Higher-Intensity Exercise Results in More Sustainable Improvements for VO2peak for Breast and Prostate Cancer Survivors
Purpose/Objectives: To examine peak volume of oxygen consumption (VO2peak) changes after a high- or low-intensity exercise intervention.
Design: Experimental trial comparing two randomized intervention groups with control.
Setting: An exercise clinic at a university in Australia.
Sample: 87 prostate cancer survivors (aged 47–80 years) and 72 breast cancer survivors (aged 34–76 years).
Methods: Participants enrolled in an eight-week exercise intervention (n = 84) or control (n = 75) group. Intervention participants were randomized to low-intensity (n = 44, 60%–65% VO2peak, 50%–65% of one repetition maximum [1RM]) or high-intensity (n = 40, 75%–80% VO2peak, 65%–80% 1RM) exercise groups. Participants in the control group continued usual routines. All participants were assessed at weeks 1 and 10. The intervention groups were reassessed four months postintervention for sustainability.
Main Research Variables: VO2peak and self-reported physical activity.
Findings: Intervention groups improved VO2peak similarly (p = 0.083), and both more than controls (p < 0.001). The high-intensity group maintained VO2peak at follow-up, whereas the low-intensity group regressed (p = 0.021). The low-intensity group minimally changed from baseline to follow-up by 0.5 ml/kg per minute, whereas the high-intensity group significantly improved by 2.2 ml/kg per minute (p = 0.01). Intervention groups always reported similar physical activity levels.
Conclusions: Higher-intensity exercise provided more sustainable cardiorespiratory benefits than lower-intensity exercise.
Implications for Nursing: Survivors need guidance on exercise intensity, because a high volume of low-intensity exercise may not provide sustained health benefits.
Jump to a section
Survivors often significantly reduce their physical activity levels after treatment and do not return to prediagnosis levels (Blanchard, Courneya, & Stein, 2008). As many as 70% of survivors do not engage in sufficient exercise to achieve health guideline recommendations (Peeters et al., 2009). Low levels of physical activity and associated losses of cardiovascular fitness increase the survivors’ risks of all-cause and disease-specific mortality (Hamer, Stamatakis, & Saxton, 2009; Irwin et al., 2008; Laukkanen, Rauramaa, Mäkikallio, Toriola, & Kurl, 2011). In addition, survivors are at higher risk of cardiovascular disease after treatment (Lakoski et al., 2013; Viale & Yamamoto, 2008). Even asymptomatic breast cancer survivors exhibit impaired cardiorespiratory fitness seven years post-treatment (Lakoski et al., 2013). However, improvements in cardiorespiratory fitness, as measured by peak volume of oxygen consumption (VO2peak), have been associated with a decrease in mortality (Blair et al., 1996; Kodama et al., 2009; Laukkanen et al., 2011) and better quality of life among breast cancer survivors (Tolentino et al., 2010). VO2peak is considered the best practical surrogate for predicting survival in any adult population (Blair et al., 1996), with reduced mortality risks seen when adults reach greater than 27.7 ml/kg per minute (Kodama et al., 2009). For context within the populations studied in the current article, research indicates that breast and prostate cancer survivors have mean VO2peak of 25.4 ml/kg per minute (Burnett, Kluding, Porter, Fabian, & Klemp, 2013) and 28.1 ml/kg per minute (Scott et al., 2015), respectively, both of which would be rated as poor in healthy adults (American College of Sports Medicine [ACSM], 2009).
In 2010, the ACSM called for researchers to examine whether different exercise intensities affected targeted health outcomes among survivors (Schmitz et al., 2010). Some studies have shown the potential for higher-intensity exercise to make larger improvements in VO2peak among survivors (Adamsen et al., 2009; De Backer et al., 2007; Quist et al., 2006). However, those studies also identified potential risks of high-intensity exercise in some populations, such as those diagnosed with hematologic cancer or a brain tumor (Adamsen et al., 2009; Quist et al., 2006). Of note, the studies employed a single intervention arm and did not make comparisons between higher- and lower-intensity exercise groups (Adamsen et al., 2009; Quist et al., 2006). To the current authors’ knowledge, the only study to compare different exercise intensity training protocols (low versus moderate intensity) in patients with cancer found no difference in improvement of cardiorespiratory fitness between the two groups after 10 weeks of training (Burnham & Wilcox, 2002). Studies examining the potential for achieving greater improvements in cardiorespiratory fitness using high-intensity exercise are scarce and, therefore, this topic warrants additional research.
To optimize an exercise program, research is needed to establish what components of a short-term exercise intervention contribute to sustainable fitness outcomes. Observational studies have found that, depending on the population sampled, people find higher-intensity exercise either motivating (Bartlett et al., 2011; Duncan, Hall, Wilson, & Jenny, 2010) or challenging (Parfitt & Hughes, 2009). Whether exercise intensity plays a role in physical activity adherence after a short-term intervention in breast and prostate cancer survivors is unknown.
The purpose of this study, therefore, is threefold. The first is to compare the cardiorespiratory fitness changes in breast and prostate cancer survivors who participated in a supervised exercise intervention at either moderate-to-high intensity or low-to-moderate intensity (see Figure 1). Both intervention groups were compared to a control group that performed their normal physical activity for eight weeks. A second aim was to examine if exercise intensity influenced the maintenance of cardiorespiratory fitness and physical activity levels postintervention. To achieve this, intervention participants were assessed at four months after the intervention. It was hypothesized that breast and prostate cancer survivors who participated in the moderate-to-high intensity exercise intervention would better sustain their improvements in cardiorespiratory fitness when compared to the group who participated in the low-to-moderate exercise training program. The final aim was to examine if exercising at either a moderate-to-high or low-to-moderate intensity was safe for survivors.
Methods
Participants were recruited through flyer distribution at hospitals in Perth, Western Australia (mailed directly to oncologists and nurses), Breast Cancer Care WA, and physicians in the Fremantle GP network. Direct mailings were sent to Western Australian Hospital Benefits Fund members. All participants met the following inclusion criteria: (a) aged 25–80 years; (b) diagnosed with stage I, II, or III breast or prostate cancer; (c) completed surgery, radiation therapy, or chemotherapy in the past five years (participants receiving adjuvant hormone therapy were still eligible); and (d) able to exercise as determined by physician consent and baseline health screening.
The research project was approved by the University of Notre Dame Australia Human Research Ethics Committee (study #011024F) and was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12611000436976) and the Australian Cancer Trials Registry. All eligible participants provided written informed consent before participating in the study activities.
The intervention was only offered at certain times, dependent on facility and staff availability. Eligible participants signed up for the intervention group that suited their availability. Once 6–10 people enrolled in an intervention group and completed the baseline assessments, the group was randomly allocated to either a low-to-moderate (LIG) or moderate-to-high (HIG) intensity exercise group. Randomization was conducted through a random number generator, with odd numbers assigned to LIG and even numbers to HIG. Participants were blinded to their allocation. Many eligible participants declined to participate in the intervention because of the time commitment required (see Figure 2). Those participants were asked to act as controls, to be assessed at baseline and at 10 weeks, and were asked to continue their normal physical activity. Because the purpose of the four-month follow-up was to assess the sustainability of the fitness outcomes from the intervention, the control group was not assessed a third time.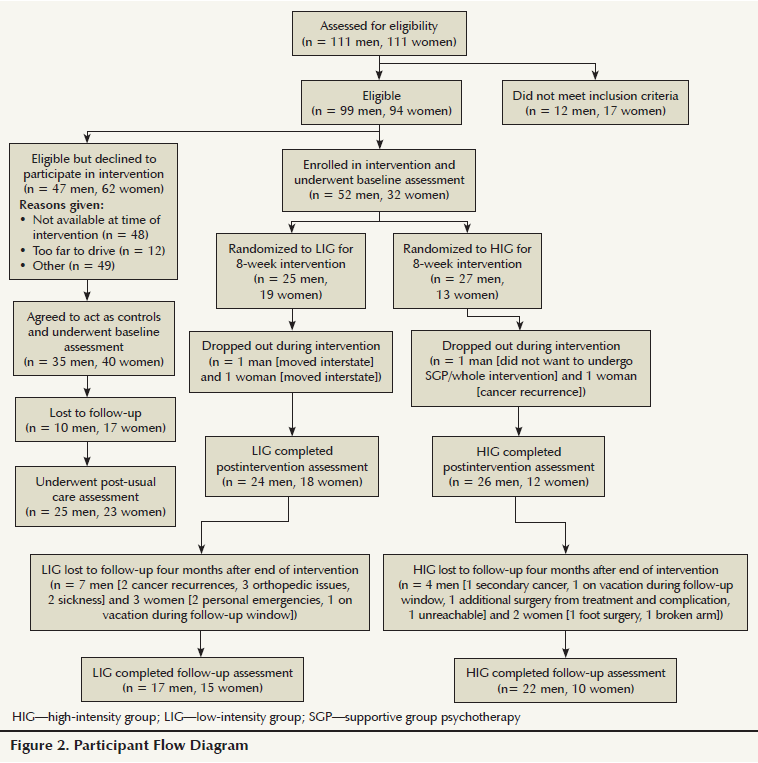
All assessments were conducted at the same time of day. Before the assessment, participants fasted for at least two hours, did not smoke for three hours, and did not exercise. Baseline measures were undertaken during the course of two days, 48–72 hours apart. On day one, resting vital signs, height, weight, and cardiorespiratory fitness were measured. Treatment data were collected from the physician referral form. On day two, the cardiorespiratory exercise test (CRET) was repeated. Dual CRETs were performed to account for any possible learning effect. In week 10, participants were reassessed on resting vital signs, height, weight, and cardiorespiratory fitness in one day. Follow-up measures were repeated four months postintervention.
Cardiorespiratory Fitness
Participants completed the CRET using a Monark Ergomedic 828E mechanically braked cycle ergometer to determine VO2peak. Oxygen uptake was measured using the Moxus Modular VO2 System metabolic cart, with VO2 and heart rate (HR) recorded continuously and averaged every 15 seconds. During the test, participants pedaled at 60 revolutions per minute, as displayed on the cycle ergometer. Verbal encouragement and feedback were given to keep participants on pace. The participants warmed up for two minutes at an initial power output of 30 watts (W), and each minute thereafter the power output increased by 30 W until they were no longer able to maintain the cadence or reached volitional fatigue. At test completion, the participants continued to breathe through the mouthpiece while pedaling at a power output of 30 W for at least one minute to cool down and to ensure the software had captured their VO2peak.
Physical Activity
Because the maintenance of cardiorespiratory fitness is dependent on continued exercise (Mujika & Padilla, 2001), participants self-reported their weekly physical activity levels using the International Physical Activity Questionnaire Short Form (IPAQ) (Craig et al., 2003) at baseline and at follow-up. From the IPAQ, total MET hours per week, hours of vigorous activity, and hours of moderate activity were calculated according to the questionnaire’s instructions.
Exercise Intervention
Exercise sessions lasted about one hour, three days per week for eight weeks, and were recorded in exercise logs. The exercise program was created and conducted by the principal investigator at the exercise clinic at the University of Notre Dame Australia (please contact the corresponding author of the current article for details). In brief, the program consisted of 12 unique workouts, which were performed in order during the first month, and then repeated with the progressed exercise intensities. On average, sessions consisted of 25 minutes of aerobic exercise, 25 minutes of resistance training, and 10 minutes of static stretching.
After the eight-week exercise intervention, the principal investigator prepared an individualized exercise program for each participant to follow, based on his or her goals and what facilities he or she may have had access to during the four-month period. This included participating in yoga or group exercise classes, joining a commercial gym, or performing exercises in their home.
Exercise Intensity
Initially, the HIG and LIG performed aerobic exercise at a HR corresponding to 75% and 60% of their VO2peak, respectively. To match the caloric expenditure between the intensity groups (O’Donovan et al., 2005), the HIG completed 80% of the minutes of aerobic exercise compared to the LIG; this was the ratio of the initial aerobic exercise intensities between the groups (60% divided by 75% = 0.8). Aerobic exercise intensity was quantified by HR because the participants completed multiple modes of aerobic exercise with various pieces of equipment and walked or jogged outside. Participants were instructed on their initial training target HR on the first day and exercised at that target for the first four weeks to allow for sufficient adaptation. Starting the fifth week of the intervention, the HIG and LIG were instructed to progress to the HR corresponding to 80% and 65% VO2peak from the baseline CRET. Participants were encouraged to maintain their target HR throughout the aerobic exercise. All data recorded from the Team2 Polar HR monitoring system were used to check for training intensity adherence. The Team2 Polar software allowed average HRs during aerobic exercise to be isolated and recorded in a data set for each participant. For every exercise session, aerobic compliance was calculated as mean HR performed during aerobic exercise divided by target HR for that session. The percentage of their target HR completed during the aerobic exercise component was labeled aerobic compliance (Ettinger et al., 1997). Relative aerobic workload was calculated as aerobic compliance multiplied by targeted percent of VO2peak (Bradfield, 1971).
Adverse Effects and Data Analysis
All adverse effects as a result of the exercise intervention were recorded in the participant’s training log. All statistical analyses were completed using SPSS®, version 20. Statistical significance was set a priori at p < 0.05. Descriptive statistics were generated for treatment variables. Demographic and treatment variables, as well as baseline outcome variables, were compared among the groups, separated by cancer type. Continuous variables were analyzed with univariate analyses of variance (ANOVAs), whereas categorical variables were analyzed with Pearson chi-square tests.
The sample size calculation for this study used the data reported by Courneya et al. (2003), which compared changes in VO2peak in a control (n = 28) and aerobic (n = 25) exercise group of breast cancer survivors. The mean difference in the response was 3.1 ml/kg per minute, with an SD of 3.8. The calculation was completed using the Power and Sample Size Program (Dupont & Plummer, 1990), which indicated that 23 experimental participants and 26 control participants were needed to be able to reject the null hypothesis that this response difference was zero with probability (power) 0.8. The type 1 error probability associated with the null hypothesis was 0.05. To adjust for not having matched pairs and possible dropouts or noncompliance, 25 participants with each cancer type were targeted to be recruited into each group (HIG, LIG, and control).
To compare change in VO2peak, a repeated measure analysis of covariance (ANCOVA) was run with age and months post-treatment as covariates. The analysis assessed if participants responded to the exercise intervention and identified differences among the controls, LIG, and HIG or breast and prostate cancer survivors (group and cancer type were used simultaneously as independent variables). To further examine specific differences among the groups or populations, three sets of delta scores were calculated as the change in VO2peak from baseline to postintervention, postintervention to follow-up, and baseline to follow-up. Each set of delta scores was analyzed with an ANCOVA, again accounting for age and months post-treatment. To ensure that changes in VO2peak were from improvements in cardiorespiratory fitness and not weight loss, body mass changes were analyzed in the same manner as VO2peak. Aerobic compliance, relative aerobic workload, and average HR during aerobic exercise were compared among groups and cancer types simultaneously using univariate ANOVAs.
To account for inherent error in self-reported physical activity (Lee, Macfarlane, Lam, & Stewart, 2011), outlying IPAQ scores (greater than three SDs from the mean) were excluded from analyses. At baseline and follow-up, total MET hours per week, hours of vigorous activity, and hours of moderate activity were compared by univariate ANOVAs, using group and cancer type as the between-subjects factors. In addition, univariate ANOVAs were used to compare changes in these scores.
Results
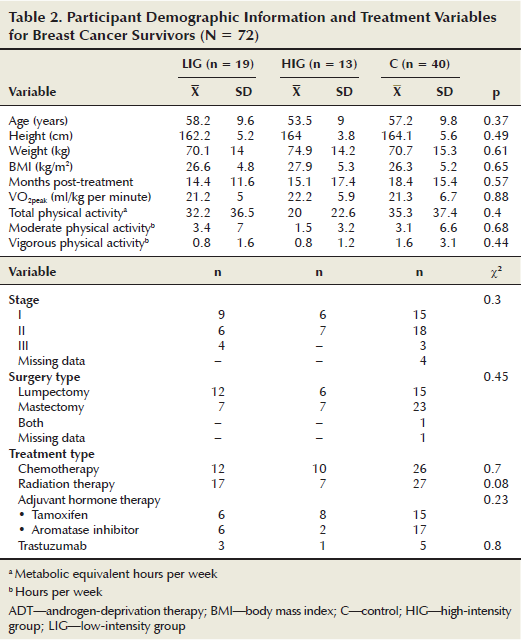
The prostate cancer survivors (n = 88, X = 65.8 years, SD = 6.6) were significantly older (p < 0.001) than the breast cancer survivors (n = 72, X = 56.8 years, SD = 9.6). This difference was expected because the typical age for diagnosis of prostate cancer is 8.1 years later than for breast cancer (Australian Institute of Health and Welfare & Australasian Association of Cancer Registries, 2010). In addition, men recorded a higher baseline VO2peak than the women, which also was expected (Ogawa et al., 1992). No significant differences were noted among cancer types on any other variable at baseline. In addition, no significant differences were noted between intervention and control groups at baseline on any of the outcome variables. Treatment, demographic, and baseline characteristics are presented in Tables 1 and 2. Cancer type was neither a significant main effect nor interaction effect on change for any outcome. Therefore, only combined cancer group (LIG, HIG, and control) comparisons are reported here.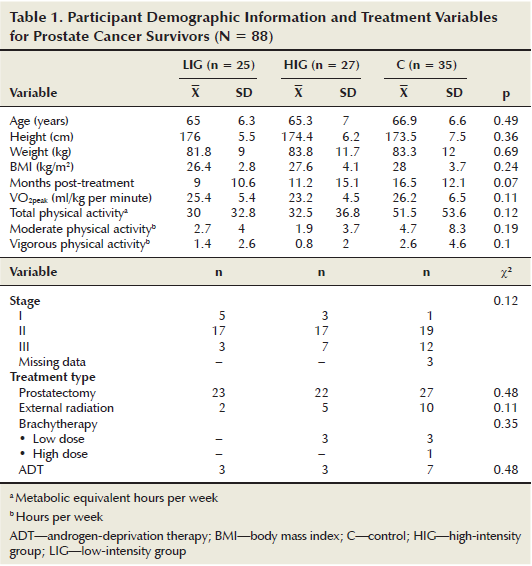
The LIG and HIG attended 87% (SD = 12.7) and 90% (SD = 11.2) of exercise sessions, respectively (p = 0.2). No adverse events were reported from the intervention. Both groups complied to within 10% of their target aerobic workload; however, a significant difference was noted between the LIG and HIG for aerobic compliance (X = 97%, SD = 8.5 and X = 90%, SD = 8.6, respectively) (p = 0.001), with the LIG exercising at their assigned intensity of 60%–65% VO2peak (X = 61%, SD = 5.3) more often than the HIG complied to their intensity level of 75%–80% VO2peak (X = 70%, SD = 6.7). This difference of only 9%, instead of the prescribed 15%, indicated that the lower-intensity aerobic exercise targets were more achievable compared to the higher-intensity aerobic exercise target. The data for three men were excluded from the aerobic compliance analysis because they had arrhythmias. All three men were in the HIG. Results of the VO2peak analysis using repeated measures ANCOVA indicated no significant change over time for all participants (p = 0.089). However, a significant interaction was noted between time and group (p = 0.013), indicating that the HIG increased their VO2peak more during the course of the study than the LIG. Analysis of the delta scores from baseline to follow-up, which showed the LIG had minimal improvement overall while the HIG significantly improved, further supports this finding (see Table 3). The ANCOVA analysis comparing the delta scores of the LIG, HIG, and control group indicated that both intervention groups improved their VO2peak compared to the control group (p < 0.001 for both LIG and HIG), but no significant difference was noted between the two intervention groups (p = 0.083). From postintervention to the four-month follow-up, the LIG decreased and the HIG maintained cardiorespiratory fitness. No significant change was noted in body mass over time, nor were any differences seen between groups on changes in body mass at any time point, indicating that the VO2peak changes were truly from improvements in cardiorespiratory fitness.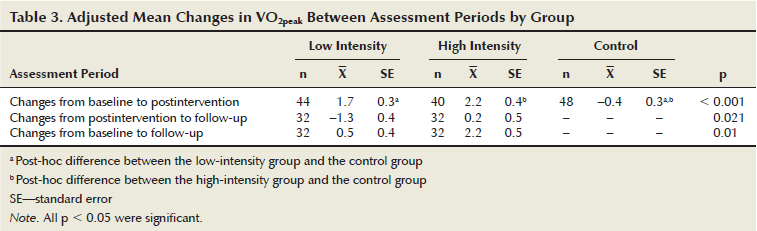
No significant differences were noted between the intervention groups for MET hours per week, hours of vigorous activity, or hours of moderate activity at either baseline or follow-up (see Table 4). And, no differences were noted between the groups for changes in all three measures. Of the participants who were unable to complete the CRET at follow-up, four men from the HIG and two from the LIG, as well as two women from the LIG, did turn in their IPAQ, which were included in the analysis.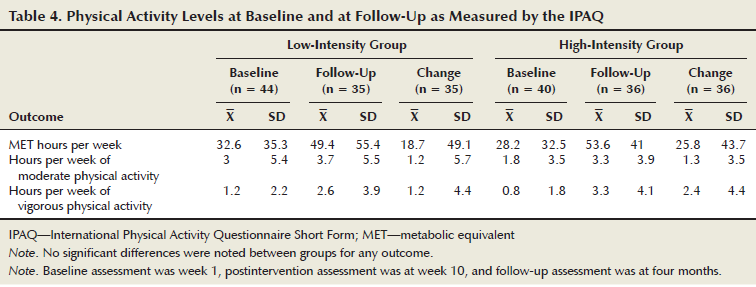
Discussion
Both exercise groups improved cardiorespiratory fitness to a similar extent after participating in the intervention, which was more than the control group. The similarity between the intervention groups was not hypothesized, however the aerobic prescription compliance analyses showed that the intervention arms were exercising at a difference of only 9% of aerobic intensity instead of the prescribed 15%. Such an outcome indicated that participants found it more challenging to sustain training at the higher intensity and that perhaps more education was needed to guide participants up to the higher intensities. When exercising at HRs corresponding to VO2peak of 61%–70%, with the duration of aerobic exercise set to make the groups isocaloric for a 15% difference, similar changes in cardiorespiratory fitness for breast and prostate cancer survivors were elicited. A difference greater than 9% in relative aerobic exercise intensity may be needed to detect a significant difference in cardiorespiratory adaptation during an eight-week exercise intervention. Burnham and Wilcox (2002), who also compared two groups at intensities 15% apart (low versus moderate), concluded that greater differences in aerobic workload are needed to see a significant difference in cardiorespiratory fitness change during a short-term exercise intervention. It may be that a 20% difference in intensity is needed to see a difference in cardiorespiratory fitness improvements between short-term exercise groups. This speculation is founded on the results of a study of patients with heart failure (Wisloff et al., 2007) who detected significantly different changes in VO2peak prescribing aerobic exercise intervals at 90%–95% HR peak versus continuous aerobic exercise at 70% HR peak. Although higher intensities are needed to promote more profound adaptations in the cardiopulmonary system, it currently is not known why patients with cancer appear to have blunted adaptations in comparison to healthy individuals.
The gains in cardiorespiratory fitness demonstrated by the participants in the current study were slightly lower than results reported in other studies involving survivors, which ranged from 2.7–3.6 ml/kg per minute (Courneya et al., 2003; De Backer et al., 2007; Quist et al., 2006). In many cases, this may be attributed to longer intervention duration (15–18 weeks) or higher training intensities of the other studies. For example, Quist et al. (2006) reported greater improvements in cardiorespiratory fitness after only six weeks. In that study, the participants were prescribed higher-intensity aerobic exercise ranging from 85%–95% HRmax. Together, these studies indicate that it may be necessary to prescribe the upper threshold of high-intensity aerobic exercise (i.e., near the anaerobic threshold) to survivors to achieve a significant cardiovascular improvement in a short-term exercise intervention. Additional studies will first be needed to ensure the safety as well as the tolerability of such practices. However, that both intervention groups improved while the control group stagnated, despite continuing their own physical activity routines, indicates that many survivors need the guidance of an exercise physiologist to improve their fitness after treatment.
The second finding of the current study was that, overall, and despite similar changes in cardiorespiratory fitness from baseline to postintervention, the improvements made by the HIG were more sustainable than those made by the LIG. Also important was the finding that the LIG members were unable to maintain the intervention gains, despite the groups reporting similar levels of physical activity at baseline and follow-up. This may indicate that both group’s subjectively thought they were performing moderate-intensity exercise, as described by the IPAQ, because they had been instructed to exercise at a certain level while being blinded to the exact prescription. It seems likely that, once habituated to the objective intensity, the participants would have associated that prescribed intensity with the psychological affective response that the exercise they had performed was the intensity they should continue (Parfitt & Hughes, 2009). The analyses of the IPAQ scores showed that both groups kept up their volume of exercise. Therefore, the difference in objective exercise intensity may be contributed to sustainability of improvements in cardiorespiratory fitness. To confirm this response in a short-term exercise intervention, future studies should create at least a 20% difference in prescribed aerobic exercise intensities between two intervention groups.
Some evidence indicates that, by following a high-intensity exercise program, cardiorespiratory fitness can be maintained with as little as one training session per week in healthy individuals (Madsen, Pedersen, Djurhuus, & Klitgaard, 1993). Mechanisms that allow for maintained performance and cardiorespiratory fitness, despite a reduction in physical activity, include sustained elevations of muscle oxidative capacity, such as higher muscle content of cytochrome c oxidase subunit 4 and GLUT4 transporter proteins (Burgomaster et al., 2007). As VO2peak has been identified as the best predictor of survival (Blair et al., 1996; Kodama et al., 2009), the results of the current study suggest that higher-intensity exercise may provide a more sustainable and greater benefit to survivor longevity than lower-intensity exercise, by allowing people to maintain a higher level of cardiorespiratory fitness.
Limitations
Limitations of this study included not having a truly randomized sample. Because of the logistical demands of the research, participants who could not participate in the intervention were drafted to act as controls. More importantly, the less than perfect compliance of participants in the HIG to their target aerobic intensity may have skewed the analyses of cardiorespiratory fitness.
Conclusion
After an eight-week exercise training intervention, breast and prostate cancer survivors improved their cardiorespiratory fitness, whether they exercised at 60% or 70% of VO2peak. However, four months after the intervention, those who had exercised at the higher intensity (i.e., 70% VO2peak) maintained their cardiorespiratory fitness, whereas those who had exercised at a lower intensity (i.e., 60% VO2peak) did not; participants had reported similar levels of physical activity after the intervention. The results of this study indicate that instructing breast and prostate cancer survivors, who are capable of exercising at a higher aerobic intensity for a relatively short-term period of eight weeks, may provide a greater sustained benefit to cardiorespiratory fitness than performing low- to moderate-intensity aerobic exercise. Regardless of intensity, many survivors likely need supervised instruction to create an exercise program that will increase their fitness level.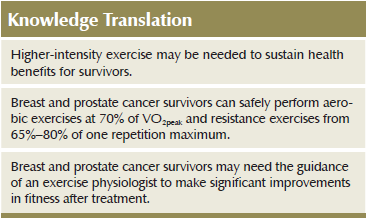
Implications for Nursing
The importance of cardiorespiratory fitness to long-term health, quality of life, and potentially increased survival time is well established (Herrero et al., 2006; Sloan, Sawada, Martin, Church, & Blair, 2009). Therefore, all healthcare providers should encourage patients, who are cleared to exercise by their physicians, to participate in regular aerobic exercise. The current ACSM recommendations (Schmitz et al., 2010) can serve as a tool for nurses to promote regular participation in physical exercise among their patients; however, patients should consult with an exercise specialist so a more specific exercise training regimen can be devised. Although some exercise is suggested to be better than none, the advice from healthcare professionals to survivors should be that quality training at higher intensities post-treatment may be required to elicit adequate sustainable cardiorespiratory improvements. Participation in regular exercise should also be encouraged.
After treatment, when side effects such as nausea have subsided, survivors should seek the guidance of an exercise physiologist specializing in meeting their needs (an exercise oncologist). The exercise oncologist can help to address specific issues, like regaining shoulder range of motion after mastectomy or pelvic floor function to reduce incontinence after radical prostatectomy. In addition, the patient will likely need guidance on how and when to progress the intensity of his or her general exercise routine, and what exercises will be safe for him or her to perform. Survivors need to progress the intensity of their exercise programs, when appropriate, to overcome the losses experienced during treatment.
References
Adamsen, L., Quist, M., Andersen, C., Moller, T., Herrstedt, J., Kronborg, D., . . . Rorth, M. (2009). Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. BMJ, 339, b3410. doi:10.1136/bmj.b3410
American College of Sports Medicine. (2009). ACSM’s guidelines for exercise testing and prescription (8th ed.). Baltimore, MD: Lippincott Williams and Wilkins.
Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. (2010). Cancer in Australia: An overview. Canberra, Australia: Australian Institute of Health and Welfare.
Bartlett, J., Close, G., MacLaren, D., Gregson, W., Drust, B., & Morton, J. (2011). High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: Implications for exercise adherence. Journal of Sports Science, 29, 547–553.
Blair, S.N., Kampert, J.B., Kohl, H.W., Barlow, C.E., Macera, C.A., Paffenbarger, R.S., & Gibbons, L.W. (1996). Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA, 276, 205–210.
Blanchard, C.M., Courneya, K.S., & Stein, K. (2008). Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. Journal of Clinical Oncology, 26, 2198–2204.
Bradfield, R.B. (1971). A technique for determination of usual daily energy expenditure in the field. American Journal of Clinical Nutrition, 24, 1148–1154.
Burgomaster, K.A., Cermak, N.M., Phillips, S.M., Benton, C.R., Bonen, A., & Gibala, M.J. (2007). Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 292, R1970–R1976.
Burnett, D., Kluding, P., Porter, C., Fabian, C., & Klemp, J. (2013). Cardiorespiratory fitness in breast cancer survivors. SpringerPlus, 2, 68.
Burnham, T., & Wilcox, A. (2002). Effects of exercise on physiological and psychological variables in cancer survivors. Medicine and Science in Sports and Exercise, 34, 1863–1867.
Courneya, K.S., Mackey, J.R., Bell, G., Jones, L.W., Field, C., & Fairey, A. (2003). Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. Journal of Clinical Oncology, 21, 1660–1668.
Craig, C.L., Marshall, A.L., Sjostrom, M., Bauman, A.E., Booth, M.L., Ainsworth, B.E., . . . Oja, P. (2003). International Physical Activity Questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise, 35, 1381–1395.
De Backer, I., Van Breda, E., Vreugdenhil, G., Nijziel, M., Kester, A., & Schep, G. (2007). High-intensity strength training improves quality of life in cancer survivors. Acta Oncologica, 46, 1143–1151.
Duncan, L., Hall, C.R., Wilson, P., & Jenny, O. (2010). Exercise motivation: A cross-sectional analysis examining its relationship with frequency, intensity, and duration of exercise. International Journal of Behavioral Nutrition and Physical Activity, 7, 7.
Dupont, W.D., & Plummer, W.D. (1990). Power and sample size calculations. A review and computer program. Controlled Clinical Trials, 11, 116–128.
Ettinger, W., Burns, R., Messier, S., Applegate, W., Rejeski, J., Morgan, T., . . . Craven, T. (1997). A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. JAMA, 277, 25–31.
Hamer, M., Stamatakis, E., & Saxton, J. (2009). The impact of physical activity on all-cause mortality in men and women after a cancer diagnosis. Cancer Causes and Control, 20, 225–231.
Herrero, F., Balmer, J., San Juan, A., Foster, C., Fleck, S., Perez, M., . . . Lucia, A. (2006). Is cardiorespiratory fitness related to quality of life in survivors of breast cancer? Journal of Strength and Conditioning Research, 20, 535–540.
Irwin, M., Smith, A., McTiernan, A., Ballard-Barbash, R., Cronin, K., Gilliland, F., . . . Bernstein, L. (2008). Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. Journal of Clinical Oncology, 26, 3958–3964. doi:10.1200/JCO.2007.15.9822
Kodama, S., Saito, K., Tanaka, S., Maki, M., Yachi, Y., Asumi, M., . . . Sone, H. (2009). Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women. JAMA, 301, 2024–2035. doi:10.1001/jama.2009.681
Lakoski, S.G., Barlow, C.E., Koelwyn, G.J., Hornsby, W.E., Hernandez, J., DeFina, L.F., . . . Jones, L.W. (2013). The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer seven years after diagnosis: The Cooper Center Longitudinal Study. Breast Cancer Research and Treatment, 138, 909–916.
Laukkanen, J., Rauramaa, R., Mäkikallio, T., Toriola, A., & Kurl, S. (2011). Intensity of leisure-time physical activity and cancer mortality in men. British Journal of Sports Medicine, 45, 125–129.
Lee, P., Macfarlane, D., Lam, T., & Stewart, S. (2011). Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 8, 115. doi:10.1186/1479-5868-8-115
Madsen, K., Pedersen, P.K., Djurhuus, M.S., & Klitgaard, N.A. (1993). Effects of detraining on endurance capacity and metabolic changes during prolonged exhaustive exercise. Journal of Applied Physiology, 75, 1444–1451.
Mujika, I., & Padilla, S. (2001). Cardiorespiratory and metabolic characteristics of detraining in humans. Medicine and Science in Sports and Exercise, 33, 413–421.
O’Donovan, G., Owen, A., Bird, S.R., Kearney, E.M., Nevill, A.M., Jones, D.W., & Woolf-May, K. (2005). Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. Journal of Applied Physiology, 98, 1619–1625.
Ogawa, T., Spina, R.J., Martin, W.H., 3rd, Kohrt, W., Schechtman, K.B., Holloszy, J.O., & Ehsani, A.A. (1992). Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation, 86, 494–503.
Parfitt, G., & Hughes, S. (2009). The exercise intensity-affect relationship: Evidence and implications for exercise behavior. Journal of Exercise Science and Fitness, 7(2, Suppl.), S34–S41.
Peeters, C., Stewart, A., Segal, R., Wouterloot, C., Scott, C.G., & Aubry, T. (2009). Evaluation of a cancer exercise program: Patient and physician beliefs. Psycho-Oncology, 18, 898–902. doi:10.1002/pon.1406
Quist, M., Rorth, M., Zacho, M., Andersen, C., Moeller, T., Midtgaard, J., & Adamsen, L. (2006). High-intensity resistance and cardiovascular training improve physical capacity in cancer patients undergoing chemotherapy. Scandinavian Journal of Medicine and Science in Sports, 16, 349–357.
Schmitz, K.H., Courneya, K.S., Matthews, C., Demark-Wahnefried, W., Galvao, D.A., Pinto, B.M., . . . Schwartz, A.L. (2010). American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise, 42, 1409–1426. doi:10.1249/MSS.0b013e3181e0c112
Scott, J., Hornsby, W., Lane, A., Kenjale, A., Eves, N., & Jones, L. (2015). Reliability of maximal cardiopulmonary exercise testing in men with prostate cancer. Medicine and Science in Sports and Exercise, 47, 27–32.
Sloan, R.A., Sawada, S.S., Martin, C.K., Church, T., & Blair, S.N. (2009). Associations between cardiorespiratory fitness and health-related quality of life. Health and Quality of Life Outcomes, 7, 47.
Tolentino, G.P., Battaglini, C.L., Araujo, S.S., Otano, A.S., Conde, D.M., Evans, E.S., & de Oliveira, R. (2010). Cardiorespiratory fitness and quality-of-life analysis posttreatment in breast cancer survivors. Journal of Psychosocial Oncology, 28, 381–398.
Viale, P., & Yamamoto, D. (2008). Cardiovascular toxicity associated with cancer treatment. Clinical Journal of Oncology Nursing, 12, 627–638.
Wisloff, U., Stoylen, A., Loennechen, J.P., Bruvold, M., Rognmo, O., Haram, P.M., . . . Skjaerpe, T. (2007). Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation, 115, 3086–3094.
About the Author(s)
Eric A. Martin, PhD, is an assistant professor in the Department of Kinesiology at California State University, Monterey Bay, in Seaside, CA; Claudio L. Battaglini, PhD, is an associate professor of exercise physiology in the Department of Exercise and Sport Science at the University of North Carolina–Chapel Hill; Beth Hands, PhD, is a director for the Institute for Health Research at the University of Notre Dame Australia in Fremantle, Western Australia; and Fiona Naumann, PhD, is a professor of exercise physiology in the School of Exercise and Nutrition Sciences at Queensland University of Technology, Australia, in Brisbane. The study was supported, in part, by a grant from Sports Medicine Australia. Martin can be reached at ealexandermartin@gmail.com, with copy to editor at ONFEditor@ons.org. (Submitted September 2014. Accepted for publication December 12, 2014.)

