Acute Aortic Dissection Following Treatment for Castration-Resistant Prostate Cancer
A 65-year-old man presents to the emergency department with increasing back pain. His history includes hypertension, peripheral neuropathy, duodenal ulcer, superior mesenteric vein thrombus, stage IIB colon cancer treated with surgery and adjuvant chemotherapy, renal cell carcinoma treated with surgery, and prostate cancer treated with surgery and radiation. He is otherwise healthy. His family history is positive for colon cancer. Physical examination found significantly elevated blood pressure and a computed tomography scan of the thoracic and lumbar spine was performed, with findings of a type B aortic dissection extending from the aberrant right subclavian artery down to the abdominal aorta.
Jump to a section
A 65-year-old man presents to the emergency department with increasing back pain. His history includes hypertension, peripheral neuropathy, duodenal ulcer, superior mesenteric vein thrombus, stage IIB colon cancer treated with surgery and adjuvant chemotherapy, renal cell carcinoma treated with surgery, and prostate cancer treated with surgery and radiation. He is otherwise healthy. His family history is positive for colon cancer. Physical examination found significantly elevated blood pressure and a computed tomography scan of the thoracic and lumbar spine was performed, with findings of a type B aortic dissection extending from the aberrant right subclavian artery down to the abdominal aorta.
Acute Aortic Dissection
The estimated incidence of acute aortic dissection is about 3 in 100,000 per year (Papadopoulos et al., 2015). Aortic dissection begins with a small tear in the lumen of the aorta. As the heart continues to pump blood through the tear, a false channel is created, which may quickly become larger than the aorta itself. If left untreated, death will often occur from a rupture in the aorta (Pyne & Apple, 2013). The most significant risk factors for aortic dissection include hypertension, age (risk increasing with age), and gender (men are at higher risk) (Golledge & Eagle, 2008; Papadopoulos et al., 2015). Other risk factors include known connective tissue disorders, smoking, direct blunt force trauma, and drug use (e.g., cocaine, amphetamines) (Nienaber & Clough, 2015). Presentation most commonly includes abrupt onset of chest and/or back pain, pulse deficit, and abnormal chest imaging; however, these symptoms are not always present (Pape et al., 2015; Ranasinghe, Strong, Boland, & Bosner, 2011). Immediate management of aortic dissection includes pain control and antihypertensive therapy with the initial goal of rapid reduction of blood pressure. Additional management is dependent on the type and location of dissection (Golledge & Eagle, 2008; Nienaber & Clough, 2015) (see Table 1).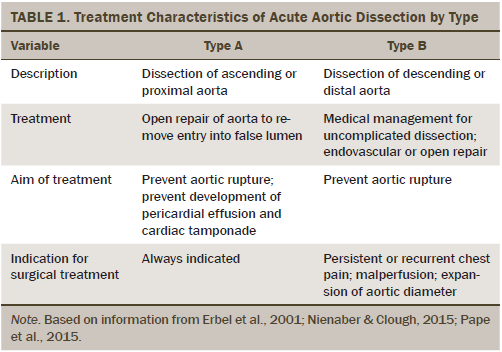
Prostate Cancer
Prostate cancer is the most common cancer among men in North America (American Cancer Society, 2016; Canadian Cancer Society, 2015). Worldwide, prostate cancer is the second most frequently diagnosed cancer and the sixth-leading cause of cancer death in males, accounting for 14% (903,500) of the total new cancer cases and 6% (258,400) of the total cancer deaths in males in 2008 (World Cancer Research Fund International, 2014).
The natural history of prostate cancer is extremely variable and is largely dependent on Gleason score, a measure of cell differentiation. With the advent of serum prostate-specific antigen (PSA) screening, the majority of prostate cancers are detected early when still small and localized to the prostate. Low-grade disease (Gleason score of 6, or well differentiated) may remain indolent for more than 10 years; in men with low-risk prostate cancer (low grade and low volume), the risk of disease progression within a decade is less than 6% (Albertsen, 2015). In contrast, about 20%–30% of men are diagnosed with high-risk prostate cancer (Gleason score of 8–10, or poorly differentiated, with a PSA greater than 20), which typically results in death within 10 years (Albertsen, 2015; Punnen & Cooperberg, 2013).
Treatment options for localized prostate cancer commonly include surgery, radiation, or active surveillance, and choice of treatment should be tailored to patient preference (Mohler et al., 2010; Wang et al., 2015). Radical prostatectomy (RP) is the treatment of choice for many men and involves removal of the entire prostate and select lymph nodes. Research indicates that about 15%–46% of men treated with RP will experience a biochemical recurrence (BCR) within 15 years (Kellogg Parsons & Partin, 2006). BCR refers to a rise in PSA following definitive treatment for prostate cancer, and the natural history of BCR is generally prolonged. A landmark study by Pound et al. (1999) found that post RP, the median time from BCR to metastatic disease was eight years, and time from metastatic disease to death was five years.
Radiation therapy for the treatment of localized prostate cancer most commonly involves external beam radiation therapy or brachytherapy. BCR is common, and one large study using the Cancer of the Prostate Strategic Urologic Research Endeavor database found recurrent disease in 63% of men treated with external beam radiation therapy (EBRT) (Argarwal, Sadetsky, Konety, Resnick, & Carroll, 2008). BCR after primary radiation therapy is also prolonged and may be detected as many as 20 years after treatment and beyond (Swanson, Riggs, & Earle, 2004).
Several treatment options are available for BCR, and treatment will depend on determining if the recurrence is localized to the prostate or involves metastatic disease (Kellogg Parsons & Partin, 2006). For patients who have previously been treated with surgery, salvage radiation may be offered. For patients previously treated with EBRT, treatment for localized recurrence may include salvage cryotherapy or salvage radical prostatectomy; however, this surgery is extremely difficult, with a high rate of complications (Williams & Choueiri, 2013).
Given that prostate cancer is largely androgen dependent, suppression of testosterone with androgen deprivation therapy (ADT) is a mainstay of treatment for metastatic disease (Sternberg, Petrylak, Madan, & Parker, 2014). ADT, also called hormone therapy, can be added to primary or salvage therapies (Williams & Choueiri, 2013). Following suppression of testosterone, either medically or surgically, tumor regression is seen, with a decline in PSA and improvement in systemic symptoms (Sternberg et al., 2014). Unfortunately, despite initial improvement, virtually all men will experience disease progression, regardless of castrate testosterone levels, to what is known as castration-resistant prostate cancer (CRPC) (Sternberg et al., 2014). The natural history of CRPC will typically include worsening of symptoms and eventually death. Disease progression in the castration-resistant state may lead to the development of serious or life-threatening complications, including anemia, urinary tract obstruction, spinal cord compression, pain, cachexia, and pathologic fractures (Sternberg et al., 2014). Several agents are now available for the treatment of CRPC, including enzalutamide (Xtandi®), abiraterone acetate (Zytiga®), cabazitaxel (Jevtana®), docetaxel (Taxotere®), sipuleucel-T (Provenge®), and radium-223. Although all have demonstrated improvement in overall survival, resistance will ultimately develop to these agents as well (Sternberg et al., 2014).
Enzalutamide is an oral androgen receptor antagonist used in men with CRPC. Enzalutamide is effective and generally well tolerated. Common side effects include fatigue, diarrhea, hot flashes, musculoskeletal pain, headaches, hypertension, and seizure (Scher et al., 2012). One large study reported hypertension was the most common severe or medically significant side effect, occurring in 7% of patients (Beer et al., 2014). Abiraterone acetate inhibits the biosynthesis of androgens and is also prescribed orally for the treatment of CRPC. Common side effects reported, similar in treatment and placebo groups, include fatigue, back pain, nausea, constipation, and bone pain. Side effects more common in patients treated with abiraterone acetate versus placebo include fluid retention, hypokalemia, and hypertension (de Bono et al., 2011).
Case Study
After the patient was diagnosed with type B acute aortic dissection, it was noted that he had been started on enzalutamide for CRPC several months earlier. Unfortunately, after starting on this new treatment, the patient was not seen for follow-up or assessed for side effects. His blood pressure was not monitored. Enzalutamide was discontinued immediately, and the patient was admitted to the cardiac unit for aggressive management of his hypertension with IV antihypertensives. Following the stabilization of blood pressure, enzalutamide was reinstated; however, the patient’s blood pressure rose significantly almost immediately. Enzalutamide was permanently discontinued; however, there was continued difficulty in controlling the blood pressure. The patient underwent endovascular repair and left carotid subclavian bypass for treatment of the aortic dissection and was discharged more than a month following initial presentation. The patient has experienced exacerbations of hypertension and remains on metoprolol (Lopressor®), nifedipine (Procardia®), and irbesartan (Avapro®). Following discharge from the hospital, the patient was started on abiraterone acetate for treatment of CRPC and was monitored closely for side effects.
Implications for Nursing and Conclusion
This case highlights the importance of nursing knowledge and assessment of potentially dangerous side effects. Cancer treatments are rapidly evolving, and oncology nurses must have a basic understanding of the treatments commonly prescribed in their setting, as well as potential side effects that may require close monitoring. Oncology nurses play an important and effective role in educating patients on their treatment and possible side effects (Koutsopoulou, Papathanassoglou, Katapodi, & Patiraki, 2010). Nurses also play a pivotal role as part of a multidisciplinary team, often serving as the main contact for the patient and medical staff, and are in the best position to observe the effects of treatment-related side effects on the patient (Sonnek & van Muilekom, 2013). Frequent nursing assessment should be routine for patients started on new treatment to ensure treatment-related side effects are managed proactively and promptly.
References
Albertsen, P.C. (2015). Observational studies and the natural history of screen-detected prostate cancer. Current Opinion in Urology, 25, 232–237. doi:10.1097/MOU.0000000000000157
American Cancer Society. (2016). Key statistics for prostate cancer. Retrieved from http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-canc…
Argarwal, P.K., Sadetsky, N., Konety, B.R., Resnick, M.I., & Carroll, P.R. (2008). Treatment failure after primary and salvage therapy for prostate cancer. Cancer, 112, 307–314. doi:10.1002/cncr.23161
Beer, T.M., Armstrong, A.J., Rathkopf, D.E., Loriot, C.N., Sternberg, C.S., Higano, P., . . . Tombal, B. (2014). Enzalutamide in metastatic prostate cancer before chemotherapy. New England Journal of Medicine, 371, 424–433.
Canadian Cancer Society. (2015). Canadian cancer statistics 2015. Retrieved from https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%…
de Bono, J., Logothetis, C.J., Molina, A., Fizazi, K., North, S., Chu, L., . . . Scher, H.I. (2011). Abiraterone and increased survival in metastatic prostate cancer. New England Journal of Medicine, 364, 1995–2005.
Erbel, R., Alfonso, F., Boileau, C., Dirsch, O., Eber, B., Haverich, A., . . . Zollikofer, C. (2001). Diagnosis and management of aortic dissection: Recommendations of the Task Force on Aortic Dissection, European Society of Cardiology. European Heart Journal, 22, 1642–1681.
Golledge, J., & Eagle, K.A. (2008). Acute aortic dissection. Lancet, 372, 55–66.
Kellogg Parsons, J., & Partin, A.W. (2006). Clinical management of rising prostate-specific antigen after radical retropubic prostatectomy. In S.R. Kirby, A.W. Partin, M.R. Feneley, & J. Kellogg Parsons (Eds.), Prostate cancer: Principles and practice (pp. 747–754). London, England: Taylor and Francis.
Koutsopoulou, S., Papathanassoglou, E.D.E., Katapodi, M.C., & Patiraki, E.I. (2010). A critical review of the evidence for nurses as information providers to cancer patients. Journal of Clinical Nursing, 19, 749–765. doi:10.1111/j.1365-2702.2009.02954.x
Mohler, J., Bahanson, R.R., Boston, B., Busby, J.E., D’Amico, A., Eastham, J.A., . . . Walsh, P.E. (2010). NCCN Clinical Practice Guidelines in Oncology: Prostate cancer. Journal of the National Comprehensive Cancer Network, 8, 162–200.
Nienaber, C.A., & Clough, R.E. (2015). Management of acute aortic dissection. Lancet, 385, 800–811. doi:10.1016/S0140-6736 (14)61005-9
Papadopoulos, D.P., Sanidas, E.A., Viniou, N.A., Gennimata, V., Chantziara, V., Barbetseas, I., & Makris, T.K. (2015). Cardiovascular hypertensive emergencies. Current Hypertension Reports, 17, 5. doi:10.1007/s11906-014-0515-z
Pape, L.A., Awais, M., Woznicki, E.M., Suzuki, T., Trimarchi, S., Evangelista, A., . . . O’Gara, P. (2015). Presentation, diagnosis and outcomes of acute aortic dissection: 17 year trends from the International Registry of Acute Aortic Dissection. Journal of the American College of Cardiology, 66, 350–358. doi:10.1016/j.jacc.2015.05.029
Pound, C.R., Partin, A.W., Eisenberger, M.A., Chan, D.W., Pearson, J.D., & Walsh, P.C. (1999). Natural history of progression after PSA elevation following radical prostatectomy. JAMA, 281, 1591–1597. doi:10.1001/jama.281.17.1591
Punnen, S., & Cooperberg, M.R. (2013). The epidemiology of high-risk prostate cancer. Current Opinion in Urology, 23, 331–336. doi:10.1097/MOU.0b013e328361d48e
Pyne, C.C., & Apple, S. (2013). Common cardiovascular disorders. In P.G. Morton & D.K. Fontaine (Eds.), Critical care nursing: A holistic approach (10th ed., pp. 374–390). Philadelphia, PA: Wolters Kluwer Health.
Ranasinghe, A.M., Strong, D., Boland, B., & Bosner, R.S. (2011). Acute aortic dissection. BMJ, 343. doi:10.1136/bmj.d4487
Scher, H.I., Fizazi, K., Saad, F., Taplin, M., Sternberg, C.N., Miller, K., . . . de Bono, J.S. (2012). Increased survival with enzalutamide in prostate cancer after chemotherapy. New England Journal of Medicine, 367, 1187–1197. doi:10.1056/NEJMoa1207506
Sonnek, F.C., & van Muilekom, E. (2013). Metastatic castration-resistant prostate cancer. Part 2: Helping patients make informed choices and managing treatment side effects. European Journal of Oncology Nursing, 17(Suppl. 1), S7–S12.
Sternberg, C.N., Petrylak, D.P., Madan, R.A., & Parker, C. (2014). Progress in the treatment of advanced prostate cancer. Retrieved from http://meetinglibrary.asco.org/content/114000117-144
Swanson, G.P., Riggs, M.W., & Earle, J.D. (2004). Long-term follow-up of radiotherapy for prostate cancer. International Journal of Radiation Oncology, Biology and Physics, 59, 406–411. doi:10.1016/j.ijrobp.2003.10.026
Wang, E.H., Gross, C.P., Tilburt, J.C., Yu, J.B., Nguyen, P.L., Smaldone, M.C., . . . Kim, S.P. (2015). Shared decision making and use of decision aids for localized prostate cancer. JAMA Internal Medicine, 175, 792–799.
Williams, S.B., & Choueiri, T.K. (2013). Management of biochemical recurrence after localized treatment for prostate cancer. In A. Klein & J.S. Jones (Eds.), Management of prostate cancer (pp. 347–359). New York, NY: Springer.
World Cancer Research Fund International. (2014). Prostate cancer statistics. Retrieved from http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/pros…
About the Author(s)
Horrill is a PhD student in the Faculty of Health Sciences at the University of Manitoba, Winnipeg, Canada. No financial relationships to disclose. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society. Horrill can be reached at tara.horrill@gmail.com, with copy to editor at ONFEditor@ons.org.

