Prevalence and Correlates of Strength Exercise Among Breast, Prostate, and Colorectal Cancer Survivors
Purpose/Objectives: To identify and compare the prevalence and correlates of strength exercise among breast, prostate, and colorectal cancer survivors.
Design: Cross-sectional, descriptive survey.
Setting: Nova Scotia, Canada.
Sample: 741 breast, prostate, and colorectal cancer survivors.
Methods: A stratified sample of 2,063 breast, prostate, and colorectal cancer survivors diagnosed from 2003–2011 were identified and mailed a questionnaire. Descriptive, chi-square, and logistic regression analyses were used to determine any correlations among the main research variables.
Main Research Variables: Strength exercise behavior; medical, demographic, and motivational correlates using the Theory of Planned Behavior.
Findings: Of 741 respondents, 23% were meeting the strength exercise guidelines of two or more days per week. Cancer survivors were more likely to meet guidelines if they were younger, more educated, had a higher income, better perceived general health, fewer than two comorbidities, and a healthy body weight. In addition, those meeting guidelines had significantly more favorable affective attitude, instrumental attitude, injunctive norm, perceived behavioral control, planning, and intention. The correlates of strength exercise did not differ by cancer site.
Conclusions: The prevalence of strength exercise is low among breast, prostate, and colorectal cancer survivors in Nova Scotia and the correlates are consistent across those survivor groups.
Implications for Nursing: Nurses should take an active role in promoting strength exercise among cancer survivors using the Theory of Planned Behavior, particularly among those survivors at higher risk of not performing strength exercise.
Jump to a section
Strength exercise, or resistance training, is any type of activity that involves the repetitive use of muscular force against an external resistance or body weight, such as weightlifting, push-ups, sit-ups, yoga, and Pilates (U.S. Department of Health and Human Services [USDHHS], 2008). Systematic reviews have documented that strength exercise improves many health outcomes in cancer survivors, including muscular strength and endurance, lean body mass, fatigue, and quality of life (Cheema, Gaul, Lane, & Fiatarone Singh, 2008; Cramp, James, & Lambert, 2010; DeBacker, Schep, Backx, Vreugdenhil, & Kuipers, 2009; Strasser, Steindorf, Wiskemann, & Ulrich, 2013). One trial even reported that strength exercise improved chemotherapy completion rate in patients with breast cancer (Courneya et al., 2007). In addition, some studies have suggested that strength exercise may result in larger improvements in quality of life than aerobic exercise in prostate cancer survivors (Segal et al., 2009). Strength exercise has even been found to be safe and feasible for cancer survivors with advanced disease (Bourke et al., 2014; Cormie, Newton, Spry, Joseph, Taafe, & Galvão, 2013; Galvão et al., 2014). These studies have led the American Cancer Society (Rock et al., 2012) and the American College of Sports Medicine (Schmitz et al., 2010) to recommend at least two days per week of strength exercise for cancer survivors.
Despite this recommendation, few studies have examined the prevalence and correlates of strength exercise among cancer survivors. Speed-Andrews et al. (2013) examined strength exercise among 600 colorectal cancer survivors and found that only about 25% reported meeting the strength exercise guidelines. In addition, the study found that colorectal cancer survivors were more likely to meet the guidelines if they were male, married, in better health, and not obese. Short et al. (2014) reported on the strength exercise behavior of 330 breast cancer survivors and found that less than 25% were meeting the strength exercise guidelines. Breast cancer survivors who had higher outcome expectancies, task self-efficacy, barrier self-efficacy, behavioral capability, social support, and goal setting were more likely to be meeting the strength exercise guidelines. Among cancer survivors, the Theory of Planned Behavior (TPB) (Ajzen, 1991) has been used extensively to explain aerobic exercise (Forbes, Blanchard, Mummery, & Courneya, 2014; Speed-Andrews et al., 2013; Trinh, Plotnikoff, Rhodes, North, & Courneya, 2012), but no study to date has used the TPB to explain strength exercise.
The TPB states that intention (or motivation) is the immediate determinant of behavior. Intention is influenced by instrumental and affective attitude (expected benefits and enjoyment from performing a behavior), injunctive and descriptive norm (expected support from others and extent to which important others perform a behavior), and perceived behavioral control (PBC) (the perceived controllability of performing a behavior). Planning is an addition to the model as a mediator between behavior and intention in an attempt to explain the intention-behavior gap (Norman & Conner, 2005; Vallance, Lesniak, Belanger, & Courneya, 2010). Several studies among various cancer survivor groups have found the TPB to be highly effective when predicting general physical activity (PA) (Belanger, Plotnikoff, Clark, & Courneya, 2012; Speed-Andrews et al., 2012; Trinh et al., 2012). A meta-analysis showed that intention and PBC had strong correlations with adhering to PA among cancer survivors (Husebø, Dyrstad, Søreide, & Bru, 2013).
The primary purpose of this study was to examine the prevalence and correlates of strength exercise among breast, prostate, and colorectal cancer survivors in Nova Scotia, Canada. To the authors’ knowledge, the current study is the first to examine the prevalence and correlates of strength exercise in prostate cancer survivors, which is arguably the survivor group with the most evidence of benefit from strength exercise (Keogh & McLeod, 2012; Strasser et al., 2013). In addition, the current study is only the second to examine the prevalence and correlates of strength exercise in breast and colorectal cancer survivors, and the first to test the TPB as a model to explain strength exercise in these survivors (Ajzen, 1991). Finally, the current study is the first to directly compare the prevalence and correlates of strength exercise across cancer survivor groups to determine if interventions to promote strength exercise may need to be targeted based on cancer site.
Based on the studies by Speed-Andrews et al. (2013) and Short et al. (2014), the authors hypothesized that the majority of survivors in Nova Scotia would not be meeting the strength exercise guidelines. Based on the evidence of benefit, the authors hypothesized that prostate cancer survivors would have the highest rate of strength exercise participation, followed by breast and colorectal cancer survivors. In terms of correlates, the authors hypothesized that the TPB would provide the strongest correlates of strength exercise across all three survivor groups. Finally, the authors hypothesized that survivors who are men (colorectal only), more educated, in better general health, and less obese would be more likely to meet the strength exercise guidelines. This comparison of the correlates across the three survivor groups was considered exploratory.
Methods
The design of this survey has been previously described (Forbes et al., 2014). The study package included a mailed, self-administered survey using a population-based, cross-sectional design. The Nova Scotia Cancer Registry (NSCR) generated a stratified sample of 700 from breast, prostate, and colorectal cancer survivors (N = 2,100) in September 2011. Participants were deemed eligible if they were (a) aged 18–80 years, (b) current residents of Nova Scotia, and (c) had a diagnosis of breast, prostate, or colorectal cancer from 2003–2011. The Halifax District Health Authority and the University of Alberta provided ethics approval. Those identified received a package containing (a) an invitation letter from the registry explaining its role in the study and how they were identified, (b) an invitation letter from the researchers explaining the purpose of the study, (c) a questionnaire, and (d) a postage-paid return envelope. If individuals were interested in participating, they were asked to complete the questionnaire and mail it in the return envelope. Participants were mailed the initial package and then a postcard reminder about three weeks later to those who had not responded in that time period.
Measures
Demographic and medical information: Self-report demographic data included age, gender, education level, marital status, income, employment status, ethnicity, and height and weight to calculate body mass index (BMI). Also collected using self-report were medical variables consisting of type of cancer, time since diagnosis, lymph node involvement, treatment type, current treatment status, previous recurrences, current disease status, and perceived general health status. Comorbidities were examined by asking participants to select from a list the conditions they were told they had (e.g., high cholesterol, diabetes, high blood pressure).
Strength exercise: To measure strength exercise behavior, the authors used a scale previously used by Speed-Andrews et al. (2013). The questionnaire asked, “Have you done any strength exercises in the past month?” with a “yes” or “no” response. Examples of strength exercise were provided for respondents, such as weightlifting, sit-ups, or push-ups. If they answered “yes,” they were instructed to complete three more questions asking what type of strength exercise they did (open-ended), how often (days per week), and the duration of each session (minutes per day). The authors used the current recommended guidelines (Rock et al., 2012; Schmitz et al., 2010; USDHHS, 2008) to determine the percentage of participants meeting the strength exercise guidelines. The guidelines state that individuals should engage in strength exercises for all major muscle groups on two or more days per week with 8–12 repetitions per exercise. The authors’ primary estimate of prevalence was participating in strength activities at least twice per week. Given that the authors did not ask about all major muscle groups or the number of repetitions, they estimated that it would take about 30 minutes to complete 8–12 repetitions for each major muscle group. Therefore, the second estimate of strength exercise prevalence was two or more days per week for at least 30 minutes per session. This secondary estimate was only used for descriptive purposes. All correlates analyses used the primary estimate of strength exercise prevalence based on frequency alone.
Theory of Planned Behavior: The TPB is generally assessed using Likert-type scale scoring from 1 (negative) to 7 (positive) that quantify a person’s attitude, subjective norm, perceived behavioral control, intention, and plan to engage in a behavior. The TPB was assessed using these standardized measures, as recommended by Ajzen (2006) and reported by Forbes et al. (2014). The items were focused on regular PA as defined for moderate and vigorous activity, but did not specifically refer to aerobic or strength exercise. Attitude was assessed using six items on a seven-point bipolar Likert-type scale for both components; instrumental (i.e., harmful/beneficial, useless/useful, and bad/good) and affective attitude (i.e., unenjoyable/enjoyable, boring/fun, and unpleasant/pleasant) using the statement, “For me, engaging in PA regularly over the next 12 weeks will be. . . .” The internal consistencies (alpha) of the attitude subscales were 0.88 and 0.83, respectively.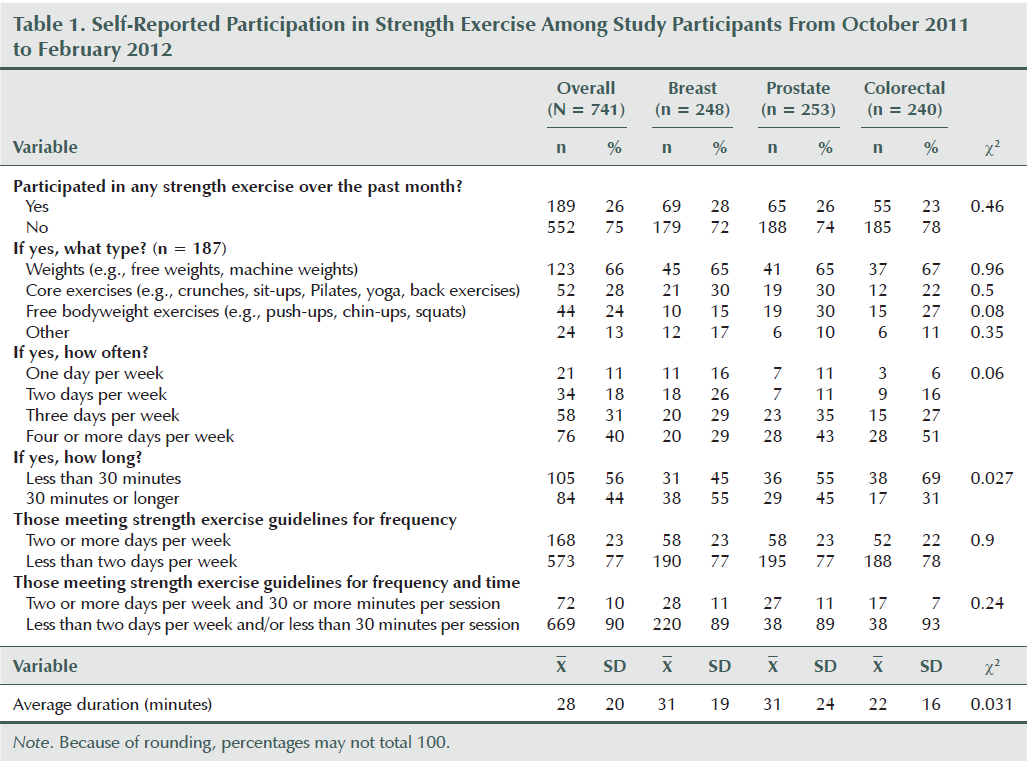
Subjective norm was measured with five items on a seven-point bipolar Likert-type scale. Three items assessed injunctive norm by asking, “I think that if I participated in regular PA over the next month, most people who are important to me will be . . .” disapproving/approving, discouraging/encouraging, or unsupportive/supportive. The other two items measured descriptive norm by asking, “I think that over the next month, most people who are important to me will be . . .” inactive/active and, “I think that over the next month, most people who are important to me will participate regularly in PA” disagree/agree. Internal consistencies (alpha) of the subjective norm subscales were 0.93 and 0.84, respectively.
PBC was measured with six items on a seven-point bipolar Likert-type scale. The items were: “If you were really motivated, participating in PA over the next month would be . . .” extremely difficult/extremely easy; “If I wanted to, I could easily engage in regular activity over the next month” strongly agree/strongly disagree; “How confident are you that you could engage in PA regularly over the month?” not at all confident/extremely confident; “If you were really motivated, how much control do you feel you would have in engaging in PA regularly over the next month?” very little control/complete control; “Whether or not I engage in PA regularly over the next month is completely up to me” strongly disagree/strongly agree; and, “How much do you feel that engaging in PA over the next month is beyond your control?” not at all/very much. Internal consistency (alpha) was 0.89 for these items.
Intention was measured with two seven-point Likert-type scale items that asked “Do you intend . . .” and “How motivated are you . . . to do regular PA over the next month,” respectively. Internal consistency (alpha) was 0.95 for these two items. Finally, planning was assessed with six items on a seven-point Likert-type scale with responses ranging from no plans to detailed plans. The first item asked, “Do you have plans for when, where, and the type of PA you will do in the next month?” The following five items expanded on the first, asking, “I have made detailed plans concerning . . .” when, where, what, how, and who regarding engagement in regular PA. Internal consistency (alpha) was 0.97 for these items.
Statistical Analyses
All statistical analyses were conducted using PASW Statistics, version 21.0. Descriptive statistics were used to determine the prevalence of strength exercise, including the frequency, duration, and type. To determine any associations between cancer site (breast, prostate, and colorectal) and meeting the strength exercise guidelines, chi-square analyses were completed. Chi-square analyses also were used to determine any differences between demographic and medical characteristics with strength exercise behavior between and within cancer sites, including exploratory tests of interactions. All demographic and medical variables were grouped based on relevant cut points or balanced statistical splits to ensure each cell had adequate numbers for analysis. The demographic variables include age (59 years and younger, 60–69, 70 and older), gender, marital status, annual income, BMI (healthy weight, 18.5–24.9; overweight, 25–29.9; obese, 30 or greater), and general health status (poor or fair, good, very good or excellent). Medical variables included disease stage (localized or metastasized), time since diagnosis (less than five years or five years or more), treatments received (surgery, radiation, chemotherapy, or hormone therapy), current cancer status (disease free or existing disease), recurrence status (yes or no), and current treatment status (not receiving treatment or receiving treatment).
TPB correlates were examined using analyses of variance (ANOVAs). The authors also explored interactions between cancer site and the correlates using ANOVAs. A p value of < 0.05 was chosen for statistical significance for the main correlates and the authors describe any interactions that were p < 0.1. Multivariate logistic regression analyses were performed using all variables that were statistically significant (p < 0.05) or borderline significant (p < 0.1) to predict the probability that a respondent would meet guidelines for strength exercises (two or more days per week). The authors used mean substitution to replace any missing data, which was less than 5% for all variables.
Results
The flow of participants through the study has been reported elsewhere (Forbes et al., 2014). Briefly, NSCR randomly generated a stratified sample of 2,100 cancer survivors (700 for each cancer type), of which 2,062 were mailed an invitation package. The survey resulted in a 36% completion rate (741 of 2,062) and a 38% response rate (741 of 1,978), which excludes the return to sender responses and deceased. The response rate did not differ by cancer site (p = 0.94).
Demographic, medical, and behavioral characteristics of the participants have been reported elsewhere (Forbes et al., 2014). The study population was mostly male (n = 405, 55%), Caucasian (n = 718, 97%), married (n = 595, 80%), not working (n = 522, 70%), and had an average age of 65.6 years. The sample was evenly distributed between breast (n = 248, 34%), prostate (n = 253, 34%) and colorectal (n = 240, 32%) cancers. Medically, 50% (n = 369) had stage II disease, mean years since diagnosis was 4.3, 90% (n = 666) had surgery, 47% (n = 351) were overweight, and 26% (n = 192) were obese.
Overall, 23% (n = 168) of the sample was meeting the strength exercise guidelines of two days or more per week. Of those meeting the guidelines, the majority were lifting weights (n = 123, 66%) followed by doing core exercises (n = 52, 28%) (e.g., sit-ups, Pilates, yoga) and free bodyweight exercises (n = 44, 24%) (e.g., push-ups, squats, chin-ups). The average session duration was 28 minutes (SD = 20). About 10% (n = 72) were meeting the criteria of two or more days per week for 30 minutes or more per session.
Strength Exercise Behavior Differences by Site
Differences in strength exercise behavior by cancer site are presented in Table 1. The only significant difference was in duration per session, with colorectal cancer survivors reporting significantly shorter duration than breast or prostate cancer survivors (69% reporting less than 30 minutes per session versus 45% and 55%, respectively; p = 0.027).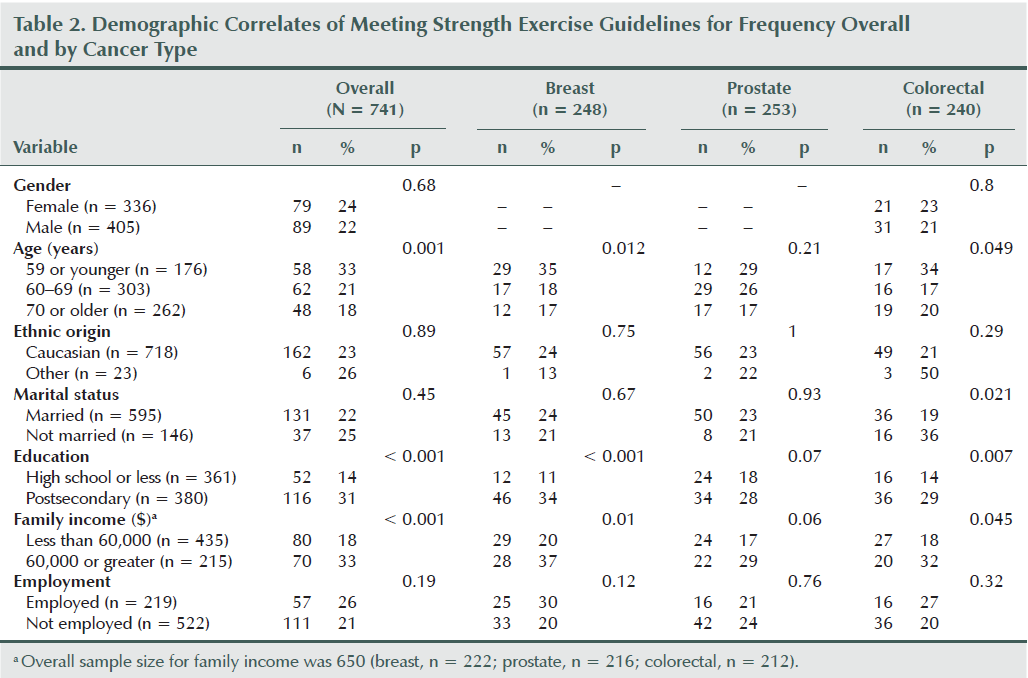
Table 2 shows detailed information regarding the associations between demographic variables and strength exercise behavior overall and within the cancer site. Overall, survivors were more likely to meet strength exercise guidelines if they were younger (p = 0.001), more educated (p < 0.001), or had a higher income (p < 0.001). The only interaction involving cancer site was a borderline significant interaction with marital status (p for interaction = 0.055) (see Figure 1). Unmarried colorectal cancer survivors were more likely to meet guidelines, whereas no difference was noted for breast and prostate cancer survivors based on marital status.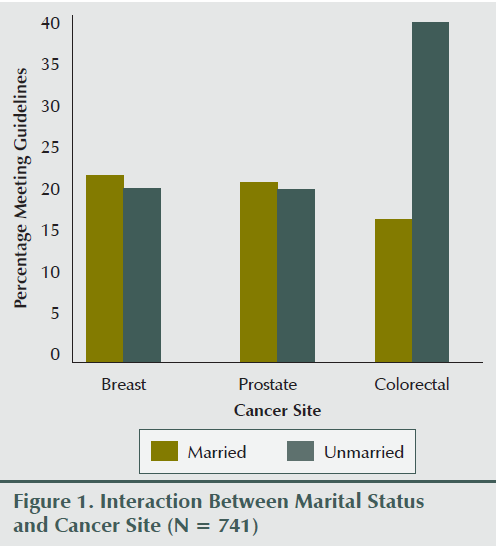
Table 3 shows detailed information regarding the associations between medical variables and strength exercise behavior overall and within cancer site. Participants were more likely to meet the strength exercise guidelines if they had better perceived general health (p < 0.001), less than two comorbidities (p = 0.01), and a normal body mass index (p = 0.001). The only interaction involving cancer site was a borderline significant interaction with time since diagnosis (p for interaction = 0.058) (see Figure 2). Breast cancer survivors were more likely to meet guidelines if their diagnosis was less than five years, whereas colorectal cancer survivors were more likely to meet guidelines if their diagnosis was more than five years ago.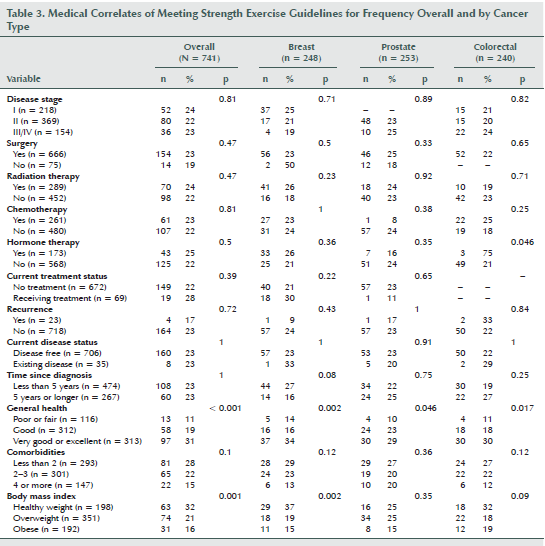
Table 4 describes differences in the TPB constructs based on meeting the strength exercise guidelines. Overall, those meeting strength guidelines had significantly higher scores for affective attitude (p < 0.001), instrumental attitude (p < 0.001), injunctive norm (p = 0.003), perceived behavioral control (p < 0.001), planning (p < 0.001), and intention (p < 0.001). Significant differences remained when results were adjusted for age, gender, marital status, disease stage, treatment type (surgery, chemotherapy, radiation, and hormone therapy), treatment status, and disease status. No significant interactions were noted based on cancer site.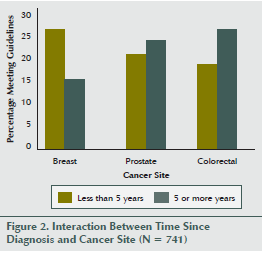
Multivariate stepwise logistic regression analysis was conducted with all TPB constructs and age (younger than age 60 years versus age 60 years or older), education level (high school or less versus postsecondary or greater), BMI (less than 25 versus 25 or greater), general health (poor or fair, good, very good or excellent) and comorbidities (less than 2, 2–3, 4 or more). Four variables entered the model and explained 15% of the variance in meeting strength exercise guidelines (p < 0.001). Survivors were more likely to be meeting strength exercise guidelines if they had stronger intentions (odds ratio [OR] = 1.61, p < 0.001) and higher education (OR = 2.08, p < 0.001) and less likely to be meeting guidelines if they were of older age (OR = 0.61, p = 0.019) and overweight or obese (OR = 0.57, p = 0.006).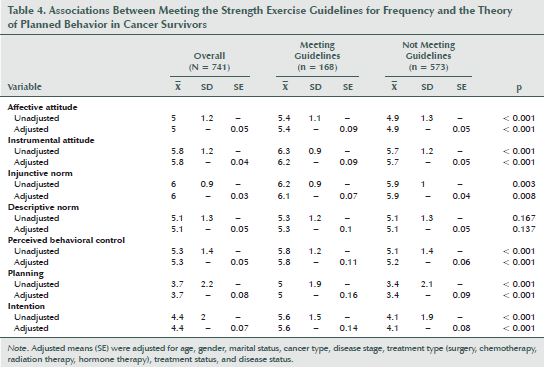
Discussion
To the authors’ knowledge, this study is the first to examine the prevalence and correlates of strength exercise in prostate cancer survivors; the first to examine the TPB constructs as correlates of strength exercise in cancer survivors; and the first to compare the prevalence and correlates across breast, prostate, and colorectal cancer survivors. About 23% of the sample was meeting the guidelines for strength exercise at least two days per week with no differences across the cancer sites. The authors are only aware of two studies that have assessed the prevalence of strength exercise among cancer survivors. Speed-Andrews et al. (2013) found that 26% of colorectal cancer survivors in Alberta were meeting strength exercise guidelines, whereas Short et al. (2014) found that 24% of breast cancer survivors in Australia were meeting guidelines. The data suggest a remarkable consistency of about 25% of cancer survivors meeting the strength exercise guidelines with very little variation across cancer sites or geographic region.
Overall, participants in the current study were more likely to be meeting the strength exercise guidelines if they were younger, more educated, and had a higher income, consistent with results among colorectal cancer survivors (Speed-Andrews et al., 2013). One borderline significant interaction between cancer site and marital status arose—the authors of the current article found that colorectal cancer survivors were more likely to be meeting guidelines if they were unmarried, whereas breast and prostate cancer survivors showed no differences based on marital status. This finding is in contrast to Speed-Andrews et al. (2013), which identified that being married was positively correlated to strength exercise behavior among colorectal cancer survivors. In addition, Short et al. (2014) did not find any differences among demographic characteristics. Given the unexpected and inconsistent association between marital status and strength exercise participation, additional research is needed before definitive conclusions can be made.
When examining medical characteristics, the authors found that having greater perceived general health, fewer comorbidities, and a healthy BMI were associated with meeting strength guidelines. Speed-Andrews et al. (2013) also found that general health and BMI were significant correlates of meeting strength guidelines among colorectal cancer survivors. As with the demographics, Short et al. (2014) found no differences based on medical characteristics for breast cancer survivors. In the current study, a borderline significant interaction was found among cancer site and time since diagnosis for meeting strength guidelines. Breast cancer survivors were more likely to meet the guidelines if they were less than five years from diagnosis, whereas colorectal cancer survivors were more likely to meet guidelines if they were five years or greater since diagnosis. It is possible that breast cancer survivors are highly motivated to improve their health soon after diagnosis or treatment, whereas colorectal cancer survivors are less motivated initially. It also is possible that the treatments for colorectal cancer are more difficult initially, which may have an impact on early strength exercise participation. Speed-Andrews et al. (2013) found a borderline significant association among colorectal cancer survivors who had an ostomy bag versus those who did not, with those without an ostomy being more likely to meet the strength guidelines.
As hypothesized, the TPB constructs were the strongest correlates of strength exercise in cancer survivors with no differences by cancer site. Those meeting guidelines had consistently higher scores for each construct when compared to those not meeting guidelines. That the differences remained after being adjusted for demographic and medical variables signifies the importance of addressing the motivational aspects of strength exercise. The authors’ logistic regression analysis indicated that those with higher intentions were 60% more likely to be engaging in strength exercise. As with aerobic exercise, strength exercise intentions should be the primary target in interventions designed to increase strength exercise behavior. Assessing a patient’s intention to engage in strength exercise and addressing concerns that may arise is an important step in recommending strength exercise to cancer survivors.
Other significant correlates in the multivariate logistic regression analysis were age, education level, and BMI classification. Cancer survivors who were younger, more educated, and had a healthy BMI were more likely to be meeting the strength exercise guidelines. Speed-Andrews et al. (2013) also found that colorectal cancer survivors who were obese and in poorer health were less likely to meet guidelines. Short et al. (2014) did not find any significant predictors among demographic or medical characteristics after controlling for constructs from social cognitive theory. The discrepancy in results may be from differences in the survivor group, the theoretical model, or country of residence.
Research in non-cancer populations has shown that age, gender, and education level are common predictors of strength exercise behavior (Humphries, Duncan, & Mummery, 2010; Kruger, Carlson, & Kohl, 2006; Loustalot, Carlson, Kruger, Buchner, & Fulton, 2013; Trost, Owen, Bauman, Sallis, & Brown, 2002). Humphries et al. (2010) conducted a study among the general population in Australia and, similar to the current study, found that younger, healthier participants were more likely to meet strength guidelines. The current study’s results indicate that interventions to promote strength exercise should target older, less educated, and obese cancer survivors. Strength exercise may be particularly beneficial for cancer survivors who are older and/or obese because of their additional comorbidities and functional decline. In addition, it may be more feasible for older and obese survivors to engage in strength exercises than aerobic exercises because of comorbidities such as musculoskeletal pain and reduced stamina.
Strengths and Limitations
The strengths of the current study include the rigorous selection of a stratified sample of cancer survivors from a population-based provincial registry, the largest sample size to date, the comparable response rate from each cancer survivor group, and the use of previously tested strength exercise measures. Limitations include the cross-sectional design, which restricts inferences of causality; the use of self-report data for the strength, demographic, and medical data; the transparent nature of the study, which may have led to selection biases; the modest response rate; and the authors’ failure to assess the TPB constructs, specifically for strength exercise.
Neglecting to assess the TPB constructs specifically for strength exercise means that participants were probably thinking of both aerobic and strength exercise when answering the social cognitive questions. The TPB explicitly notes that every behavior is unique in terms of target, action, context, and time. Attitudes toward strength exercise may be very different than attitudes toward aerobic exercise. Given this principle, the authors likely underestimated the association between the TPB and strength exercise. Future studies of strength exercise should explicitly measure the TPB constructs for performing strength exercise.
Implications for Nursing
Results from the current study show that interventions are needed to promote strength exercise in cancer survivors. Nurses may promote strength exercise to cancer survivors by using TPB as a framework. Any interventions that strengthen intentions to engage in strength exercise will be effective. Such interventions can focus on educating cancer survivors about the benefits of strength exercise, how to make strength exercise enjoyable, obtaining social support for strength exercise, and overcoming common barriers. In cases of cancer survivors who may be at higher risk of injuries or health problems from strength exercise, nurses should refer these survivors to qualified physiotherapists or certified exercise specialists at the cancer center or in the community. Nurses may have the greatest impact by providing special attention to promote strength exercise in older, less educated, and more overweight or obese cancer survivors.
[[{"type":"media","view_mode":"media_original","fid":"19571","attributes":{"alt":"","class":"media-image","height":"188","typeof":"foaf:Image","width":"365"}}]]
Conclusion
The authors found that the prevalence of strength exercise among survivors in Nova Scotia was low and did not vary among prostate, breast, and colorectal cancer survivors. In addition, stronger intentions, higher education, younger age, and healthy body weight were independent correlates of meeting the strength exercise in guidelines with very little evidence of variation by cancer site. The data suggest that interventions to increase strength exercise in breast, prostate, and colorectal cancer survivors should focus on maximizing motivation for strength exercise with special attention to less educated, older, and overweight or obese survivors, but with minimal concern for cancer site. Because there still is very little research on the prevalence and correlates of strength exercise in cancer survivors, more studies are needed to determine the reliability of these results.
References
Ajzen, I. (1991). The Theory of Planned Behavior. Organizational Behavior and Human Decision Processes, 50, 179–211.
Ajzen, I. (2006). Constructing a TPB questionnaire: Conceptual and methodological considerations. Retrieved from http://www.unibielefeld.de/ikg/zick/ajzen%20construction%20a%20tpb%20qu…
Belanger, L.J., Plotnikoff, R.C., Clark, A.M., & Courneya, K.S. (2012). Determinants of physical activity in young adult cancer survivors. American Journal of Health Behavior, 36, 483-494. doi:10.5993/AJHB.36.4.5
Bourke, L., Gilbert, S., Hooper, R., Steed, L.A., Joshi, M., Catto, J.W.F., . . . Rosario, D.J. (2014). Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: A randomised controlled trial. European Urology, 65, 865–872.
Cheema, B., Gaul, C.A., Lane, K., & Fiatarone Singh, M.A. (2008). Progressive resistance training in breast cancer: A systematic review of clinical trials. Breast Cancer Research and Treatment, 109, 9–26.
Cormie, P., Newton, R.U., Spry, N., Joseph, D., Taaffe, D.R., & Galvão, D.A. (2013). Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer and Prostatic Diseases, 16, 328–335.
Courneya, K.S., Segal, R.J., Mackey, J.R., Gelmon, K., Reid, R.D., Friedenreich, C.M., . . . McKenzie, D.C. (2007). Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. Journal of Clinical Oncology, 25, 4396–4404.
Cramp, F., James, A., & Lambert, J. (2010). The effects of resistance training on quality of life in cancer: A systematic literature review and meta-analysis. Supportive Care in Cancer, 18, 1367–1376.
DeBacker, I.C., Schep, G., Backx, F.J., Vreugdenhil, G., & Kuipers, H. (2009). Resistance training in cancer survivors: A systematic review. International Journal of Sports Medicine, 30, 703–712.
Forbes, C.C., Blanchard, C.M., Mummery, W.K., & Courneya, K.S. (2014). A comparison of physical activity correlates across breast, prostate and colorectal cancer survivors in Nova Scotia, Canada. Supportive Care in Cancer, 22, 891–903.
Galvão, D.A., Spry, N., Denham, J., Taaffe, D.R., Cormie, P., Joseph, D., . . . Newton, R.U. (2014). A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 radar. European Urology, 65, 856–864.
Humphries, B., Duncan, M.J., & Mummery, W.K. (2010). Prevalence and correlates of resistance training in a regional Australian population. British Journal of Sports Medicine, 44, 653–656.
Husebø, A.M.L., Dyrstad, S.M., Søreide, J.A., & Bru, E. (2013). Predicting exercise adherence in cancer patients and survivors: A systematic review and meta-analysis of motivational and behavioural factors. Journal of Clinical Nursing, 22(1–2), 4–21.
Keogh, J.W.L., & MacLeod, R.D. (2012). Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: A systematic review. Journal of Pain and Symptom Management, 43, 96–110.
Kruger, J., Carlson, S., & Kohl, H., III. (2006). Trends in strength training—United States, 1998–2004. Morbidity and Mortality Weekly Report, 55, 769–772.
Loustalot, F., Carlson, S.A., Kruger, J., Buchner, D.M., & Fulton, J.E. (2013). Muscle-strengthening activities and participation among adults in the United States. Research Quarterly for Exercise and Sport, 84, 30–38.
Norman, P., & Conner, M. (2005). The Theory of Planned Behavior and exercise: Evidence for the mediating and moderating roles of planning on intention-behavior relationships. Journal of Sport and Exercise Psychology, 27, 488–504.
Rock, C.L., Doyle, C., Demark-Wahnefried, W., Meyerhardt, J., Courneya, K.S., Schwartz, A.L., . . . Gansler, T. (2012). Nutrition and physical activity guidelines for cancer survivors. CA: A Cancer Journal for Clinicians, 62, 242–274.
Schmitz, K.H., Courneya, K.S., Matthews, C., Demark-Wahnefried, W., Galvão, D.A., Pinto, B.M., . . . Schwartz, A.L. (2010). American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise, 42, 1409–1426.
Segal, R.J., Reid, R.D., Courneya, K.S., Sigal, R.J., Kenny, G.P., Prud’Homme, D.G., . . . Slovinec D’Angelo, M.E. (2009). Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. Journal of Clinical Oncology, 27, 344–351.
Short, C.E., James, E.L., Vandelanotte, C., Courneya, K.S., Duncan, M.J., Rebar, A., & Plotnikoff, R.C. (2014). Correlates of resistance training in post-treatment breast cancer survivors. Supportive Care in Cancer, 22, 2757–2766. doi:10.1007/s00520-014-2273-5
Speed-Andrews, A.E., McGowan, E.L., Rhodes, R.E., Blanchard, C.M., Culos-Reed, S.N., Friedenreich, C.M., & Courneya, K.S. (2013). Correlates of strength exercise in colorectal cancer survivors. American Journal of Health Behavior, 37, 162–170.
Speed-Andrews, A.E., Rhodes, R.E., Blanchard, C.M., Culos-Reed, S.N., Friedenreich, C.M., Belanger, L.J., & Courneya, K.S. (2012). Medical, demographic and social cognitive correlates of physical activity in a population-based sample of colorectal cancer survivors. European Journal of Cancer Care, 21, 187–196.
Strasser, B., Steindorf, K., Wiskemann, J., & Ulrich, C.M. (2013). Impact of resistance training in cancer survivors: A meta-analysis. Medicine and Science in Sports and Exercise, 45, 2080–2090. doi:10.1249/MSS.0b013e31829a3b63
Trinh, L., Plotnikoff, R.C., Rhodes, R.E., North, S., & Courneya, K.S. (2012). Correlates of physical activity in population-based sample of kidney cancer survivors: An application of the theory of planned behavior. International Journal of Behavioral Nutrition and Physical Activity, 9, 96.
Trost, S.G., Owen, N., Bauman, A.E., Sallis, J.F., & Brown, W. (2002). Correlates of adults’ participation in physical activity: Review and update. Medicine and Science in Sports and Exercise, 34, 1996–2001.
U.S. Department of Health and Human Services. (2008). 2008 physical activity guidelines for Americans. Retrieved from http://www.health.gov/paguidelines/pdf/paguide.pdf
Vallance, J., Lesniak, S.L., Belanger, L.J., & Courneya, K.S. (2010). Development and assessment of a physical activity guidebook for the colon health and life-long exercise change. Journal of Physical Activity and Health, 7, 794–801.
About the Author(s)
Cynthia C. Forbes, MSc, is a doctoral student in the Department of Physical Education and Recreation at the University of Alberta in Edmonton; Chris M. Blanchard, PhD, is the chair for Cardiovascular Disease and Physical Activity at Canada Research Chairs in Ottawa, Ontario, and a professor in the Department of Medicine at Dalhousie University in Halifax, Nova Scotia; and W. Kerry Mummery, PhD, is a professor and dean and Kerry S. Courneya, PhD, is a professor, both in the Department of Physical Education and Recreation at the University of Alberta, all in Canada. This research was supported, in part, by the President’s Grant for Creative and Performing Arts from the Human Performance Scholarship Fund. Courneya can be reached at kerry.courneya@ualberta.ca, with copy to editor at ONFEditor@ons.org. (Submitted July 2014. Accepted for publication September 14, 2014.)

