An Integrative Review of the Role of the Oral and Gut Microbiome in Oral Health Symptomatology During Cancer Therapy
Problem Identification: Both chemotherapy and radiation therapy cause considerable symptom burden on patients’ oral health, influencing nutritional status and quality of life. The role of the oral and gut microbiome in oral health alterations during cancer therapy is an emerging area of science in symptom management.
Literature Search: PubMed®, CINAHL®, and Scopus® were searched for articles published from January 2000 through July 2020.
Data Evaluation: Articles published in English that were focused on chemotherapy and/or radiation therapy were included in the review.
Synthesis: Of the 22 identified studies, 12 described oral health symptoms during chemotherapy and radiation therapy for head and neck cancer. Ten studies assessed symptoms during treatment for a variety of solid tumors and blood cancers, with four of these describing microbial interventions for the management of oral mucositis. Interventions varied, but the results supported the benefits of probiotics and synbiotics in reducing mucositis severity. Overall, less diverse oral and gut microbiome environments were associated with increased severity of oral health symptomatology.
Implications for Practice: Additional research is needed to determine how the oral and gut microbiome and microbial interventions may be used to improve oral health management during cancer treatment.
Jump to a section
Researchers expect there to be 29.5 million cancer cases per year globally by 2040 (International Agency for Research on Cancer, 2020). Incidence of cancer cases continues to increase because of the aging population, screening and treatment advancements, and increasing health inequities (Miller et al., 2019). Accompanied with advanced treatment protocols, concerns have been raised related to the side effects of systemic chemotherapy and radiation therapy (RT) directed near the oral cavity, particularly detrimental changes to oral health.
Background
Systemic or cytotoxic chemotherapy damages rapidly dividing cells (e.g., normal cells lining the alimentary canal), causing inflammation and an inability to grow new cells in the oral cavity (National Cancer Institute, 2016). In turn, this creates oral health alterations in the form of cluster symptoms, including oral mucositis (OM), xerostomia, pain, and oral sensory alterations. Similarly, RT leads to direct cell damage and breakdown, so RT directed to the mouth or neck can cause injury to the cells in these areas, including salivary glands and sensory receptors (National Cancer Institute, 2016). Therefore, OM, xerostomia, and disturbances in oral sensations (e.g., true taste, retronasal olfaction, altered touch, temperature, pain sensations) are commonly related to cancer treatment (Murtaza et al., 2017). Although research is limited, the literature supports that OM occurs in about 40% of patients receiving chemotherapy (Elting et al., 2003). In addition, about 70% of patients receiving chemotherapy report oral sensory alterations (Zabernigg et al., 2010). Nearly all patients undergoing RT to the head and neck area develop OM (Villa & Sonis, 2015). Overlapping injuries from both chemotherapy and RT lead to increased severity of oral health symptoms (Rosenthal et al., 2014). The oral and gut microbiome, which is composed of trillions of microorganisms within the human body, is being studied as part of the etiology of cancers and symptomatology during cancer treatment (Orlandi et al., 2019).
Consideration of healthy oral and gut microbiome composition is essential prior to exploring the impact of microbial dysbiosis (i.e., an imbalance of microbial community) on oral health symptoms. The oral and gut microbiome represents two of the most diverse bacterial environments in the human body (Integrative HMP Research Network Consortium, 2019). Optimal oral microbiome composition is relatively consistent across individuals, with a complex interaction of proinflammatory and anti-inflammatory microbial activities coexisting to maintain homeostasis (Kilian et al., 2016). Healthy oral microbiome environments are characterized by a diverse microbial population consisting of large numbers of different microbial species (Kilian et al., 2016). Optimal gut microbiome composition also consists of a variety of balanced microbial species. Gut microbiome composition is generally more susceptible to changes in response to diet and the environment than the oral microbiome (Lu et al., 2019; Rinninella et al., 2019). Research highlights a strong relationship between diverse gut microbiota and optimal health, including oral health (Rinninella et al., 2019; Xu et al., 2020). Alteration of microbes from homeostatic ranges influences and is influenced by inflammatory processes. It is essential to examine the mechanism of action behind oral and gut microbial changes during cancer treatment and how these influence oral health symptoms.
The oral and gut microbiome are linked physically along the alimentary canal and microscopically through oral microbe displacement to the gut microbiome (Olsen & Yamazaki, 2019). Systemic cancer therapies influence proliferating cells—including their microbial components—along the entire alimentary canal, and the oral and gut microbiome affects oral health (Helmink et al., 2019; Kilian et al., 2016; Xu et al., 2020). Therefore, it is necessary to examine the roles of both environments in oral health symptomatology during cancer treatment. Chemotherapy and RT can disturb the oral and gut microbiome along the alimentary canal and are thought to contribute to oral symptomology using mechanistic underpinnings and trajectory of events, leading to the development of oral and gut mucositis. Cancer therapies contribute to this trajectory through heightened inflammation (Villa & Sonis, 2015). Gram-negative oral microbes are more abundant with ulceration, whereas normalized bacterial ratios, with less gram-negative bacteria, are associated with mucositis resolution (Sonis, 2009). In brief, cancer therapies lead to cell and DNA damage, disrupting the oral and gut microbiome composition in areas of rapid cell growth (Villa & Sonis, 2015). The mechanism of action involved in general mucositis and overall inflammation and damage from cancer therapies has been connected to the microbiome environment. Chemotherapy-induced OM has been linked to oxidative stress and release of inflammatory cytokines (Peterson et al., 2016). This inflammatory response activates the ceramide pathway, leading to tissue breakdown and increased epithelial permeability (Peterson et al., 2016). Increased epithelial permeability and tissue breakdown provide a gateway for the release of microbes into the bloodstream. Dysbiosis in the oral and gut regions has been characterized by increased levels of gram-negative bacteria. Toxins released by gram-negative bacteria due to cellular wall lipopolysaccharide heighten the inflammatory response, leading to further damage and symptom severity (Kelly et al., 2015; Maldonado et al., 2016) (see Figure 1). 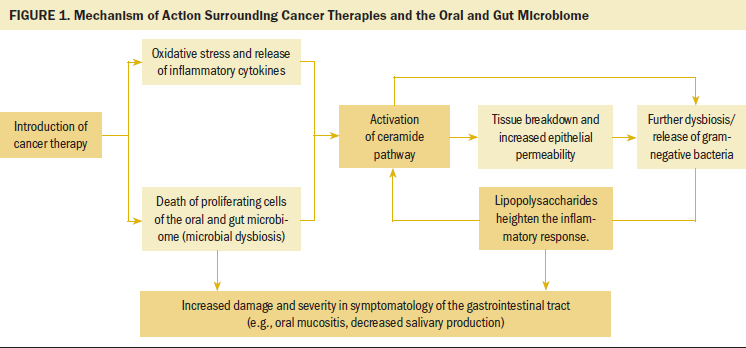
A systematic review by Wardill et al. (2020) explored the emerging role of baseline oral and gut microbiome dysbiosis and its influence on oral symptom development. Clinical research has suggested a predictive relationship between oral and gut microbiome composition and mucositis severity; however, its utility in oncology nursing practice is unknown. Therefore, it is necessary to examine oral health symptoms and their relation to oral and gut microbiome composition during cancer treatment.
Cancer therapies can lead to alterations in oral sensations, changing the flavor profile of foods and beverages, the desire to eat and drink, the quality of the eating experience, nutritional health, and, ultimately, overall health and quality of life (Duffy, 2019). Oral health symptoms occur in a cluster and are interrelated. For example, RT commonly leads to xerostomia as a result of salivary gland injury, and limited saliva production directly affects the ability to experience true taste (i.e., salt, sweet, sour, bitter, and umami) and retronasal olfaction (primary component of food flavor), as well as causes altered taste sensations (e.g., persistent tastes, dysgeusia) and pain sensations (Duffy, 2019). Patients have reported lower quality of life associated with the oral health symptoms of cancer treatment (Shavi et al., 2015). Patients who develop OM are also more likely to experience delays in cancer treatment and longer hospitalizations (Elting et al., 2003). Efforts to maintain good oral health have implications for treatment adherence, quality of life, and healthcare costs. Innovative approaches to oral health management, including microbiome exploration, are expanding to meet this need.
Previous research has examined the impact of the oral and gut microbiome on oral health symptomatology in patients receiving cancer treatment, predominantly for head and neck cancer (Al-Qadami et al., 2019; Orlandi et al., 2019). The current review seeks to expand on these findings to other cancer populations and, through an integrative method, extend to nursing implications. The purpose of this review was to explore the state of the science concerning the role of the oral and gut microbiome in oral health symptomatology during cancer treatment. The review aimed to understand the association between oral and gut microbiome composition and the prevalence and severity of oral health symptomatology during cancer therapy, as well as the role of microbial interventions (e.g., probiotics or synbiotics usage) in oral health symptom management. Reviewing the current literature that links the oral and gut microbiome and the oral health of patients undergoing cancer treatment is essential for clarifying and advancing this science to determine implications for nursing practice.
Methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for manuscript development were followed (Moher et al., 2009). Consideration of oral health symptoms was guided by the Theory of Unpleasant Symptoms given the complexity of physiological, psychological, and situational factors involved in the symptom experience (Lenz et al., 1995). Although guided by the totality of the theory, this review used an adaptation of the Theory of Unpleasant Symptoms, with a primary focus on physiological aspects of the role of oral and gut microbiome in oral health symptom assessment.
Eligibility Criteria
Studies were included if (a) the study cohorts included participants who were aged older than 18 years and who had received chemotherapy and/or RT for treatment of cancer, (b) oral or gut microbiome specimens were collected, and (c) measurements of oral health were conducted (e.g., OM, xerostomia, hydration status, appetite, oral sensory, other oral health conditions). Studies were excluded if they only reviewed literature or presented an in vitro model.
Search Strategy
Searches were conducted in the PubMed®, CINAHL®, and Scopus® electronic databases to identify relevant articles for review. Keywords and terms were established with the assistance of a university librarian who specializes in health sciences research. Search terms included microbiome, cancer, chemotherapy, radiation therapy, oral health, and oral mucositis. Searches were conducted from January 2000 through July 2020, and searches were limited to studies published in English. The last search was conducted on July 17, 2020. A hand search of authors and references of articles that met the full-text inclusion criteria was performed.
Data Collection
Database results were screened for inclusion and exclusion criteria. Relevant articles were reviewed for quality by two independent reviewers.
Quality Appraisal
Based on the integrative review methodology of Whittemore and Knafl (2005), each study was assessed by two independent reviewers for quality and risk of bias using the Mixed Method Appraisal Tool (MMAT) (Hong, Pluye, et al., 2019). The MMAT includes two screening questions for each study and five criteria for each study type. An overall quality score was created from the study type–specific criteria, meaning the score was 20% if one criterion was met, and the score was 100% if all five criteria were met.
Data Synthesis
Articles were analyzed in stages by first organizing any data related to the study purposes into an evidence table. Data were extracted for comparison of study design, study participants, type of microbiome data, cancer type, cancer treatment type, and oral health data. Studies were then grouped according to the data, particularly by which oral health measurement and microbiome sampling location were included. These groups were synthesized, and relationships among studies were identified.
Findings
Study Selection
A total of 476 articles were identified through database searching in PubMed, CINAHL, and Scopus (see Figure 2). Eight additional articles were identified via hand search that met the inclusion criteria. After duplicates were removed (n = 122) and articles were excluded via title and abstract review (n = 334), 28 articles were reviewed for full-text eligibility. On full-text screening, six articles were excluded for the following reasons: review article (n = 2), in vitro model (n = 2), no oral health measurement (n = 1), and no microbiome sampling (n = 1). Articles that met the inclusion criteria during full-text screening were then assessed for quality using the MMAT (n = 22). Most articles had a quality score of at least 80%. No articles were eliminated due to issues with methodological rigor. 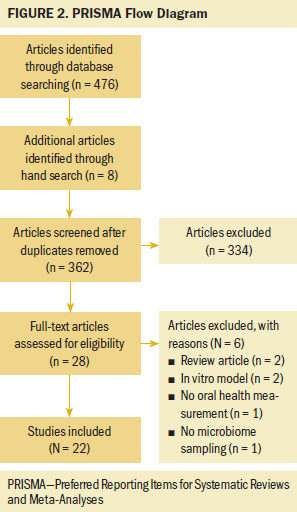
Study Characteristics
A sample of 1,032 participants across 22 studies was included in this integrative review. The sample included participants diagnosed with head and neck cancer, including nasopharyngeal carcinoma and oral squamous cell carcinoma (n = 12), breast cancer (n = 2), multiple myeloma (n = 1), esophageal cancer (n = 1), and multiple cancer diagnoses (n = 6). Cancer treatment types included RT (n = 9), chemotherapy (n = 9), concurrent chemotherapy and RT (CCRT) (n = 1), RT or CCRT (n = 2), and chemotherapy or RT (n = 1). The types of oral health data that were collected from participants included OM (n = 13), oral candidiasis (n = 2), and a combination of multiple oral health measurements (e.g., OM, salivary flow, xerostomia, gingival inflammation, oral sensory disturbances) (n = 7). Twenty studies obtained oral microbiome samples from participants, whereas two studies collected gut microbiome samples. Fourteen studies analyzed microbiota samples using DNA sequencing methods, whereas eight studies used culture methods.
Synthesis of Findings
The 22 articles that met the full-text eligibility criteria and quality appraisal were placed into an abstraction tool. Demographic information and study-specific data were abstracted from the articles. As similarities and differences emerged, articles were grouped into various categories for synthesis. Groupings were based on study elements, including type of oral health symptom, type of microbiome sample, and presence of a microbial intervention. A qualitative depiction of the results was integrated into an adapted Theory of Unpleasant Symptoms (see Figure 3). 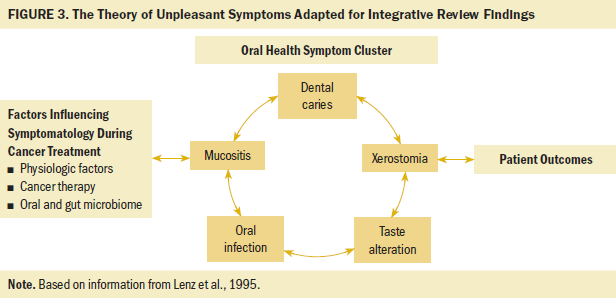
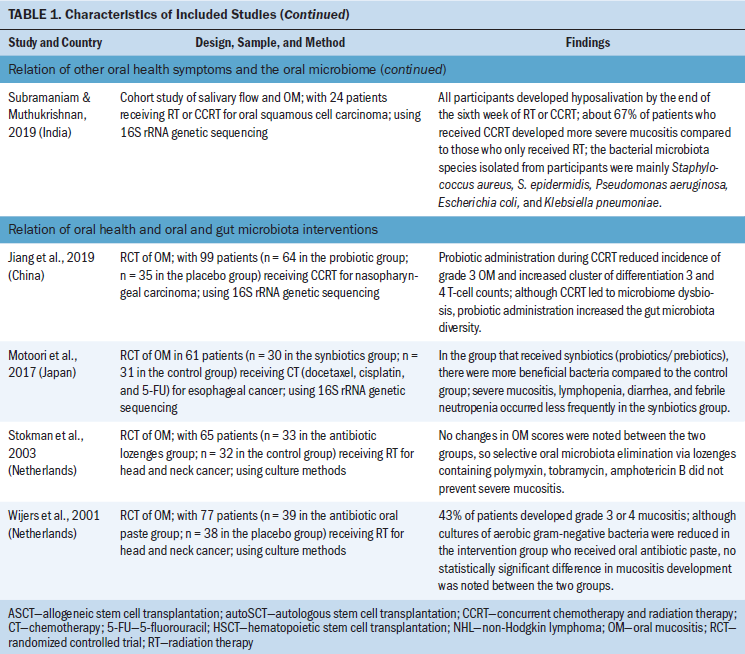
Various associations between oral health measurements and microbiota composition emerged from this review. The association between gut microbiota and oral health focused on OM and microbial interventions, which included the use of beneficial bacteria-containing products. The relationship between oral microbiota and oral health during cancer treatment included associations of OM, oral candidiasis, and other measurements of oral health. Characteristics of each of the included studies and their major results are depicted in Table 1. Included studies that reported alpha diversity trends (i.e., the estimation of the richness of different species present in a microbiome sample) and their associated oral health symptom development are further detailed in Table 2. Although beta diversity (i.e., the estimation of the differences in bacterial species between samples) was included in some studies, it was not as widely reported across studies as alpha diversity. Therefore, only alpha diversity was included in the research synthesis. Table 3 demonstrates the propensity toward gram-negative bacterial microbes that were associated with adverse oral health outcomes in the included studies. 
Relation of Oral Mucositis and the Oral Microbiome
Most studies included in the review discussed the association between OM and oral microbiota composition during cancer therapies (Gaetti-Jardim et al., 2018; Hong, Sobue, et al., 2019; Hou et al., 2018; Laheij et al., 2019; Lee et al., 2020; Mougeot et al., 2020; Napeñas et al., 2010; Shouval et al., 2020; Vesty et al., 2020; Vidal-Casariego et al., 2015; Zhu et al., 2017). Eight studies examined the relationship between OM and oral microbes in the setting of RT treatment for head and neck cancer. Enterobacteriaceae, Prevotella, Fusobacterium, and other gram-negative bacteria were associated with OM across multiple studies (Gaetti-Jardim et al., 2018; Hou et al., 2018; Vesty et al., 2020). One study noted that the abundance of oral microbiota species usually associated with periodontal disease was highest at the onset of severe OM (Hou et al., 2018). In a study by Vidal-Casariego et al. (2015), a risk factor for OM development included bacterial colonization of various gram-negative species prior to RT. The results of these studies suggest an association between OM development and gram-negative bacteria. 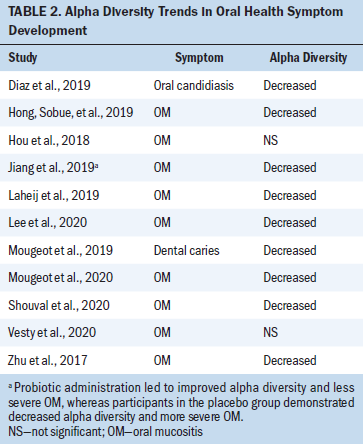
Patients receiving conditioning chemotherapy prior to a stem cell transplantation are also at risk for OM. Studies reported that decreased alpha diversity of the oral microbiome was correlated with more severe OM development across cohorts of patients undergoing stem cell transplantation (Laheij et al., 2019; Lee et al., 2020; Mougeot et al., 2020; Shouval et al., 2020). The onset of oral microbiome dysbiosis during chemotherapy, including decreased alpha diversity, occurred earlier in those who developed OM in this population (Laheij et al., 2019; Lee et al., 2020). Patients who developed moderate to severe OM were more likely to have increased Gammaproteobacteria and Escherichia-Shigella oral microbes and decreased Haemophilus parainfluenza (mucosal surfaces protectant) oral microbes compared to those with mild to no OM (Mougeot et al., 2020). Although associations between decreased microbial diversity and severe OM were noted in the stem cell transplantation population, findings varied with regard to potentially problematic microbial species. 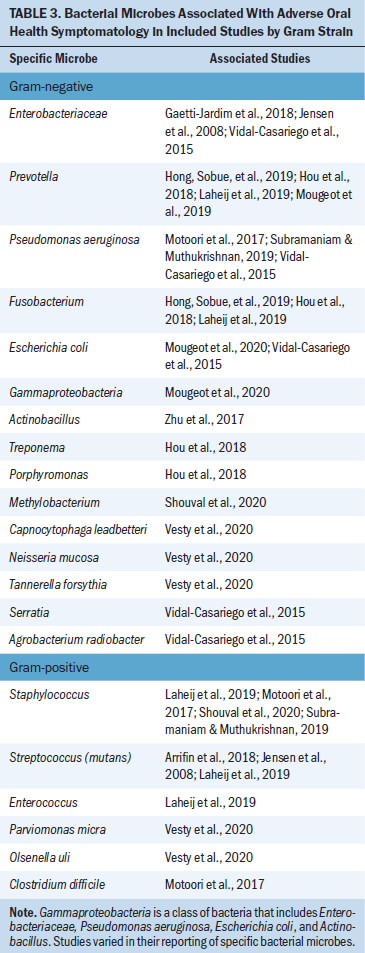
The relationship between oral microbiome composition and OM was explored in participants undergoing treatment for other cancers, particularly chemotherapy administration. Hong, Sobue, et al. (2019) demonstrated that chemotherapy administration across multiple solid tumor populations—particularly 5-fluorouracil administration—was associated with dysbiosis of the oral mucosa, with similar changes as inflammatory processes. Certain gram-negative oral microbes were associated with OM across multiple studies, including increased Fusobacterium, Prevotella, and Gammaproteobacteria, which includes Enterobacteriaceae and Pseudomonas aeruginosa. Decreased alpha diversity was also associated with more severe OM.
Relation of Oral Candidiasis and the Oral Microbiome
Another oral health measurement in the reviewed studies was the presence of oral candidiasis (Arrifin et al., 2018; Bulacio et al., 2012; Diaz et al., 2019; Glažar et al., 2017). Three articles used a culture method for identification of Candida and other fungal strains. Candida albicans emerged as the prominent strain implicated in oral candidiasis development across patient populations receiving both monotherapies of chemotherapy and RT. Oral candidiasis development occurred in patients across multiple tumor types, but appeared more common in the head and neck population (Glažar et al., 2017). Candida albicans was reported to be susceptible to common and inexpensive antifungal medications, such as fluconazole and ketoconazole (Bulacio et al., 2012).
Relation of Other Oral Health Symptoms and the Oral Microbiome
The relationship between other oral health measurements and oral microbiota during cancer treatment was examined across studies (Jensen et al., 2008; Mougeot et al., 2019; Subramaniam & Muthukrishnan, 2019). Two additional studies included other oral health symptoms as a secondary measurement (Arrifin et al., 2018; Gaetti-Jardim et al., 2018). Oral health symptoms, including gingival inflammation, taste disturbances, salivary flow, xerostomia, and dental caries, were apparent across head and neck and breast cancer populations receiving RT or chemotherapy. Oral microbes associated with poor oral health, such as Candida, Enterobacteriaceae, and Streptococcus mutans, were associated with adverse oral health symptoms during cancer treatment, including xerostomia and dental caries. Abiotrophia defectiva was decreased in a group of participants receiving RT for head and neck cancer where tooth decay increased, which is noteworthy because this species is usually present in control participants who have a healthy oral cavity (Mougeot et al., 2019). In addition to the impact of RT, the association between moderate-dose chemotherapy and oral health and microbiome changes was reported. Jensen et al. (2008) noted that oral microbiome composition became more acidophilic during moderate-dose chemotherapy, and increased presence of Streptococcus mutans was associated with gingival inflammation and taste disturbances. These studies suggest that a shift in oral microbiome, characterized by an increase in disease-related microbes and decrease in health-associated microbes, appears to be associated with detrimental oral health effects.
Relation of Oral Health and Oral Microbiota Interventions
Two included studies were randomized clinical trials (RCTs) that explored the influence of antibiotic paste and lozenges on oral microbiome composition and oral health during RT for head and neck cancer. Stokman et al. (2003) conducted an RCT of 65 participants undergoing RT for head and neck cancer, in which the intervention group received lozenges containing polymyxin, tobramycin, and amphotericin B, and the control group received placebo lozenges. Both Candida species and gram-negative bacilli were significantly decreased in the intervention group. However, no differences in OM scores were noted between the treatment and placebo groups, suggesting that selective oral microbiota elimination via antibacterial lozenges did not prevent the progression of OM (Stokman et al., 2003). Wijers et al. (2001) examined the influence of an antibiotic oral paste on OM severity in an RCT of 77 participants undergoing RT for head and neck cancer. Although oral cultures of aerobic gram-negative bacteria were reduced in the intervention group, no statistically significant difference in OM development was noted between the two groups (Wijers et al., 2001). Neither antibacterial lozeneges nor pastes were effective in reducing OM in these RCTs.
Relation of Oral Health and Gut Microbiota Interventions
Two of the included studies were RCTs that examined the influence of probiotics and synbiotics (a combination of probiotics and prebiotics) on gut microbiome composition and oral health during cancer therapy. Probiotics include live, beneficial microorganisms found in foods or supplements, whereas prebiotics are fiber substances that promote beneficial bacteria in the microbiome (Ambalam et al., 2016). Jiang et al. (2019) conducted a study of 99 participants undergoing CCRT for nasopharyngeal carcinoma, with gut microbiota sampling and OM measurements. One group received a combination probiotic of Bifidobacterium longum, Lactobacillus lactis, and Enterococcus faecium, and the control group received a placebo. Probiotic administration during CCRT resulted in a reduced incidence of grade 3 OM compared to the placebo group. Although CCRT led to microbiome dysbiosis, probiotic administration restored the gut microbiome to homeostatic levels present in a healthy control group (Jiang et al., 2019). In addition, researchers reported that CCRT decreased the number of immune cells in participants, whereas the probiotic significantly decreased the reduction rates of multiple T-cell counts. Researchers suggested that the mechanism of enhanced immunity from probiotics stems from microbes influencing T- cell and glycoprotein production, which have a protective effect on the oral mucosa (Jiang et al., 2019).
In a study of 61 participants undergoing chemotherapy for esophageal cancer, Motoori et al. (2017) compared a group that received synbiotics in the form of Bifidobacterium breve strain Yakult and Lactobacillus casei strain Shirota and a control group that received Bioferma containing Streptococcus faecalis. The numbers of beneficial bacteria present in gut microbiota samples were increased in the group that received synbiotics compared to the control group, including Bifidobacterium and Lactobacillus. Severe OM also occurred significantly less frequently in the synbiotics group compared to the control group. Harmful bacteria, including Clostridium difficile, Staphylococcus, and Pseudomonas were more prevalent in the control group (Motoori et al., 2017). These RCTs both suggested that use of probiotics and synbiotics increased gut microbiome diversity and reduced OM severity in populations receiving cancer therapies (Jiang et al., 2019; Motoori et al., 2017).
Discussion
Based on this integrative review, most research involving oral health and the microbiome has centered on head and neck cancer populations receiving RT. OM was the focus of oral health management in the studies examining treatment for head and neck cancer, and it was included as a measurement in nine of the studies focused on head and neck cancer and in 17 studies overall. Microbiome data focused on oral samples and were associated with oral health measurements, including OM and candidiasis. Generally, increased gram-negative bacterial microbes and decreased alpha diversity were associated with adverse oral health outcomes during cancer therapy (Jiang et al., 2019; Laheij et al., 2019; Motoori et al., 2017).
Oral Microbiome and Oral Health Symptoms
Oral microbiome changes, including dysbiosis such as decreased diversity, were reported across included studies (Hong, Sobue, et al., 2019; Laheij et al., 2019; Lee et al., 2020; Shouval et al., 2020; Zhu et al., 2017). In general, some studies reported that certain oral disease-related gram-negative species were associated with more severe OM, including Fusobacterium, Prevotella, Enterobacteriaceae, and Pseudomonas aeruginosa. Some studies discussed a connection between harmful oral microbial species (those associated with poor oral health) and detriments to oral health, such as OM and dental caries (Hou et al., 2018; Mougeot et al., 2019). These findings are consistent with a comprehensive review, which found pathogenic and proinflammatory species to be more common in the head and neck cancer population compared to healthy controls (Chattopadhyay et al., 2019). A systematic review of the impact of chemotherapy on the oral microbiome reported that patients undergoing chemotherapy have increased gram-negative bacteria in their oral microbiome (Villafuerte et al., 2018) and concluded that the composition of microbes represented dysbiosis, which is consistent with the propensity toward increased gram-negative bacteria in oral health alterations reported in this review.
Within the oral microbiome, an abundance of potentially problematic species can lead to poor oral health. Streptococcus mutans is implicated in dental plaque and caries, Porphyromonas gingivalis is a gram-negative bacteria linked to periodontal disease, and Lactobacillus is integral in lactic acid production, which can cause dental caries (Lu et al., 2019). Research has also suggested a link between oral microbiota changes, including increased Candida albicans and OM severity during RT for head and neck cancer (Orlandi et al., 2019). The findings of this review are consistent with previous research because an abundance of disease-related microbes appeared to be associated with the severity of adverse oral health symptoms.
A connection between certain microbiota and cancer development, including the possible role of Porphyromonas gingivalis and Pseudomona aeruginosa in the carcinogenesis of oral cancer, has been previously explored (Kakabadze et al., 2020). In the current clinical integrative review, those microbes were associated with multiple adverse oral health symptoms (OM and xerostomia), highlighting their persistent detriment during treatment. Currently, there is insufficient evidence to support widespread microbial testing as part of a symptom risk assessment during cancer therapy. However, as this research expands, individual microbiome composition and symptomatology may become key in identifying patients at risk for severe side effects.
Two studies in this review involved oral microbial interventions, including bacteria-containing products to alter the microbiome, for OM management. The use of antibiotic paste or lozenges did not appear to improve or prevent OM in patients undergoing cancer treatment. This finding is consistent with a previous systematic review reporting a lack of evidence to support their use (Saunders et al., 2020). Additional research in oral antimicrobials is warranted before clinical implementation.
Gut Microbiome and Oral Health Symptoms
Research has indicated that the composition of the gut microbiome is an important factor in oral health (Xu et al., 2020). Two included studies examined the effect of probiotics and synbiotics on the gut microbiome and oral health during cancer therapies. These studies explored gut microbial interventions for OM prevention and supported the use of probiotics or synbiotics to prevent severe mucositis (Jiang et al., 2019; Motoori et al., 2017). Jiang et al. (2019) further explored the mechanism behind improved immunity in the form of T-cell counts following probiotic administration. Although cancer therapies decreased the immune response, as indicated by a reduction in T-cell counts, probiotics appeared to correct the immune response by introducing microbes that enhanced the immune system. Probiotic microbes are thought to influence T cell and glycoprotein production, which has a protective effect on the oral mucosa, explaining the less severe OM in the intervention group (Jiang et al., 2019). A systematic review by Shu et al. (2020) of probiotics in the prevention and treatment of OM suggested that probiotics lessen OM severity; however, researchers called for further RCTs in this area.
Supporting a connection between gut microbiome composition and oral health outcomes, Gori et al. (2019) reviewed a link between gut microbiota composition and the efficacy and toxicity of cancer therapy, including antineoplastic agents and immunotherapy. They concluded that manipulation of the gut microbiome could reduce toxicity and improve treatment effectiveness in people with cancer (Gori et al., 2019). In addition, researchers have reported a similar relationship between the gut microbiome and RT response, including the possibility of altering microbiota to reduce RT-induced OM (Al-Qadami et al., 2019). A study including expert opinion and systematic reviews has supported further investigation of gut microbial manipulation techniques (Ervin et al., 2020).
Because optimal gut microbiome composition consists of diverse species, the ability to manipulate microbiome composition to improve cancer therapy outcomes is an important new area of research (Rinninella et al., 2019). Gut microbiome composition is prone to changes related to diet and the environment, so education for patients on food consumption and carcinogen avoidance during cancer therapies may be integral to improving outcomes (Rinninella et al., 2019). Although evidence is limited, this review supports future research in the form of larger, multisite clinical trials evaluating the role of the gut microbiome and alterations in the abundance of specific microbes related to oral health during cancer treatment.
This review also highlights the lack of consideration of psychological and situational factors influencing the symptom experience across the microbiome-focused research. In accordance with the Theory of Unpleasant Symptoms, consideration of patient factors is essential to understanding and treating oral health symptoms during cancer therapies. The patient experience should be included as a measurement in future oral health symptom research.
Strengths and Limitations
Strengths of this review include the use of PRISMA reporting guidelines throughout the review process and manuscript development. In addition, a quality assessment was conducted by two independent reviewers.
Limitations of this review include variation in the microbiome extraction and sequencing strategies across the studies reviewed. The reviewed studies were conducted in different countries with small sample sizes, which may limit the generalizability. Oral health measurements were collected with high variability, impeding the synthesis of findings.
Implications for Nursing
Symptom management during cancer treatment is predominantly led by oncology nurses. Patient education regarding oral health includes proper oral hygiene, hydration, pretreatment dental evaluation, repair of correctable caries and extraction of uncorrectable diseased teeth, and dietary modifications (National Cancer Institute, 2016; Nekhlyudov et al., 2017). In addition to national guidelines, innovative oral health management strategies are emerging. Based on the current evidence, widespread microbiota testing for oral health management does not appear to be warranted. However, probiotic and synbiotic products may become increasingly common and provide additional options to alleviate detrimental oral symptoms. It is essential for nurses to understand the mechanistic underpinnings of these possible new treatment strategies because they are key patient educators in oncology. Implications for nurses include being vigilant in oral health screenings prior to and continuously throughout therapy; inquiring about patients’ dietary intake patterns (e.g., probiotics), hygiene practices, and oral health history; and consistently intaking patient-reported outcomes regarding oral health changes. Each nursing strategy should be integrated prior to treatment initiation and continue through survivorship. 
Conclusion
The role of microbiota in oral health symptomatology during chemotherapy and RT is multifaceted and poised to affect clinical practice. Nurses are in a unique position to improve oral health symptom management during cancer treatment through patient education and vigilant symptom screening. The findings of this review support the need for additional research on the connection between oral and gut microbiota and oral health changes during cancer treatment. The Theory of Unpleasant Symptoms can guide the development of this research, focusing on multiple factors influencing symptom experience. Efforts to improve oral health symptom management during cancer treatment will benefit patients’ quality of life.
About the Author(s)
Hayley J. Dunnack, MS, RN, CMSRN, OCN®, is a PhD candidate, Michelle P. Judge, PhD, RD, is an associate professor, and Xiaomei Cong, PhD, RN, FAAN, is a professor and the associate dean for research and scholarship, all in the School of Nursing at the University of Connecticut in Storrs; Andrew Salner, MD, FACR, is the Ned C. and Janet C. Rice Chair of the Hartford HealthCare Cancer Institute at Hartford Hospital and director of the Helen and Harry Gray Cancer Center, both in Hartford, CT, and a clinical professor in the School of Medicine at the University of Connecticut in Farmington; and Valerie B. Duffy, PhD, RD, is a professor and director of the graduate program in the Department of Allied Health Sciences in the College of Agriculture, Health, and Natural Resources, and Wanli Xu, PhD, RN, is an assistant professor in the School of Nursing, both at the University of Connecticut in Storrs. No financial relationships to disclose. Dunnack, Judge, Cong, and Xu contributed to the conceptualization and design. Dunnack and Cong completed the data collection. Dunnack, Judge, Cong, and Salner provided analysis. All authors contributed to the manuscript preparation. Dunnack can be reached at hayley.dunnack@uconn.edu, with copy to ONFEditor@ons.org. (Submitted October 2020. Accepted November 18, 2020.)




