Improving Adherence to Adjuvant Endocrine Therapy in Breast Cancer Through a Therapeutic Educational Approach: A Feasibility Study
Purpose/Objectives: To develop and test the feasibility of a tailored therapeutic educational program, with the aim of improving adherence to oral endocrine adjuvant chemotherapy in women with breast cancer.
Design: A qualitative study to identify educational needs and a feasibility study assessing the efficacy of the program.
Setting: A comprehensive cancer center, the Lucien Neuwirth Cancer Institute in Saint-Priest-en-Jarez, France.
Sample: Two consecutive samples (N = 11, N = 6) of women taking adjuvant oral endocrine chemotherapy for breast cancer.
Methods: A mixed qualitative and quantitative method was used. The participants’ representations of disease and treatment were explored through one-on-one interviews and then translated into educational needs, which were used to develop a tailored therapeutic education program. The pilot study evaluated the reach and efficacy using before-and-after comparisons.
Main Research Variables: Educational objectives, knowledge, trust in the treatment, and anxiety.
Findings: Five educational objectives (acquiring knowledge, improving communication skills, managing anxiety, managing side effects, and improving adherence) were identified through 11 interviews. A three-session program was developed. Eight of the 23 patients invited to participate in a pilot study accepted, and six completed the intervention. Knowledge improved from 38.9 of 100 preintervention to 69.4 of 100 postintervention (p = 0.045). Trust in treatment showed a trend to improvement from 5.5 of 10 to 8 of 10 (p = 0.14), but anxiety did not change significantly; anxiety went from 6 to 7 (p = 0.88).
Conclusions: Results from the feasibility study showed promising efficacy for the educational objectives and provided information about how the program could be improved.
Implications for Nursing: Tailored educational programs conducted by trained nurses may help patients to adhere to and live with the effects of endocrine therapy.
Jump to a section
Adjuvant endocrine therapy is the standard treatment recommended for women with hormone receptor–positive early breast cancer (American Cancer Society & National Comprehensive Cancer Network, 2006). The aim of this therapy is to prevent disease recurrence and improve overall survival (Baum et al., 2002; Coates et al., 2007; Cuzick et al., 2010; Early Breast Cancer Trialists’ Collaborative Group, 1998). This therapy is given to 60% of all women diagnosed with early breast cancer, representing a substantial number of women who receive long-term oral treatment.
Although adjuvant endocrine therapy has many potential benefits, it can also lead to side effects (e.g., menopausal symptoms, arthralgia, weight gain, osteoporosis), which may have a greater impact on women’s quality of life than clinicians believe (Fellowes, Fallowfield, Saunders, & Houghton, 2001). Adherence to tamoxifen (Nolvadex®) treatment has been estimated to be from 25%–96% and to aromatase inhibitors to be from 62%–79% (Barron, Connolly, Bennett, Feely, & Kennedy, 2007; Partridge, Avorn, Wang, & Winer, 2002; Partridge et al., 2008; Partridge, Wang, Winer, & Avorn, 2003; Ruddy, Mayer, & Partridge, 2009; Ziller et al., 2009). Adherence decreases with longer treatment periods, resulting in as much as 50% nonadherence after four years of treatment (Partridge et al., 2003). Nonadherence is an issue that has to be addressed because each decrease in treatment adherence can lead to reduction in treatment efficacy (Haynes, McDonald, Garg, & Montague, 2002). A model to understand nonadherence has been built that incorporates treatment-, patient-, and healthcare system–related reasons (Ruddy et al., 2009). The treatment-related factors include characteristics of the treatment that make it difficult to take (e.g., high rates of side effects, complexity of regimens). The patient-related factors include individual characteristics of the patient (e.g., history, beliefs, age). The healthcare system–related factors are related to the interaction between the system and patients (Atkins & Fallowfield, 2006; Partridge et al., 2002; Verbrugghe, Verhaeghe, Lauwaert, Beeckman, & Van Hecke, 2013). Interventions targeting these factors using different approaches (e.g., educational, behavioral, multidimensional) have been demonstrated to be more effective to enhance adherence (Haynes, Ackloo, Sahota, McDonald, & Yao, 2008; McDonald, Garg, & Haynes, 2002). Therapeutic educational programs in oncology have been shown to be effective in improving the management of side effects (Porter, 1998), including cancer treatment–related fatigue (Yates et al., 2005) and pain (Aubin et al., 2006; Koller, Miaskowski, De Geest, Opitz, & Spichiger, 2013), as well as the post-treatment care plan (Bergin et al., 2015; Jefford et al., 2011; Park, Bae, Jung, & Kim, 2012; Schlairet, Heddon, & Griffis, 2010). Educational counseling has been shown to be effective in improving adherence rate for non-oncologic pathologies (Beney, Bero, & Bond, 2000). Although some interventions to improve adherence to adjuvant endocrine therapies in patients with breast cancer have been reported (Hadji et al., 2013; von Blanckenburg, Schuricht, Albert, Rief, & Nestoriuc, 2013; Yu et al., 2012; Ziller et al., 2013), a therapeutic educational program has yet to be validated.
Guidelines for the development of educational interventions recommend that the program should take into consideration the patients’ characteristics to be tailored to the population (Cancer Patient Education Network, 2013). These characteristics can be identified through qualitative studies assessing individual educational needs (Department of Health, 2005; Régnier-Denois, Rousset-Guarato, Nourissat, Bourmaud, & Chauvin, 2010).
In the current study, the authors assumed that a therapeutic educational program (developed according to current guidelines and with the main objective of increasing treatment adhesion) would improve adherence and other intermediate outcomes, such as knowledge about and trust in the treatment, as well as reduce anxiety among patients receiving adjuvant endocrine therapy.
The objectives of this study were (a) to develop a standardized, tailored therapeutic educational program aimed at improving adhesion to endocrine adjuvant chemotherapy in women with early breast cancer and (b) to test the feasibility of the developed program in a pilot study.
Methods
Development of the Therapeutic Educational Program
The authors followed the standardized five-step method proposed by the National Cancer Institute and the Cancer Patient Education Network to develop the therapeutic educational program (Cancer Patient Education Network, 2013; World Health Organization, 1998).
The first step was the identification of topics to be covered during the therapeutic educational program. Patients’ representation of their disease, belief in the efficacy of the treatment, and perception of side effects can be different from that perceived by healthcare professionals. The first step was a qualitative survey, using an anthropologic approach.
The topics covered were (a) beliefs and level of knowledge about the treatment and its effects, (b) treatment experience, (c) management of side effects, (d) choices, (e) relationships with relatives, and (f) needs and expectations. A content analysis of the interviews was performed. The authors used the Martin and Savary (2015) method of educational diagnosis to translate representations into educational needs. This method is based on a schema, which visually describes (from an unsatisfactory situation) one or more educational objectives that lead to the expected situation. This method describes the common thread leading the education plan by taking into account existing resources.
The second step was the construction of the program. Educational objectives for each educational need were identified. The program was developed around these objectives by a multidisciplinary team composed of an educationalist, a healthcare educator, a sociologist, and a methodologist. The specific objectives of the program were to (a) increase patients’ knowledge about the disease and the efficacy of adjuvant endocrine therapy, (b) strengthen trust in the treatment, and (c) reduce anxiety about the disease and treatment. The authors aimed to improve adherence and the ability to manage side effects.
The therapeutic educational program consisted of three two-hour group sessions for 5–10 patients. The same healthcare educator led all of the sessions, which were organized outside of the comprehensive cancer center to separate health care from education. The sessions were programmed seven days apart, so the complete program was three weeks long.
Evaluation of the Feasibility of the Program
A feasibility study was done to assess the program under real-life conditions. This feasibility study included assessment of the process quality, or reach, and the intermediate efficacy. The reach of the program was the third step. Reach was assessed using data collected at each session. Data collected included the patients’ acceptance and completion rates for the program, as well as patients’ and instructors’ satisfaction with the program content.
The fourth step was the intermediate efficacy, which was assessed using data collected at the beginning of the first session and at the end of the last session (for a before-and-after comparison). The last step, which involves assessing the efficacy of the final program on a large scale, has not yet been carried out. The study was approved by the ethics committee of University Hospital of Saint-Étienne in France on March 30, 2011 (No. IORG004981). All participants provided written informed consent at the start of the study.
Study Participants
Patients were recruited from the Lucien Neuwirth Cancer Institute in Saint-Priest-en-Jarez, France, from January to July 2012. All patients aged 18 years or older, being treated for early breast cancer, and receiving oral adjuvant endocrine therapy (tamoxifen or aromatase inhibitors) were considered for participation. Early breast cancer was defined as cancer that has not spread beyond the breast or the axillary lymph nodes (in situ or stage I, IIA, IIB, or IIIA breast cancer). Women with severe psychiatric disease or who lived too far from the center were excluded. Patients were identified, and eligibility was assessed during the consultation with the oncologist. All eligible patients were invited to participate in the study. The first patients were recruited for the qualitative study, with sampling continuing until data saturation was achieved. Enrollment then stopped until the interviews had been analyzed and the program had been constructed. Another recruitment period then began to include in the feasibility study all consecutive women consulting their oncologist. The reason for refusal was collected for all eligible patients who refused to participate.
Measurement
In the qualitative study, required data were gathered directly from patients. Original hypotheses were based on the literature. Semistructured interviews followed an interview guide, which was enriched as the interviews were performed. Each interview covered the following topics: beliefs and level of knowledge about the treatment and its effects, treatment experience, management of side effects, relationships with relatives and physicians, and needs and expectations.
Semistructured interviews were carried out by a nurse specially trained for socioanthropologic interviews. Face-to-face, in-depth individual interviews were conducted to explore patients’ representations. The interviews lasted one hour and were audio recorded.
In the feasibility study, several methods were used to assess the reach. At the beginning of each session of the program, the number of participants attending was noted. At the end of each session, the participants were asked to complete a questionnaire to express their satisfaction with the session (D’Ivernois & Gagnayre, 2008), what they felt was missing, and what was difficult to understand. The healthcare educator who led the session was asked to complete another questionnaire to assess the content of the session.
For each of the educational objectives, specific tools were used to evaluate the intermediate efficacy. The participants completed the tools before the first session and at the end of the last session. The first tool was a quiz constructed for this program and based on current medical literature and guidelines. This tool was double-checked and validated by a methodologist and an oncologist, and it aimed to assess patients’ knowledge about breast cancer, as well as efficacy of endocrine therapy and its side effects. This tool was comprised of 18 statements that the participants were asked to code as “correct,” “incorrect,” or “I do not know.” The second tool assessed trust in the treatment, using a visual analog scale ranging from “I have no faith in it at all” to “I have total faith in it.” Visual analog scales are believed to be reliable, are increasingly used in the education field, and have been validated in other settings, such as symptom control (Bendon, Johnson, Judge, Wall, & Johnson, 2014; Khalil, Feldman, & Bridger, 2003; Lesage & Berjot, 2011; Price, Mackenzie, Metlay, Camargo, & Gonzales, 2011; Pritchard, 2010; Waldorff et al., 2012). The third tool, the Hospital Anxiety and Depression Scale (HADS) was developed to identify anxiety disorders and depression among nonpsychiatric patients (Zigmond & Snaith, 1983). This scale has been validated and has demonstrated its ability to assess anxiety and depression in patients with cancer and the general population (Bjelland, Dahl, Haug, & Neckelmann, 2002). In addition, the scale has been translated and validated in eight languages. Fourteen questions are rated from 0–3, and the anxiety and depression subscale scores range from 0–21. The cutoff for each subscale was eight.
Sample Size
The socioanthropologic qualitative study precluded any sample size calculation. The inclusion process was planned to continue until data saturation was reached. For the evaluation study, the authors estimated that 22 women were needed to achieve type I error of 0.05%, power of 80%, expected initial knowledge of 50 points (SD = 16), and expected increase in knowledge of 20 points.
Qualitative Content Analysis
First, recorded interviews were transcribed verbatim. Transcripts were read, and line-by-line analysis was conducted to extract significant statements from the interviews, following established guidelines for a thematic analysis (Creswell, 2007). These statements were used to generate specific codes, and each transcript was then coded using this thematic coding scheme. The themes emerging from the first interviews helped to refine the interview guide used for the next set of interviews, with these latter interviews informing the next set, and so on. Data analysis was performed simultaneously and continually with the data collection to identify data saturation. The information was categorized into five main themes (educational needs) based on the objectives of the study. The context of the interviews, the codes, and the extracted categories were reviewed by the head of the research team, an anthroposociologist and expert in qualitative research.
Statistical Analysis
The authors performed descriptive analyses of the variables collected from the feasibility study, using frequency (percentages) or medians (interquartiles) as appropriate. The before-and-after median scores, measured with the specific tools, were compared using the non-parametrical Wilcoxon test with a significance threshold of p < 0.05. All analyses were performed using SAS®, version 9.1.
Results
Population
All 11 patients included in the qualitative study completed the face-to-face interview. They were aged from 44–75 years (see Table 1). Four were receiving tamoxifen, and seven were receiving aromatase inhibitors. The qualitative portion of the study covered identification of educational needs and construction of the program.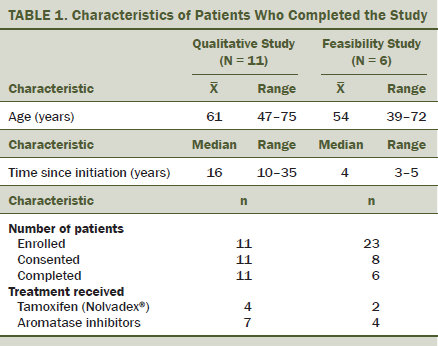
Twenty-three eligible patients were screened and invited to participate in the feasibility study. Only eight consented to participate in the therapeutic educational program and came to the first session; six of these patients attended all three sessions. The main reasons given by the 15 women who refused to participate in the therapeutic educational program included the need to return to work, no means of transportation, and too tired. The authors were unable to collect the reasons for withdrawals. Those who completed all sessions were aged from 25–63 years. Two were receiving tamoxifen, and four were receiving aromatase inhibitors. The feasibility portion of the study covered reach assessment and intermediate efficacy assessment.
Educational Diagnosis
The qualitative study enabled the identification of five educational needs in this population (see Table 2). The first was the lack of knowledge about the disease and risk of recurrence, as well as a lack of understanding about how the treatment worked. The patients did not understand its efficacy in reducing recurrence rate and what that really meant, or how it caused side effects and what they were. The second concerned the high level of anxiety about the possibility of recurrence, particularly when the process of recurrence was misunderstood. The patients expressed anxiety because of mistrust in the endocrine therapy, which was based on uncertainty about efficacy and side effects. The third educational need was the feeling of loneliness caused by the disease and the long-term adjuvant treatment. They expressed feelings of isolation from their relatives, who did not understand why they remained anxious after the disease was gone. They also felt isolated from the oncologist because the oncologist often underestimated the side effects and their impact on the patients’ quality of life. The fourth need concerned occasional nonadherence, which was particularly distressing to participants who were afraid that if they missed one pill, the therapy would no longer be effective. The fifth and final need was for the development of skills for the management of side effects to maintain the quality of life and autonomy. 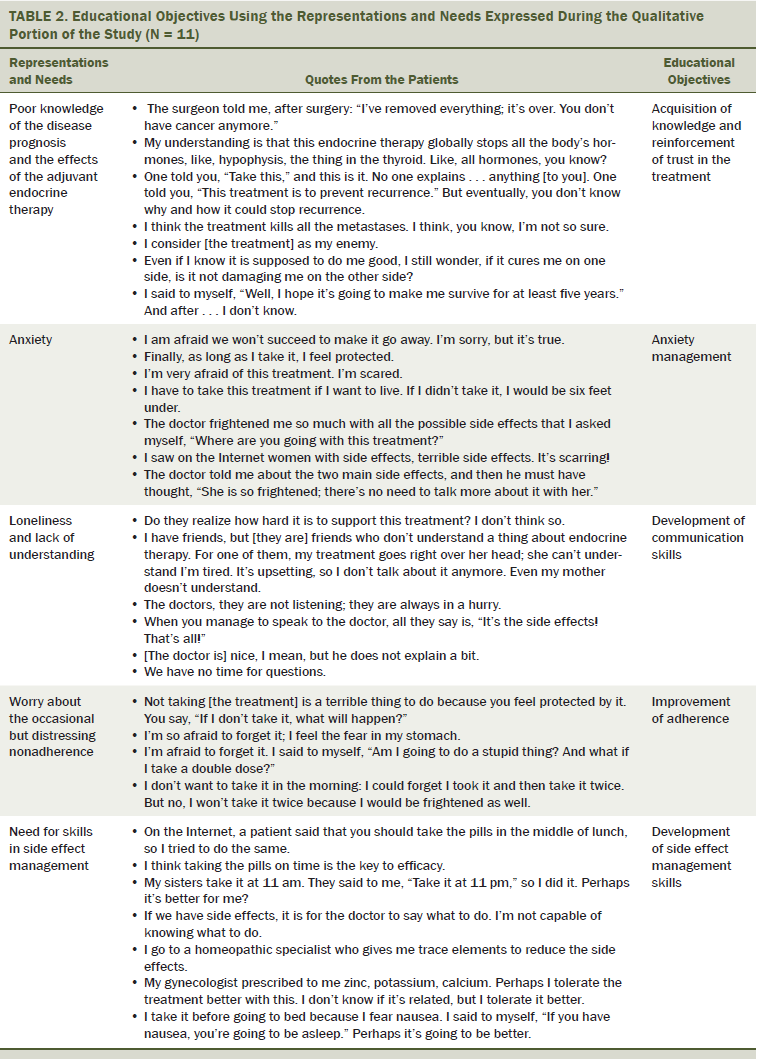
Construction of the Therapeutic Educational Program
The therapeutic educational program was designed to address these five educational objectives. The program was comprised of three two-hour group sessions, with a week between each session. The first session dealt with knowledge about the disease and treatment and increasing trust about the treatment. The second session covered side effects and adherence management, and the third session was about communication. Anxiety was addressed in every session.
Evaluation of Reach
Because only eight participants agreed to attend the training program, the authors organized one group, and the feasibility study was done during three weeks. The participants were asked about their level of satisfaction for each session. At the end of the last session, most of the women were satisfied with the program content (see Table 3). Some women said that not enough sessions were in the program and that the sessions were not long enough. Two women stated that they would have liked one more session.
The healthcare educator said that the program was adapted to the needs and the population, with the exception of the second session. The educator felt that this session was too dense and, therefore, did not reach the targeted educational objectives. The educator suggested a fourth session and noted that the tools were relevant and the rhythm coherent. 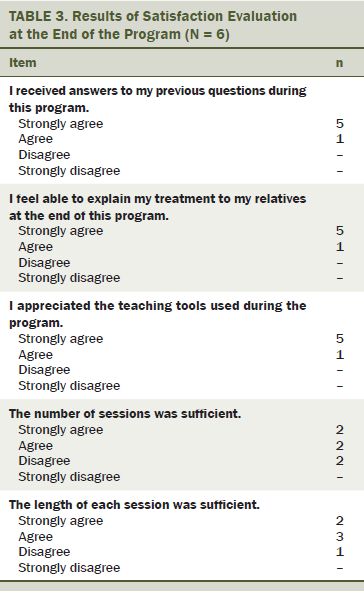
Evaluation of the Intermediate Efficacy
The questionnaires filled out by the six participants were used in the evaluation of three educational objectives (i.e., increased knowledge, increased trust in the treatment, and anxiety management) (see Table 4). Participants had a statistically significant increase in knowledge about their disease and the adjuvant endocrine treatment after the sessions (from 38.9 of 100 to 69.4 of 100, p = 0.045). However, no change seemed to occur in the level of anxiety (from 6 of 21 to 7 of 21 rated on the anxiety subscale of the HADS). A nonsignificant trend occurred in favor of an increased trust in the treatment (from 5.5 of 10 to 8 of 10) at the end of the program. 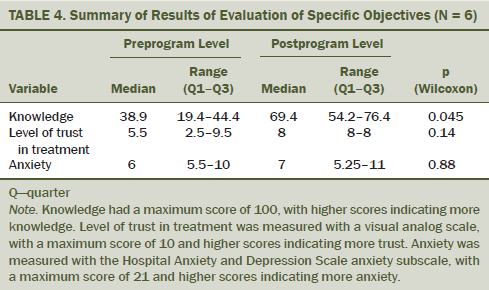
Discussion
The results from this feasibility study suggest that a therapeutic educational program, tailored to patients and developed according to current guidelines, could be implemented for women receiving adjuvant endocrine therapy for early breast cancer. The results also suggest that such a program could have an impact on at least two of the main factors influencing adherence to treatment (i.e., knowledge and trust in the treatment). Another main result of the study is the low level of program’s attendance, which is a weakness that has to be taken into account to improve it before deciding to implement and disseminate it broadly.
This educational program was developed following guidelines and using a standardized methodology, which may help in improving its quality and the potential benefit for patients (Cancer Patient Education Network, 2013). Integrating key criteria into this structured education program (e.g., formal assessment of needs, trained professionals integration, identification of indicators that can assist in assessing the process quality and efficacy of the program) may help to reinforce its quality and satisfaction by patients. In addition, description of the construction process may help in enhancing its reproducibility. The results obtained in the qualitative study enabled the authors to have a better understanding about patients’ behavior. They also allowed for confirmation that the factors identified as influencing nonadherence were consistent with those reported in the literature (i.e., not understanding the reason for therapy, not knowing about the therapy’s benefit or its side effects, poor communication with healthcare providers, and worry about cancer recurrence) (Atkins & Fallowfield, 2006; Partridge et al., 2002; Verbrugghe et al., 2013).
These results also allowed for identification of other unknown factors. By taking into consideration all of the factors identified, the authors developed a program tailored to the needs of the targeted population. Although tailoring programs to the targeted patients is widely recommended and known to increase the efficacy in patient’s education, few of the previous studies tailored their programs in this way (Cancer Patient Education Network, 2013).
For practical reasons, the authors did not assess all of the educational objectives in the feasibility study. The principal objective (i.e., improved adherence) will be evaluated in the future in a comparative, large-scale study. For the intermediate objectives, the authors developed specific tools. Very few tools exist that can be used to assess the efficacy of a therapeutic education program on specific educational objectives (Pasquier et al., 2013). The authors developed a questionnaire to assess the participants’ knowledge based on what was presented in the program. A visual analog scale was identified as the most appropriate tool to assess the participants’ trust in the treatment.
Some studies have evaluated the efficacy of interventions to improve adherence to adjuvant endocrine treatment (Davidson, Vogel, & Wickerham, 2007; Monnier, 2007; Xue, Sun, & Li, 2011), but educational programs have not been tested. Validated, standardized, and tailored educational programs that take into account factors of nonadherence are lacking (Atkins & Fallowfield, 2006; Davidson et al., 2007; Doggrell, 2011; Gold & McClung, 2006; Hadji, 2010; Hugtenburg, Timmers, Elders, Vervloet, & van Dijk, 2013; Osterberg & Blaschke, 2005). The current study helps to fill this gap (Feldman-Stewart et al., 2013a, 2013b; Friese et al., 2013; Hartigan, 2003).
Limitations
One limitation of this study is the low participation rate (26%). An explanation would be that it was not the oncologist who invited the patients to participate in the educational program; instead, a member of the educational team that the participants did not know and probably did not trust invited participation. This limitation underlines the absolute necessity of running programs in collaboration with all healthcare providers. The second limitation is that only six patients completed the educational program, which may limit statistical power of the study. However, despite this small number, the authors observed a statistically significant increase in the patients’ knowledge and a nonsignificant trend for increased trust in the treatment. Therefore, baseline knowledge was lower (35 of 100 versus 50 of 100) and the increase after the program was higher (30 points versus 20 points) than initially assumed in sample size calculation. Another limitation is that the authors did not evaluate the efficacy of the program to improve adherence to treatment, which is the principal objective of the program. However, the authors performed a feasibility study to assess the practicability of running a large-scale comparative study with long-term follow-up. Another limitation is that patients could have answered the questionnaires with social desirability bias. This bias cannot be excluded for the satisfaction assessment. Finally, regarding the tools used to measure the intermediate efficacy criteria, two of the tools (the quiz and visual analog scale) had not been validated and could be subject to bias despite the attention taken with their construction process.
Based on the results from this feasibility study, the authors have modified the educational program according to the theory of the “plan, do, study, act” model (Langley et al., 2009), taking into consideration the participation rate and comments received from the attendees and the instructors. The educational program will be prescribed by the oncologists at the same time that the adjuvant endocrine therapy is prescribed to reinforce the link between care and education and to encourage participation and confidence in the program. In addition, a fourth session will be included to lighten the sessions. To improve the effect on anxiety management, the authors have included a module on relaxation techniques taught by a trained doctor.
Implications for Oncology Nursing
This program is an opportunity for oncology nurses to provide pragmatic, standardized, and tailored education to patients receiving adjuvant endocrine therapy. The tailoring part of this study led to the identification of patients’ specific needs and representations related to adherence to this specific treatment. Even in the survivorship phase, patients are anxious about the risk of cancer recurrence, but they may not know that their treatment is precisely prescribed to reduce this risk. Nonadherence is often related to a lack of efficacy knowledge, the inability to manage incapacitating side effects, and poor caregiver and physician commitment. Those needs should be emphasized in everyday practice to give support to patients (Cannon, Watson, Roth, & LaVergne, 2014).
The standardized and transparent process of this educational program construction allowed oncology nurses to master the content and the implementation of this program. This study also highlighted the strengths and weakness of the program, so nurses who would want to implement or develop a program in this setting could make use of this pilot study. Acting on knowledge, treatment trust and anxiety seem to be major issues in this setting. Group sessions appear to be highly useful in transmitting and sharing solutions to manage side effects. However, the educational role of the nurse in the care setting needs to be formalized and acknowledged as a cornerstone in the patient care pathway (McCabe & Jacobs, 2008). This may be the main issue in the resolution of the participation problem.
Conclusion
The current study provides the first standardized inventory of the development and feasibility testing of an educational program to improve treatment adherence and side effect management for women receiving adjuvant endocrine therapy for early breast cancer. The authors’ approach enabled understanding of participants’ behaviors and needs, which led to the development of a program tailored to these specific needs. The results from the feasibility study showed promising efficacy for the specific educational objectives that were assessed and provided important information about how the program could be improved. The efficacy of this modified educational program on patients’ treatment adherence will be assessed in a large-scale, prospective, controlled study.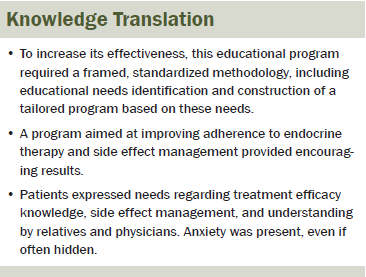
References
American Cancer Society & National Comprehensive Cancer Network. (2006). Breast cancer: Treatment guidelines for patients. Retrieved from http://screening.iarc.fr/doc/Breast_VIII.pdf
Atkins, L., & Fallowfield, L. (2006). Intentional and non-intentional non-adherence to medication amongst breast cancer patients. European Journal of Cancer, 42, 2271–2276. doi:10.1016/j.ejca.2006.03.004
Aubin, M., Vézina, L., Parent, R., Fillion, L., Allard, P., Bergeron, R., . . . Giguère, A. (2006). Impact of an educational program on pain management in patients with cancer living at home. Oncology Nursing Forum, 33, 1183–1188. doi:10.1188/06.ONF.1183-1188
Barron, T.I., Connolly, R., Bennett, K., Feely, J., & Kennedy, M.J. (2007). Early discontinuation of tamoxifen: A lesson for oncologists. Cancer, 109, 832–839. doi:10.1002/cncr.22485
Baum, M., Budzar, A.U., Cuzick, J., Forbes, J., Houghton, J.H., Klijn, J.G., & Samoud, T. (2002). Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet, 359, 2131–2139.
Bendon, C.L., Johnson, H.P., Judge, A.D., Wall, S.A., & Johnson, D. (2014). The aesthetic outcome of surgical correction for sagittal synostosis can be reliably scored by a novel method of preoperative and postoperative visual assessment. Plastic and Reconstructive Surgery, 134, 775e–786e. doi:10.1097/PRS.0000000000000633
Beney, J., Bero, L.A., & Bond, C. (2000). Expanding the roles of outpatient pharmacists: Effects on health services utilisation, costs, and patient outcomes. Cochrane Database of Systematic Reviews, 2000, CD000336. doi:10.1002/14651858.CD000336
Bergin, R.J., Grogan, S.M., Bernshaw, D., Juraskova, I., Penberthy, S., Mileshkin, L.R., . . . Schofield, P.E. (2015). Developing an evidence-based, nurse-led psychoeducational intervention with peer support in gynecologic oncology. Cancer Nursing, 29, E19–E30. doi:10.1097/NCC.0000000000000263
Bjelland, I., Dahl, A.A., Haug, T.T., & Neckelmann, D. (2002). The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research, 52, 69–77.
Cancer Patient Education Network. (2013). Establishing comprehensive cancer patient education programs: Standards of practice. Retrieved from http://www.cancerpatienteducation.org/docs/CPEN/Educator%20Resources/CP…
Cannon, C.A., Watson, L.K., Roth, M.T., & LaVergne, S. (2014). Assessing the learning needs of oncology nurses. Clinical Journal of Oncology Nursing, 18, 577–580. doi:10.1188/14.CJON.577-580
Coates, A.S., Keshaviah, A., Thürlimann, B., Mouridsen, H., Mauriac, L., Forbes, J.F., . . . Goldhirsch, A. (2007). Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. Journal of Clinical Oncology, 26, 486–492. doi:10.1200/JCO.2006.08.8617
Creswell, J.W. (2007). Qualitative inquiry and research design: Choosing among five approaches (2nd ed.). Thousand Oaks, CA: Sage.
Cuzick, J., Sestak, I., Baum, M., Buzdar, A., Howell, A., Dowsett, M., & Forbes, J.F. (2010). Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncology, 11, 1135–1141. doi:10.1016/S1470-2045(10)70257-6
Davidson, B., Vogel, V., & Wickerham, L. (2007). Oncologist-patient discussion of adjuvant hormonal therapy in breast cancer: Results of a linguistic study focusing on adherence and persistence to therapy. Journal of Supportive Oncology, 5, 139–143.
Department of Health. (2005). Structured patient education in diabetes: Report from the Patient Education Working Group. Retrieved from https://www.diabetes.org.uk/Documents/Reports/StructuredPatientEd.pdf
D’Ivernois, J.F., & Gagnayre, M. (2008). Apprendre à éduquer le patient: Approche pédagogique. Paris, France: Maloine.
Doggrell, S.A. (2011). Adherence to oral endocrine treatments in women with breast cancer: Can it be improved? Breast Cancer Research and Treatment, 129, 299–308. doi:10.1007/s10549-011-1578-z
Early Breast Cancer Trialists’ Collaborative Group. (1998). Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet, 351, 1451–1467.
Feldman-Stewart, D., Madarnas, Y., Mates, M., Tong, C., Grunfeld, E., Verma, S., . . . Brundage, M. (2013a). Information for decision making by post-menopausal women with hormone receptor positive early-stage breast cancer considering adjuvant endocrine therapy. Breast, 22, 919–925. doi:10.1016/j.breast.2013.04.020
Feldman-Stewart, D., Madarnas, Y., Mates, M., Tong, C., Grunfeld E., Verma, S., . . . Brundage, M. (2013b). Information needs of post-menopausal women with hormone receptor positive early-stage breast cancer considering adjuvant endocrine therapy. Patient Education and Counseling, 93, 114–121. doi:10.1016/j.pec.2013.03.019
Fellowes, D., Fallowfield, L.J., Saunders, C.M., & Houghton, J. (2001). Tolerability of hormone therapies for breast cancer: How informative are documented symptom profiles in medical notes for “well-tolerated” treatments? Breast Cancer Research and Treatment, 66, 73–81.
Friese, C.R., Pini, T.M., Li, Y., Abrahamse, P.H., Graff, J.J., Hamilton, A.S., . . . Griggs, J.J. (2013). Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Research and Treatment, 138, 931–939. doi:10.1007/s10549-013-2499-9
Gold, D.T., & McClung, B. (2006). Approaches to patient education: Emphasizing the long-term value of compliance and persistence. American Journal of Medicine, 119(Suppl. 1), S32–S37. doi:10.1016/j.amjmed.2005.12.021
Hadji, P. (2010). Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Critical Reviews in Oncology/Hematology, 73, 156–166. doi:10.1016/j.critrevonc.2009.02.001
Hadji, P., Blettner, M., Harbeck, N., Jackisch, C., Lück, H.J., Windemuth-Kieselbach, C., . . . Kreienberg, R. (2013). The Patient’s Anastrozole Compliance to Therapy (PACT) Program: A randomized, in-practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Annals of Oncology, 24, 1505–1512. doi:10.1093/annonc/mds653
Hartigan, K. (2003). Patient education: The cornerstone of successful oral chemotherapy treatment. Clinical Journal of Oncology Nursing, 7(Suppl.), 21–24. doi:10.1188/03.CJON.S6.21-24
Haynes, R.B., Ackloo, E., Sahota, N., McDonald, H.P., & Yao, X. (2008). Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews, 2008, CD000011. doi:10.1002/14651858.CD000011.pub3
Haynes, R.B., McDonald, H., Garg, A.X., & Montague, P. (2002). Interventions for helping patients to follow prescriptions for medications. Cochrane Database of Systematic Reviews, 2002, CD000011. doi:10.1002/14651858.CD000011
Hugtenburg, J.G., Timmers, L., Elders, P.J., Vervloet, M., & van Dijk, L. (2013). Definitions, variants, and causes of nonadherence with medication: A challenge for tailored interventions. Patient Preference and Adherence, 7, 675–682. doi:10.2147/PPA.S29549
Jefford, M., Lotfi-Jam, K., Baravelli, C., Grogan, S., Rogers, M., Krishnasamy, M., . . . Schofield, P. (2011). Development and pilot testing of a nurse-led posttreatment support package for bowel cancer survivors. Cancer Nursing, 34, E1–E10. doi:10.1097/NCC.0b013e3181f22f02
Khalil, H.S., Feldman, M., & Bridger, M.W. (2003). A simple assessment of quality of life in head and neck cancer patients: What can it tell us? Revue de Laryngologie Otologie Rhinonologie, 124, 211–214.
Koller, A., Miaskowski, C., De Geest, S., Opitz, O., & Spichiger, E. (2013). Results of a randomized controlled pilot study of a self-management intervention for cancer pain. European Journal of Oncology Nursing, 17, 284–291. doi:10.1016/j.ejon.2012.08.002
Langley, G.J., Moen, R., Nolan, K.M., Nolan, T.W., Norman, C.L., & Provost, L.P. (2009). The improvement guide: A practical approach to enhancing organizational performance (2nd ed.). San Francisco, CA: Jossey Bass.
Lesage, F.X., & Berjot, S. (2011). Validity of occupational stress assessment using a visual analogue scale. Occupational Medicine, 61, 434–436. doi:10.1093/occmed/kqr037
Martin, J.P., & Savary, E. (2013). Formateur d’adultes: Se professionnaliser, exercer au quotidien. Lyon, France: Chronique sociale.
McCabe, M.S., & Jacobs, L. (2008). Survivorship care: Models and programs. Seminars in Oncology Nursing, 24, 202–207. doi:10.1016/j.soncn.2008.05.008
McDonald, H.P., Garg, A.X., & Haynes, R.B. (2002). Interventions to enhance patient adherence to medication prescriptions: Scientific review. JAMA, 288, 2868–2879.
Monnier, A. (2007). Clinical management of adverse events in adjuvant therapy for hormone-responsive early breast cancer. Annals of Oncology, 18(Suppl. 8), viii36–viii44. doi:10.1093/annonc/mdm264
Osterberg, L., & Blaschke, T. (2005). Adherence to medication. New England Journal of Medicine, 353, 487–497. doi:10.1056/NEJMra050100
Park, J.H., Bae, S.H., Jung, Y.S., & Kim, K.S. (2012). Quality of life and symptom experience in breast cancer survivors after participating in a psychoeducational support program: A pilot study. Cancer Nursing, 35, E34–E41. doi:10.1097/NCC.0b013e318218266a
Partridge, A.H., Avorn, J., Wang, P.S., & Winer, E.P. (2002). Adherence to therapy with oral antineoplastic agents. Journal of the National Cancer Institute, 94, 652–661.
Partridge, A.H., LaFountain, A., Mayer, E., Taylor, B.S., Winer, E., & Asnis-Alibozek, A. (2008). Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. Journal of Clinical Oncology, 26, 556–562. doi:10.1200/JCO.2007.11.5451
Partridge, A.H., Wang, P.S., Winer, E.P., & Avorn, J. (2003). Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. Journal of Clinical Oncology, 21, 602–606.
Pasquier, F., Feutrier, C., Charpiat, B., Vagnarelli, S., Schlienger, I., & Leboucher, G. (2013). Observance thérapeutique aux antirétroviraux et évaluations pédagogiques des programmes d’éducation thérapeutique: Pas de corrélation systématique. À propos d’un cas. Education Thérapeutique Du Patient, 5, 401–407. doi:10.1051/tpe/2012017
Porter, H.B. (1998). Effectiveness and efficiency of nurse-given cancer patient education. Canadian Oncology Nursing Journal, 8, 229–240.
Price, E.L., Mackenzie, T.D., Metlay, J.P., Camargo, C.A., Jr., & Gonzales, R. (2011). A computerized education module improves patient knowledge and attitudes about appropriate antibiotic use for acute respiratory tract infections. Patient Education and Counseling, 85, 493–498. doi:10.1016/j.pec.2011.02.005
Pritchard, M. (2010). Measuring anxiety in surgical patients using a visual analogue scale. Nursing Standard, 25(11), 40–44.
Régnier-Denois, V., Rousset-Guarato, V., Nourissat, A., Bourmaud, A., & Chauvin, F. (2010). Contribution of a preliminary socio-anthropological survey to the development of a therapeutic patient education programme for patients receiving oral chemotherapy. Education Thérapeutique Du Patient, 2(Suppl.), S101–S107. doi:10.1051/tpe/2010010
Ruddy, K., Mayer, E., & Partridge, A. (2009). Patient adherence and persistence with oral anticancer treatment. CA: A Cancer Journal for Clinicians, 59, 56–66. doi:10.3322/caac.20004
Schlairet, M., Heddon, M.A., & Griffis, M. (2010). Piloting a needs assessment to guide development of a survivorship program for a community cancer center. Oncology Nursing Forum, 37, 501–508. doi:10.1188/10.ONF.501-508
Verbrugghe, M., Verhaeghe, S., Lauwaert, K., Beeckman, D., & Van Hecke, A. (2013). Determinants and associated factors influencing medication adherence and persistence to oral anticancer drugs: A systematic review. Cancer Treatment Reviews, 39, 610–621. doi:10.1016/j.ctrv.2012.12.014
von Blanckenburg, P., Schuricht, F., Albert, U.S., Rief, W., & Nestoriuc, Y. (2013). Optimizing expectations to prevent side effects and enhance quality of life in breast cancer patients undergoing endocrine therapy: Study protocol of a randomized controlled trial. BMC Cancer, 18, 426. doi:10.1186/1471-2407-13-426
Waldorff, F.B., Buss, D.V., Eckermann, A., Rasmussen, M.L., Keiding, N., Rishoj, S., . . . Waldemar, G. (2012). Efficacy of psychosocial intervention in patients with mild Alzheimer’s disease: The multicentre, rater blinded, randomised Danish Alzheimer Intervention Study (DAISY). BMJ, 345, e4693. doi:0.1136/bmj.e4693
World Health Organization. (1998). Therapeutic patient education: Continuing education programmes for health care providers in the field of prevention of chronic diseases. Retrieved from http://www.euro.who.int/__data/assets/pdf_file/0007/145294/E63674.pdf
Xue, D., Sun, H., & Li, P.-P. (2011). Long-term chinese herbs decoction administration for management of hot flashes associated with endocrine therapy in breast cancer patients. Chinese Journal of Cancer Research, 23, 74–78. doi:10.1007/s11670-011-0074-7
Yates, P., Aranda, S., Hargraves, M., Mirolo, B., Clavarino, A., McLachlan, S., & Skerman, H. (2005). Randomized controlled trial of an educational intervention for managing fatigue in women receiving adjuvant chemotherapy for early-stage breast cancer. Journal of Clinical Oncology, 23, 6027–6036. doi:10.1200/JCO.2005.01.271
Yu, K.D., Zhou, Y., Liu, G.Y., Li, B., He, P.Q., Zhang, H.W., . . . Shen, Z.Z. (2012). A prospective, multicenter, controlled, observational study to evaluate the efficacy of a patient support program in improving patients’ persistence to adjuvant aromatase inhibitor medication for postmenopausal, early stage breast cancer. Breast Cancer Research and Treatment, 134, 307–313. doi:10.1007/s10549-012-2059-8
Zigmond, A.S., & Snaith, R.P. (1983). The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica, 67, 361–370.
Ziller, V., Kalder, M., Albert, U.S., Holzhauer, W., Ziller, M., Wagner, U., & Hadji, P. (2009). Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Annals of Oncology, 20, 431–436. doi:10.1093/annonc/mdn646
Ziller, V., Kyvernitakis, I., Knöll, D., Storch, A., Hars, O., & Hadji, P. (2013). Influence of a patient information program on adherence and persistence with an aromatase inhibitor in breast cancer treatment—The COMPAS study. BMC Cancer, 4, 407.
About the Author(s)
Bourmaud is a medical doctor, Rousset is an educational program manager, and Regnier-Denois is a medical anthropologist, all in the Hygee Centre–Public Health Department; Collard is a medical oncologist, Jacquin is a medical oncologist, Merrouche is the department head, and Lapoirie is an educational nurse, all in the Medical Oncology Department; and Tinquaut is a statistician in the Hygee Centre–Public Health Department, all at the Lucien Neuwirth Cancer Institute in Saint-Priest-en-Jarez, France; Lataillade is a clinical nurse specialist in the Teaching Department for Therapeutics for Chronic Diseases at the Geneva University Hospital in Geneva, Switzerland; and Chauvin is the department head of the Hygee Centre–Public Health Department at the Lucien Neuwirth Cancer Institute. Translation and editorial support was provided by Margaret Haugh at MediCom Consult through funding from the Hygee Research Centre in France. Bourmaud, Rousset, Regnier-Denois, Lataillade, and Chauvin contributed to the conceptualization and design. Rousset and Lapoirie completed the data collection. Tinquaut provided the statistical support. Bourmaud, Rousset, Regnier-Denois, and Lataillade contributed to the analysis. Bourmaud, Rousset, Regnier-Denois, Collard, Jacquin, Merrouche, Lapoirie, Tinquaut, Lataillade, and Chauvin contributed to the manuscript preparation. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society. Bourmaud can be reached at aurelie.bourmaud@icloire.fr, with copy to editor at ONFEditor@ons.org. Submitted December 2014. Accepted for publication July 11, 2015.

