis, W.W., . . . Clauser, S.B. (2009). Impact of cancer on health-related quality of life of older Americans. Journal of the National Cancer Institute, 101(12), 860–868. https://doi.org/10.1093/jnci/djp123
Revicki, D., Hays, R.D., Cella, D., & Sloan, J. (2008). Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. Journal of Clinical Epidemiology, 61(2), 102–109. https://doi.org/10.1016/j.jclinepi.2007.03.012
Rivera, J.N., Borger, T., Sizemore, Y., & Burris, J.L. (2023). Similarities and differences across the underlying dimensions of social functioning in rural and nonrural cancer survivors: A mixed-methods study. Journal of Rural Health, 39(2), 434–443. https://doi.org/10.1111/jrh.12721
Rowland, J.H., & Bellizzi, K.M. (2014). Cancer survivorship issues: Life after treatment and implications for an aging population. Journal of Clinical Oncology, 32(24), 2662–2668. https://doi.org/10.1200/jco.2014.55.8361
Sauer, C., Weis, J., Faller, H., Junne, F., Hönig, K., Bergelt, C., . . . Maatouk, I. (2019). Impact of social support on psychosocial symptoms and quality of life in cancer patients: Results of a multilevel model approach from a longitudinal multicenter study. Acta Oncologica, 58(9), 1298–1306. https://doi.org/10.1080/0284186x.2019.1631471
Seiler, A., & Jenewein, J. (2019). Resilience in cancer patients. Frontiers in Psychiatry, 10, 208. https://doi.org/10.3389/fpsyt.2019.00208
Selim, A.J., Rogers, W., Fleishman, J.A., Qian, S.X., Fincke, B.G., Rothendler, J.A., & Kazis, L.E. (2009). Updated U.S. population standard for the Veterans RAND 12-Item Health Survey (VR-12). Quality of Life Research, 18(1), 43–52. https://doi.org/10.1007/s11136-008-9418-2
Sequist, T.D. (2021). Improving the health of the American Indian and Alaska Native population. JAMA, 325(11), 1035–1036. https://doi.org/10.1001/jama.2021.0521
Shippee, T.P., Duan, Y., Olsen Baker, M., & Angert, J. (2020). Racial/ethnic disparities in self-rated health and sense of control for older adults receiving publicly funded home- and community-based services. Journal of Aging and Health, 32(10), 1376–1386. https://doi.org/10.1177/0898264320929560
Siegel, R.L., Giaquinto, A.N., & Jemal, A. (2024). Cancer statistics, 2024. CA: A Cancer Journal for Clinicians, 74(1), 12–49. https://doi.org/10.3322/caac.21820
Smith, A.W., Reeve, B.B., Bellizzi, K.M., Harlan, L.C., Klabunde, C.N., Amsellem, M., . . . Hays, R.D. (2008). Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financing Review, 29(4), 41–56.
Stover, A.M., Mayer, D.K., Muss, H., Wheeler, S.B., Lyons, J.C., & Reeve, B.B. (2014). Quality of life changes during the pre- to postdiagnosis period and treatment-related recovery time in older women with breast cancer. Cancer, 120(12), 1881–1889. https://doi.org/10.1002/cncr.28649
Teo, I., Krishnan, A., & Lee, G.L. (2019). Psychosocial interventions for advanced cancer patients: A systematic review. Psycho-Oncology, 28(7), 1394–1407. https://doi.org/10.1002/pon.5103
Trevino, K.M., Nelson, C.J., Saracino, R.M., Korc-Grodzicki, B., Sarraf, S., & Shahrokni, A. (2020). Is screening for psychosocial risk factors associated with mental health care in older adults with cancer undergoing surgery? Cancer, 126(3), 602–610. https://doi.org/10.1002/cncr.32564
Utley, M., Adeyanju, T., Bernardo, B., Paskett, E.D., & Krok-Schoen, J.L. (2022). The association between mental health, social support and physical health outcomes among older female cancer survivors. Journal of Geriatric Oncology, 13(6), 834–838. https://doi.org/10.1016/j.jgo.2022.04.001
Weaver, K.E., Geiger, A.M., Lu, L., & Case, L.D. (2013). Rural–urban disparities in health status among US cancer survivors. Cancer, 119(5), 1050–1057. https://doi.org/10.1002/cncr.27840
Williams, G.R., Chen, Y., Kenzik, K.M., McDonald, A., Shachar, S.S., Klepin, H.D., . . . Bhatia, S. (2020). Assessment of sarcopenia measures, survival, and disability in older adults before and after diagnosis with cancer. JAMA Network Open, 3(5), e204783. https://doi.org/10.1001/jamanetworkopen.2020.4783
Williams, G.R., Mackenzie, A., Magnuson, A., Olin, R., Chapman, A., Mohile, S., . . . Holmes, H. (2016). Comorbidity in older adults with cancer. Journal of Geriatric Oncology, 7(4), 249–257. https://doi.org/10.1016/j.jgo.2015.12.002
Yoo, G.J., Musselman, E., Lee, Y.-S., & Yee-Melichar, D. (2014). Addressing health disparities among older Asian Americans: Data and diversity. Generations, 38(4), 74–81.
Zandbergen, N., de Rooij, B.H., Vos, M.C., Pijnenborg, J.M.A., Boll, D., Kruitwagen, R.F.P.M., . . . Ezendam, N.P.M. (2019). Changes in health-related quality of life among gynecologic cancer survivors during the two years after initial treatment: A longitudinal analysis. Acta Oncologica, 58(5), 790–800. https://doi.org/10.1080/0284186x.2018.1560498



