Cardiovascular Disease Risk and Breast Cancer Outcomes: A Pilot Study
Purpose/Objectives: To assess feasibility of using electronic health records for profiling multiple cardiovascular disease (CVD) risk factors in women with breast cancer at diagnosis and five years post-treatment, and to explore relationships among CVD risk factors and breast cancer outcomes.
Design: Retrospective, descriptive.
Setting: A comprehensive cancer center in the southwestern United States.
Sample: 200 women with stage 0–III breast cancer.
Methods: A record review using an instrument to profile multiple CVD risk factors and breast cancer outcomes.
Main Research Variables: CVD risk factors, such as blood pressure (BP) and hemoglobin A1C (HbA1C), and breast cancer outcomes, such as metastasis.
Findings: Most data on CVD risk factors were undocumented. Even BP values to assess hypertension were missing in 35% of women at breast cancer diagnosis. Women with poor outcomes had trends toward higher blood glucose and HbA1C than women with good outcomes.
Conclusions: The study failed to comprehensively capture CVD risk factors in women with breast cancer because of missing data. Glucose control may be associated with breast cancer outcomes.
Implications for Nursing: Better documentation of shared risk factors for CVD and breast cancer is needed. Prospective studies are needed to evaluate shared CVD risk factors and breast cancer outcomes because of missing health record information.
Jump to a section
Breast cancer and cardiovascular disease (CVD) are two important health problems in women. The American Cancer Society, the American Heart Association, and the American Diabetes Association joined forces in 2004 to promote health and reduce risk factors for cancer, CVD (including stroke), and diabetes—the three most common causes of death in the United States (Eyre, Kahn, & Robertson, 2004). Evidence suggests that these diseases share common risk factors. Interventions directed at primary prevention, including public awareness of healthy lifestyles, should reduce morbidity and mortality for all three diseases (Eyre et al., 2004).
Emerging data are suggestive that breast cancer and CVD share more risk factors than previously believed. For example, diabetes, a well-established risk factor for CVD, is associated with an increased risk of breast cancer and poor outcomes (Coughlin, Calle, Teras, Petrelli, & Thun, 2004; Larsson, Mantzoros, & Wolk, 2007). In addition, inflammation, a long-known risk factor for CVD, has emerged as a risk factor for cancer (Colotta, Allavena, Sica, Garlanda, & Mantovani, 2009). Promotion of a healthy lifestyle to reduce what were once considered traditional CVD risk factors may actually prevent breast cancer or improve breast cancer survival. However, no comprehensive study has been conducted on shared multiple risk factors for these two diseases and a potential relationship with breast cancer outcomes. To fill the gap, the aim of this pilot study was to begin exploring multiple CVD risk factors and breast cancer outcomes in women who have been diagnosed with breast cancer.
The objectives of this study were (a) to assess the feasibility of using the health record for profiling prevalence of multiple CVD risk factors (increased age, dyslipidemia, high body mass index [BMI], diabetes, smoking, hypertension, family history of premature coronary artery disease, estrogen therapy, sedentary lifestyle, inflammation, and depression) in women at the time of the initial diagnosis and five years post-treatment, and relate these to breast cancer outcomes (tumor recurrence, stage progression, metastasis, and death) at five years post-treatment; and (b) to explore possible relationships among multiple CVD risk factors and breast cancer outcomes.
Methods
Design, Sample, and Settings
Using a descriptive design, a retrospective health record review of 200 women diagnosed with breast cancer at a comprehensive cancer center in the southwestern region of the United States was conducted. Records of women who initially were diagnosed with stage 0–III breast cancer from 2002–2005 and had five years of follow-up care at the study site or died within the five years following the initial diagnosis were included. Women who initially were diagnosed with stage IV breast cancer were excluded because of associated poor outcomes (e.g., average survival of 2.5–3.5 years), which was significantly different than those with stage 0–III breast cancer. Those younger than age 21 years were not included because childhood breast cancer and CVD are extremely rare and are not representative of these diseases. No women were excluded on the basis of ethnicity. Data were collected after institutional review board approval was obtained through the Human Subjects Protection Program at the University of Arizona.
Instruments
Based on a review of the literature (Chobanian et al., 2003; National Cancer Institute, 2014; Radford et al., 2005), two of the authors (SFW and CJM) developed a data collection instrument to profile prevalence of multiple CVD risk factors and breast cancer outcomes. Variables related to CVD risk factors were identified and operationally defined (see Table 1). These variables were obtained from health record entries closest to initial breast cancer diagnosis and at five years post-treatment. Other variables included stage of breast cancer at initial diagnosis and five years post-treatment, as well as treatment modalities. Poor outcomes were defined as tumor recurrence, stage progression, metastasis, and death within the five-year window after initial breast cancer diagnosis. Additional variables included tumor characteristics, menopausal status, hereditary cancer (i.e., BRCA1 and BRCA2 mutation status), and years of overall and disease-free survival.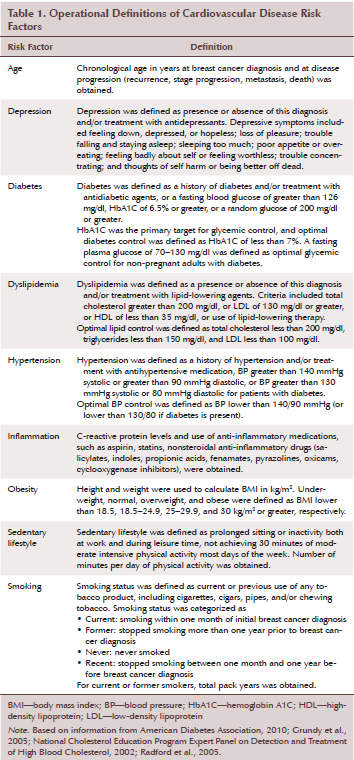
Content validity of this data collection instrument was supported by two doctorally prepared nurse researchers, a nurse practitioner board certified in cardiac care, and two doctorally prepared nurse researchers in cancer nursing, who reviewed and judged the instrument to be a measure of CVD risk factors and breast cancer outcomes.
Records were identified using the International Classification of Disease 9th edition clinical modification (ICD-9-CM) category 174.9, malignant neoplasm of breast (female), unspecified. Once women with breast cancer were identified, the authors randomly selected 200 records for review using a systematic sampling method. If a randomly selected woman had an initial diagnosis of stage IV breast cancer, another record was randomly selected for review.
Statistical Analysis
SPSS®, version 16.0, was used for data entry and statistical analysis. To ensure that all data were collected in the same manner, the authors used the created instrument to collect data on the same seven subjects to determine the percentage of agreement among data collectors. Once agreement among data collectors was greater than 95% in more than two consecutive agreement checks, data collection began. Before data were analyzed, the data were checked for outliers by inspecting the values in a frequency distribution, with scrutiny of the highest and lowest values.
Feasibility of using the health record for profiling prevalence of multiple CVD risk factors was determined by presence or absence of documentation of relevant CVD risk factors. A priori to data collection for this study, feasibility of using the health record for presence of CVD risk factors was defined as information being documented at least 90% of the time. Descriptive statistics and frequencies were used to describe the prevalence of multiple CVD risk factors in women with breast cancer. Exploratory analytic techniques, including multiple and logistic regression analyses, were used to explore the relationships between multiple CVD risk factors and breast cancer outcomes. A level of significance of p < 0.05 was considered statistically significant.
Results
Sample Characteristics
Records from 200 women with stage 0–III breast cancer were reviewed. The ages of these women at breast cancer diagnosis ranged from 25–88 years, with a mean age of 55.7 years (SD = 12.8). The sample included 35 women in stage 0 (18%), 53 in stage I (27%), 76 in stage II (38%), and 16 in stage III (8%). Two (1%) were invasive (stage unknown) and 18 (9%) were missing stage information (see Table 2). Of the 200 women, information on race or ethnicity was only available on 108 (54%). The racial or ethnic composition of these 108 women was 90 Caucasians (83%), 13 Hispanics (12%), 3 Asians (3%), and 2 Native Americans (2%).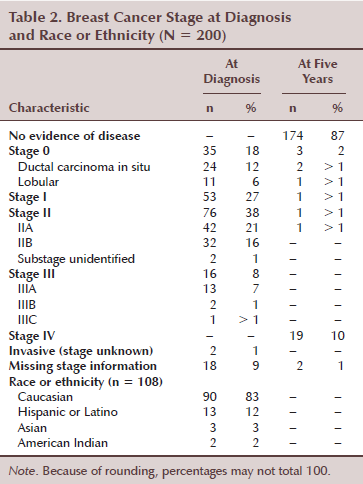
Feasibility of Profiling Cardiovascular Disease Risk Factors Using Health Records
The authors defined feasibility of using the health record for profiling multiple CVD risk factors as information being documented at least 90% of the time. Using this definition, information related to CVD risk factors at breast cancer diagnosis did not achieve 90% in any category (see Table 3). Even parameters used to calculate BMI and blood pressure (BP) values for hypertension were missing in 75 (38%) and 70 (35%) women, respectively, at breast cancer diagnosis. At five years after breast cancer diagnosis, presence of multiple CVD risk factors in health records was determined to be feasible for only two of the risk factors, BMI and hypertension. Here, information on height, weight, and BP was found in the health records of more than 90% of the women.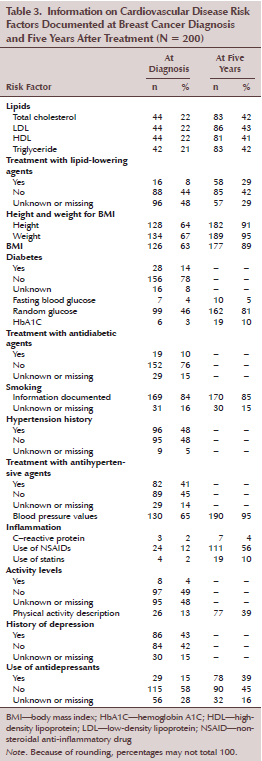
The records reviewed had other important deficiencies. First, information pertaining to lipids was deficient, which is significant because, historically, lipid values have served as standard measures of CVD risk. All lipid measures were documented in 42 (20%) and 81 (40%) women at breast cancer diagnosis and at five years after breast cancer diagnosis, respectively, although at least 81 women had a history of dyslipidemia. Secondly, although diabetes is a well-established risk factor for CVD, information on fasting and random blood glucose was documented in 7 (4%) and 99 (50%) women at breast cancer diagnosis, respectively. These, however, improved to 10 (5%) and 162 (81%) at five years after breast cancer diagnosis. Despite at least 28 women (14%) having a history of diabetes, hemoglobin A1C (HbA1C) levels were only available in 6 (3%) and 19 (10%) women at breast cancer diagnosis and at five years after breast cancer diagnosis, respectively.
Chronic inflammation is considered to be a fundamental mechanism underlying CVD. C-reactive protein (CRP), a common clinical inflammatory marker and a strong predictor of CVD risk (Libby, Ridker, & Hansson, 2009), was only documented in three women (2%). Use of anti-inflammatory medications, such as nonsteroidal anti-inflammatory drugs and statins, was reported in 24 (12%) and 4 (2%) women at breast cancer diagnosis, respectively. Documentation of anti-inflammatory medication use was reported in 111 (56%) and 19 (10%) women at five years after breast cancer diagnosis.
Physical activity level was documented in 8 (13%) and 77 (39%) women at breast cancer diagnosis and at five years after breast cancer diagnosis, respectively. The descriptors used to characterize physical activity became more diverse and better elucidated at five years after breast cancer diagnosis. Finally, two relevant CVD risk factors, history of smoking and depression, were well documented (169 [85%] and 86 [43%], respectively) in the health records.
Relationships Among Cardiovascular Disease Risk Factors and Breast Cancer Outcomes
Control of modifiable cardiovascular disease risk at breast cancer diagnosis: Among the 44 women with available lipid values, many did not have optimally controlled lipids levels (see Table 4). Optimally controlled total cholesterol, low-density lipoprotein (LDL), and triglycerides were found in 18 (41%), 10 (23%), and 30 (71%) women, respectively. All women had optimal high-density lipoprotein (HDL). Among the 126 women with available parameters to calculate BMI, 70 (56%) were overweight or obese. With limited availability of fasting blood glucose values (n = 7), all were below 125 mg/dl (range = 68–111 mg/dl). Random blood glucose values were available in 99 women. These values were below 200 mg/dl in 97 (98%) of the women. HgA1C values were only available in six women (values range of 5.4%–7.8%), and four of these women had optimal HbA1C control of less than 7%. The majority of women either never smoked (n = 108, 64%) or were former smokers (n = 43, 25%). Fifteen women (9%) were current smokers with three (2%) abstaining from smoking between one month and one year before breast cancer diagnosis. Of the 129 women with available BP values, 40 (31%) had suboptimal control of hypertension. Finally, out of only 29 women with documentation, 23 women (79%) reported having 30 minutes of moderate activity most days of week. Of note, this information was unknown or missing in 85% of women.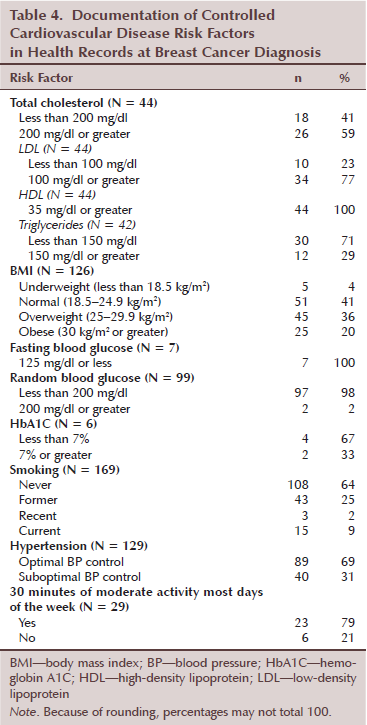
Breast cancer outcomes: Outcome data at five years after breast cancer diagnosis were available in 185 women. Among these, 22 (11%) had poor outcomes, defined as progression to stage IV breast cancer/metastatic disease (n = 20, 11%) and/or death within five years postdiagnosis (n = 11, 6%).
The authors compared multiple CVD risk factors at both breast cancer diagnosis and at five years after diagnosis with breast cancer outcomes (see Table 5). Although the authors used a small sample size, women with poor outcomes had indicators of poor glucose control as well as increased triglyceride levels at breast cancer diagnosis. At breast cancer diagnosis, the authors identified a trend for associations between poor outcomes and increased fasting blood glucose (p = 0.074) and elevated fasting triglycerides (p = 0.092). Women with good outcomes (n = 6) had a mean fasting blood glucose level of 86.83 mg/dl (SD = 9.95) as compared to the one woman with a poor outcome who had a fasting blood glucose of 111 mg/dl (p = 0.054). Mean triglyceride level was 227 mg/dl (SD = 205.06) among women with poor outcomes (n = 2) compared to 133.1 mg/dl (SD = 68.18) in women with good outcomes (n = 39) (p = 0.092). At five years after breast cancer diagnosis, a trend was noted for associations between poor outcomes and increased fasting blood glucose (p = 0.054) and elevated HbA1C (p = 0.074). Mean fasting blood glucose level was 111.25 mg/dl (SD = 15.65) among women with poor outcomes (n = 4) compared to 89.33 mg/dl (SD = 14.67) in women with good outcomes (n = 6) (p = 0.054). Mean HbA1C level was 7.3% (SD = 1%) among women with poor outcomes (n = 2) compared to 6.25% (SD = 0.7%) in women with good outcomes (n = 16) (p = 0.074).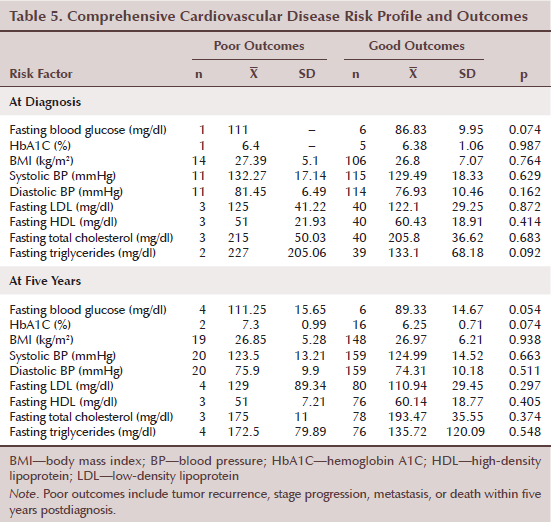
Variables that did not reach statistical significance for poor outcomes for breast cancer, with the small sample size, included BMI, BP values, and other lipid parameters. Of note, regarding BMI, the authors did not detect a statistically significant difference between women with poor and good outcomes (p = 0.764); however, both groups had higher than normal BMIs (mean = 27.39 kg/m2, SD = 5.1, n = 14; mean = 26.8 kg/m2, SD = 7.07, n = 106, respectively).
Discussion
Using this retrospective study in which data on CVD risk factors were extracted from health records of 200 women at breast cancer diagnosis and at five years after diagnosis, the authors reached two important conclusions. First, most data relevant to CVD risk factors were undocumented in women diagnosed with breast cancer. Secondly, evidence suggests that some CVD risk factors, such as fasting blood glucose and HbA1C, may be associated with breast cancer outcomes.
Feasibility of Profiling Cardiovascular Disease Risk Factors Using Health Records
In the current study, data were completed on the staging of breast cancer and age as a demographic variable; however, race and ethnicity information is surprisingly low. The authors found that most data relevant to CVD risk factors were undocumented. Increased obesity, hypertension, and increased inflammation are well-documented risk factors for CVD; however, these data were missing in the health records reviewed. At breast cancer diagnosis, parameters used to calculate BMI and BP values for hypertension and obesity were missing in 35% of women. Physical activity level was only documented in 13% and 39% of women at breast cancer diagnosis and at five years after breast cancer diagnosis, respectively. CRP data, which serve as clinical markers for inflammation, were documented in only three of 200 women. Therefore, a prospective study on CVD risk factors in women with breast cancer is needed to comprehensively capture CVD risk profiles in women with breast cancer. Although the health records are not a feasible method to extract CVD risk factors, which the authors defined as information being documented at least 90% of the time, individual CVD risk factors warrant further comments and discussions.
Blood sugar and diabetes: Data from large prospective epidemiologic studies have shown that diabetes is a risk factor for breast cancer development (Coughlin et al., 2004) and, in some studies, a risk factor for mortality from breast cancer (Coughlin et al., 2004; Jee et al., 2005). To re-emphasize, in the current study, women with poor breast cancer outcomes had trends toward higher fasting blood glucose and HbA1C, an indicator of poor blood sugar control, compared to women with good outcomes. The mean HbA1C level in women with poor outcomes was 7.3% (SD = 1) compared to 6.25% (SD = 0.71) in those with good outcomes when measured at five years after breast cancer diagnosis. Findings, however, from a large study from Sweden (Miao Jonasson et al., 2012) did not show an association between high HbA1C and incidences of all cancers, including breast cancer. In that study, an HbA1C cutoff value of 7.5% was used to correlate the incidence of cancers, although the American Diabetes Association (2010) recommended that the optimal HbA1C level be equal to or less than 7%, as these levels have been associated with reduced microvascular and neuropathic complications. Additional research is warranted to determine if high HbA1C level or poor blood sugar control is related to breast cancer outcomes.
Dyslipidemia: The authors of the current study found that, in this group of women with breast cancer, of those with reported laboratory values, the majority did not have well-controlled total cholesterol and LDL levels: 59% had a cholesterol level of 200 mg/dl or greater and 77% had an LDL level of 100 mg/dl or greater. The findings of the current study are consistent with the work of Abdelsalam, Hassan, and Sadig (2012), who showed that elevated total cholesterol and LDL levels are associated with all stages of breast cancer. Of note, in the current study, all women had HDL levels of 35 mg/dl or greater, which is known to have protective effects against CVD. Elevated HDL levels also were reported in women with breast cancer in the study by Abdelsalam et al. (2012), although they did not find this to be associated with breast cancer risk.
The current study also revealed potentially useful information regarding elevated triglyceride levels at breast cancer diagnosis and poor breast cancer outcomes, although the relationship between triglyceride level and poor breast cancer outcomes only approached statistical significance (p = 0.092). This trend is consistent with an epidemiology study on metabolic syndrome and outcomes following early-stage breast cancer, suggesting an increase in risk of a second primary breast cancer with high triglyceride levels (Calip et al., 2014). Calip et al. (2014) hypothesized a mechanism involving the lipoprotein lipase, but recommended continued studies in larger samples to further elucidate the mechanisms.
Physical activity: A meta-analysis of 12,108 patients with breast cancer showed that physical activity pre- and postdiagnosis reduced mortality from breast cancer (Ibrahim & Al-Homaidh, 2011). However, in the current study, physical activity level was only documented in 8 (13%) and 77 (39%) women at breast cancer diagnosis and at five years after breast cancer diagnosis, respectively. Therefore, associating physical activity with breast cancer outcomes was difficult, particularly because the descriptors used to characterize physical activity were diverse and better elucidated in the health record at five years after breast cancer diagnosis. Descriptors ranged from “does not exercise” to “excessive exercise,” with walking as the most frequently reported activity. Specific descriptors, such as tennis, kickboxing, horseback riding twice a week, and golfing 60 rounds per year, were provided in the reviewed health records. Standardization and quantification of these physical activities to better evaluate the relationship between physical activity and breast cancer outcomes in these women would be beneficial.
Inflammation: Inflammation is an important mechanism underlying development of atherosclerosis and progression of CVD. Inflammation has now emerged as an important risk factor for the development and progression of many cancers (Colotta et al., 2009; Mantovani, Allavena, Sica, & Balkwill, 2008). A hypothesis from Howe, Subbaramaiah, Hudis, and Dannenberg (2013) suggested that inflammation mediates the effects of obesity and increased adiposity on breast cancer development and poor outcomes. Unfortunately, despite current scientific investigation on inflammation, the important inflammatory CVD marker, CRP, was only measured in 3 of 200 women in the current study. In addition, because statin drugs have anti-inflammatory properties, its use in only 4 of 81 women with a history of dyslipidemia is of particular concern.
Obesity: The authors did not detect a statistically significant difference between BMI and breast cancer outcomes (p = 0.764); however, women in this study, regardless of having poor or good outcomes, had higher than normal BMIs. Although hypotheses have been proposed to explain the relationship between obesity and breast cancer via inflammation, as mentioned earlier, current data are not consistently supportive of obesity as a risk factor for development of breast cancer and poor outcomes (Cheraghi, Poorolajal, Hashem, Esmailnasab, & Doosti Irani, 2012; de Azambuja et al., 2010; Ladoire et al., 2014; Protani, Coory, & Martin, 2010). For example, Ladoire et al. (2014) suggested that obesity has no impact on breast cancer prognosis when modern adjuvant chemotherapy, at the appropriate dose intensity, is delivered. In contrast, a meta-analysis by Protani et al. (2010) showed poorer survival among obese compared with non-obese women with breast cancer, which was similar for overall and breast cancer– specific survival.
Conclusions
Breast cancer is the most commonly diagnosed cancer, excluding skin cancer, in women in the United States (Siegel, Miller, & Jemal, 2015). Improvements in early detection and treatment have resulted in significant survival gains, with the current five-year survival rate reported to be 91% (Siegel et al., 2015). Ongoing chronic illness management across major diseases is warranted; however, few studies have been designed to address this. Based on the known substantial incidence of CVD in women, which is typically underdiagnosed, and the potential for cardiovascular-related adverse effects of breast cancer therapy, evaluating CVD risk factors in women with breast cancer is particularly important. Data suggest that women with early-stage breast cancer are more likely to die of CVD than recurrent cancer (Schairer, Mink, Carroll, & Devesa, 2004). Therefore, prevention and optimal management of CVD risk factors prior to and after receiving breast cancer treatment are of paramount importance.
An important limitation in the current study was the lack of data on African American women, which was consistent with the ethnic composition of the community studied. In addition, the authors found that their retrospective electronic health record review failed to comprehensively capture CVD risk factor profiles in women with breast cancer because of missing pertinent data. For example, CRP reports were documented in only three patients. However, the importance of inflammation and cancer has only been emphasized in recent years and, therefore, a record review conducted at present time and five years from now on follow-up may yield better documented data on inflammation and CVD risk factor in these patients.
Despite the limitations of this study, the authors have obtained evidence suggesting some CVD risk factors, such as fasting blood glucose and HbA1C, may contribute to breast cancer outcomes. Although electronic records were not a feasible source for profiling multiple CVD risk factors in women with breast cancer in this retrospective review, the study provides supportive evidence for the need to perform a prospective study, which has important implication for nursing. A significant implication of this work is that assessment and documentation of emerging CVD risk factors, such as markers of inflammation and blood glucose control, may be critical in the evaluation of patients with breast cancer to ensure optimal care. Better documentation of information related to shared risk factors for CVD and breast cancer should be included in the health records.
The authors gratefully acknowledge Alison T. Stopeck, MD, for her careful review and insightful suggestions regarding this article.
References
Abdelsalam, K.E., Hassan, I.K., & Sadig, I.A. (2012). The role of developing breast cancer in alteration of serum lipid profile. Journal of Research in Medical Sciences, 17, 562–565.
American Diabetes Association. (2010). Standards of medical care in diabetes—2010. Diabetes Care, 33(Suppl. 1), S11–S61. doi:10.2337/dc10-S011
Calip, G.S., Malone, K.E., Gralow, J.R., Stergachis, A., Hubbard, R.A., & Boudreau, D.M. (2014). Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Research and Treatment, 148, 363–377. doi:10.1007/s10549-014-3157-6
Cheraghi, Z., Poorolajal, J., Hashem, T., Esmailnasab, N., & Doosti Irani, A. (2012). Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: A meta-analysis. PLoS One, 7, e51446. doi:10.1371/journal.pone.0051446
Chobanian, A.V., Bakris, G.L., Black, H.R., Cushman, W.C., Green, L.A., & Izzo, J.L., Jr. (2003). The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA, 289, 2560–2572. doi:10.1001/jama.289.19.2560
Colotta, F., Allavena, P., Sica, A., Garlanda, C., & Mantovani, A. (2009). Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis, 30, 1073–1081. doi:10.1093/carcin/bgp127
Coughlin, S.S., Calle, E.E., Teras, L.R., Petrelli, J., & Thun, M.J. (2004). Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. American Journal of Epidemiology, 159, 1160–1167. doi:10.1093/aje/kwh161
de Azambuja, E., McCaskill-Stevens, W., Francis, P., Quinaux, E., Crown, J.P., Vicente, M., . . . Piccart-Gebhart, M. (2010). The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: The experience of the BIG 02–98 trial. Breast Cancer Research and Treatment, 119, 145–153. doi:10.1007/s10549-009-0512-0
Eyre, H., Kahn, R., & Robertson, R.M. (2004). Preventing cancer, cardiovascular disease, and diabetes: A common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. CA: A Cancer Journal for Clinicians, 54, 190–207.
Grundy, S.M., Cleeman, J.I., Daniels, S.R., Donato, K.A., Eckel, R.H., & Franklin, B.A. (2005). Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation, 112, 2735–2752. doi:10.1161/CIRCULATIONAHA.105.169404
Howe, L.R., Subbaramaiah, K., Hudis, C.A., & Dannenberg, A.J. (2013). Molecular pathways: Adipose inflammation as a mediator of obesity-associated cancer. Clinical Cancer Research, 19, 6074–6083. doi:10.1158/1078-0432.CCR-12-2603
Ibrahim, E.M., & Al-Homaidh, A. (2011). Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Medical Oncology, 28, 753–765. doi:10.1007/s12032-010-9536-x
Jee, S.H., Ohrr, H., Sull, J.W., Yun, J.E., Ji, M., & Samet, J.M. (2005). Fasting serum glucose level and cancer risk in Korean men and women. JAMA, 293, 194–202. doi:10.1001/jama.293.2.194
Ladoire, S., Dalban, C., Roche, H., Spielmann, M., Fumoleau, P., Levy, C., . . . Ghiringhelli, F. (2014). Effect of obesity on disease-free and overall survival in node-positive breast cancer patients in a large French population: A pooled analysis of two randomised trials. European Journal of Cancer, 50, 506–516. doi:10.1016/j.ejca.2013.11.013
Larsson, S.C., Mantzoros, C.S., & Wolk, A. (2007). Diabetes mellitus and risk of breast cancer: A meta-analysis. International Journal of Cancer, 121, 856–862. doi:10.1002/ijc.22717
Libby, P., Ridker, P.M., & Hansson, G.K. (2009). Inflammation in atherosclerosis: From pathophysiology to practice. Journal of the American College of Cardiology, 54, 2129–2138. doi:10.1016/j.jacc.2009.09.009
Mantovani, A., Allavena, P., Sica, A., & Balkwill, F. (2008). Cancer-related inflammation. Nature, 454, 436–444. doi:10.1038/nature07205
Miao Jonasson, J., Cederholm, J., Eliasson, B., Zethelius, B., Eeg-Olofsson, K., & Gudbjornsdottir, S. (2012). HbA1C and cancer risk in patients with type 2 diabetes—A nationwide population-based prospective cohort study in Sweden. PlOS ONE, 7, e38784.
National Cancer Institute. (2014). Surveillance, epidemiology, and end results (SEER) Program. Retrieved from http://canques.seer.cancer.gov/cgi-bin/cq_submit?dir=devcan2011&db=1&rp…^4^1^3^57^0^20&dec=2&template=null
National Cholesterol Education Program Expert Panel on Detection and Treatment of High Blood Cholesterol. (2002). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation, 106, 3143–3421.
Protani, M., Coory, M., & Martin, J.H. (2010). Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Research and Treatment, 123, 627–635. doi:10.1007/s10549-010-0990-0
Radford, M.J., Arnold, J.M., Bennett, S.J., Cinquegrani, M.P., Cleland, J.G., Havranek, E.P., . . . Redberg, R.F. (2005). ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Failure Society of America. Circulation, 112, 1888–1916.
Schairer, C., Mink, P.J., Carroll, L., & Devesa, S.S. (2004). Probabilities of death from breast cancer and other causes among female breast cancer patients. Journal of the National Cancer Institute, 96, 1311–1321. doi:10.1093/jnci/djh253
Siegel, R.L., Miller, K.D., & Jemal, A. (2015). Cancer statistics, 2015. CA: A Cancer Journal for Clinicians, 65, 5–29. doi:10.3322/caac.21254
About the Author(s)
Shu-Fen Wung, PhD, RN, ACNP-BC, FAAN, is an associate professor and Joseph T. Hepworth, PhD, is an associate research scientist, both in the College of Nursing at the University of Arizona in Tucson; Danielle Sparenga, MS, RN, ACNP-BC, is an acute care nurse practitioner at Arizona Arrhythmia Consultants in Scottsdale; and Carrie J. Merkle, PhD, RN, FAAN, is an associate professor in the College of Nursing at the University of Arizona. Funding was provided through the ONS Foundation Small Grants program. Wung can be reached at wung@arizona.edu, with copy to editor at ONFEditor@ons.org. (Submitted January 2015. Accepted for publication April 7, 2015.)

