Nursing Roles in Cardiac Safety: Romidepsin in Patients With T-Cell Lymphoma
Purpose/Objectives: To provide information to help nurses mitigate cardiac risks among patients receiving romidepsin (Istodax®), a histone deacetylase (HDAC) inhibitor approved by the U.S. Food and Drug Administration for the treatment of relapsed/refractory cutaneous and peripheral T-cell lymphoma.
Data Sources: Clinical studies of romidepsin represented the primary data sources. Supporting references included class information on HDAC inhibitors, as well as data regarding the impact of electrolyte imbalances and antiemetic treatment on electrocardiogram (ECG) data.
Data Synthesis: Cardiac concerns during treatment with romidepsin are multifactorial. Electrolyte deficiencies, which are associated with ECG abnormalities and dysrhythmias, are common among patients with T-cell lymphoma. In addition, clinically insignificant changes in the corrected QT interval reported with romidepsin are primarily attributable to concomitant use of prophylactic antiemetics and likely exaggerated by transient increases in heart rate.
Conclusions: Data support the cardiac safety of romidepsin while cautioning about the need for nurses’ vigilance regarding consistent electrolyte supplementation, appropriate antiemetic selection, and heart rate monitoring.
Implications for Nursing: By recognizing drug-related and non–drug-related influences on cardiac safety during treatment with romidepsin, as well as other anticancer agents, nurses can identify risks, report them, and recommend appropriate interventions, which, ultimately, facilitates improved patient outcomes.
Jump to a section
Romidepsin (Istodax®) is a novel, potent class 1 selective histone deacetylase (HDAC) inhibitor (Bolden, Peart, & Johnstone, 2006; Bradner et al., 2010; Tan, Cang, Ma, Petrillo, & Liu, 2010). In 2009, the drug received U.S. Food and Drug Administration (FDA) approval for the treatment of cutaneous T-cell lymphoma (CTCL) in patients who have received one or more prior systemic therapy. In 2011, romidepsin was approved for the treatment of peripheral TCL (PTCL) in patients who have received one or more prior therapy (Celgene Corporation, 2014) after pivotal phase II studies exhibited durable disease responses with manageable toxicity (Coiffier et al., 2012; Whittaker et al., 2010). In patients with PTCL, the objective response rate (ORR) was 25% (33/130), including 15% with confirmed/unconfirmed complete response and a median duration of response (DOR) of 28 months (Coiffier et al., 2014). In patients with CTCL, the ORR was 34% (33/96), including 6% with CR and a median DOR of 15 months (Whittaker et al., 2010).
Other HDAC inhibitors have received FDA approval. Vorinostat (Zolinza®) (Merck & Co, Inc., 2013) and belinostat (Beleodaq®) (Spectrum Pharmaceuticals, 2014) are also approved by the FDA for the treatment of relapsed/refractory (R/R) CTCL and PTCL, respectively, whereas panobinostat (Farydak®) is approved by the FDA in combination with bortezomib (Velcade®) and dexamethasone (Baycadron®) for the treatment of patients with multiple myeloma who have received two or more prior regimens (including bortezomib and an immunomodulatory agent) (Novartis Pharmaceuticals Corporation, 2015). In addition, HDAC inhibitors are actively undergoing clinical investigation for a multitude of malignancies (Khan & La Thangue, 2012; New, Olzscha, & La Thangue, 2012). Because of the increased use of HDAC inhibitors, as well as the likelihood of additional indications and more widespread use going forward, identifying and managing potential adverse effects is paramount.
Although the most common drug-related adverse events (AEs) with romidepsin were gastrointestinal or asthenic conditions, which were primarily mild or moderate (Celgene Corporation, 2014; Coiffier et al., 2012; Whittaker et al., 2010), various electrocardiogram (ECG) changes were reported with several HDAC inhibitors. As a result, a class effect on cardiac safety was suggested (Kristeleit, Fong, Aherne, & de Bono, 2005; Marsoni, Damia, & Camboni, 2008; Molife et al., 2007) despite limited systematic corrected QT (QTc) studies of HDAC inhibitors.
Early studies of romidepsin raised concerns about cardiac safety, which were largely resolved with follow-up studies and proper patient management. However, many providers continue to express concern about potential cardiotoxicity. In this article, data for romidepsin will be used to highlight key roles nurses play in minimizing cardiac risks in patients with cancer.
Early Cardiac Data
In a phase I dose-finding study of romidepsin in advanced or refractory cancers (N = 37), reversible grade 1–2 ST/T wave changes and mild reversible dysrhythmias (including asymptomatic atrial bigeminy [with preexisting sinus bradycardia], 3-s sinus pause during sleep, and asymptomatic 5-beat run of ventricular tachycardia) were reported, none of which were considered to be dose limiting or definitely related to romidepsin (Sandor et al., 2002). Above the maximum-tolerated dose (MTD), one patient experienced an episode of atrial fibrillation without recurrence when retreated at the MTD. No clinically significant changes in left ventricular ejection fraction (LVEF) and troponin I or other evidence of myocardial damage were reported (Sandor et al., 2002). In a separate phase I dose-finding study in advanced cancers (N = 33), minor, reversible ECG changes were reported in 10 patients, with no changes in cardiac enzymes or LVEF (Marshall et al., 2002). Because of sudden death occurring in a few patients across several subsequent early clinical studies, concerns of cardiac safety were heightened. However, each patient was found to have comorbidities that were independent risk factors for sudden death, including severe valve pathology, severe atherosclerotic heart disease, sarcoidosis, and uncontrolled hypertension (Noonan et al., 2013; Piekarz et al., 2006, 2009; Shah et al., 2006; Stadler, Margolin, Ferber, McCulloch, & Thompson, 2006). Because of nausea and vomiting, prophylactic antiemetics were routinely given beginning at romidepsin 3.5 mg/m2, the same dose at which QTc changes were first observed.
QT Prolongation
The QT interval is the time from the start of the Q wave to the end of the T wave on an ECG tracing; QT represents the time taken for ventricular depolarization and repolarization. By definition, the QT interval shortens at faster heart rates and lengthens at slower heart rates; QTc uses one of several formulas developed to estimate the QT interval at a heart rate of 60 beats per minute (bpm), allowing for comparison of QT values at different heart rates. QTc prolongation is associated with an increased risk of arrhythmias, including a life-threatening polymorphic ventricular tachycardia termed torsades de pointes (Roden, 1997). Symptoms associated with QTc prolongation emerge as a result of the related arrhythmias and may include syncope (can occur while awake or asleep, resulting in noisy gasping during sleep), seizures, or sudden cardiac arrest (Mayo Clinic Staff, 2015).
Abnormally prolonged QTc can be congenital (congenital long QT syndrome [Romano-Ward syndrome] and Jervell and Lange-Nielsen syndrome) (Mizusawa, Horie, & Wilde, 2014) or acquired. In the third National Health and Nutrition Examination Survey (NHANES), increased age, female gender, hypokalemia, a history of thyroid disease, hypertension, and myocardial infarction were independently associated with prolonged QTc in U.S. adults aged older than 40 years (Benoit, Mendelsohn, Nourjah, Staffa, & Graham, 2005). Hypomagnesemia was not measured in NHANES; however, along with hypokalemia, hypomagnesemia has been shown to be associated with ECG abnormalities (El-Sherif & Turitto, 2011; Napolitano, Priori, & Schwartz, 1994; Piekarz et al., 2006).
In NHANES, receiving a known QT-prolonging medication in the previous month was associated with a greater than two-fold increase in the odds of QTc prolongation (Benoit et al., 2005). Many medications are known to prolong QTc, including but not limited to commonly used concomitant medication types, such as antibiotics (e.g., azithromycin [Zithromax®], ciprofloxacin [Cipro®], erythromycin [Ilotycin®], levofloxacin [Levaquin®], and moxifloxacin [Vigamox®]); antidepressants (e.g., citalopram [Celexa®], escitalopram [Lexapro®]); antiemetics (e.g., ondansetron [Zofran®]); antifungals (e.g., fluconazole [Diflucan®], pentamidine [NebuPent®]); antipsychotics (e.g., chlorpromazine [Thorazine®], droperidol [Inapsine®], haloperidol [Haldol®], pimozide [Orap®], thioridazine [Mellaril®]); and opiates (e.g., methadone [Methadose®]). A frequently updated list can be found at www.crediblemeds.org.
Of particular relevance to romidepsin are ECG changes caused by antiemetics, particularly given that prophylactic antiemetics are required with each romidepsin dose to prevent nausea and vomiting (Celgene Corporation, 2014; Iyer & Foss, 2015). Preferred antiemetics are not provided in the prescribing information, and selection is made by individual providers or set by institutional standards. Serotonin 5-hydroxytryptamine 3 (5-HT3) receptor antagonists have been shown to be efficacious (Jin et al., 2013; Rawlinson et al., 2012; Salvo et al., 2012; Tang & Malone, 2012) and are the most common antiemetics used in oncology practice (Brygger & Herrstedt, 2014). However, 5-HT3 receptor antagonists, particularly ondansetron, are also associated with changes in ECG parameters, including QTc intervals (Brygger & Herrstedt, 2014; Keefe, 2002; Navari & Koeller, 2003). As a result of cardiac AEs secondary to ondansetron, the FDA (2016) withdrew the approval of high-dose ondansetron (32 mg). Granisetron (Kytil®) may have less of an impact on QT, and the next-generation agent palonosetron (Aloxi®) has been shown to not significantly increase QT (Gonullu, Demircan, Demirag, Erdem, & Yucel, 2012; Keefe, 2002; Yavas, Dogan, Yavas, Araz, & Ata, 2012). In addition, oral administration of 5-HT3 antiemetics (if available) should be preferred to IV dosing because of lower peak concentrations (Brygger & Herrstedt, 2014). For nurses managing patients on romidepsin, awareness of issues relating to concomitant medications is important. Concomitant medications can affect safety (including cardiac safety) and efficacy of agents, so a thorough review with the treating physician is crucial. Exclusion or stopping of select concomitant medications or additional monitoring for patients receiving certain concomitant medications may be required (see Table 1).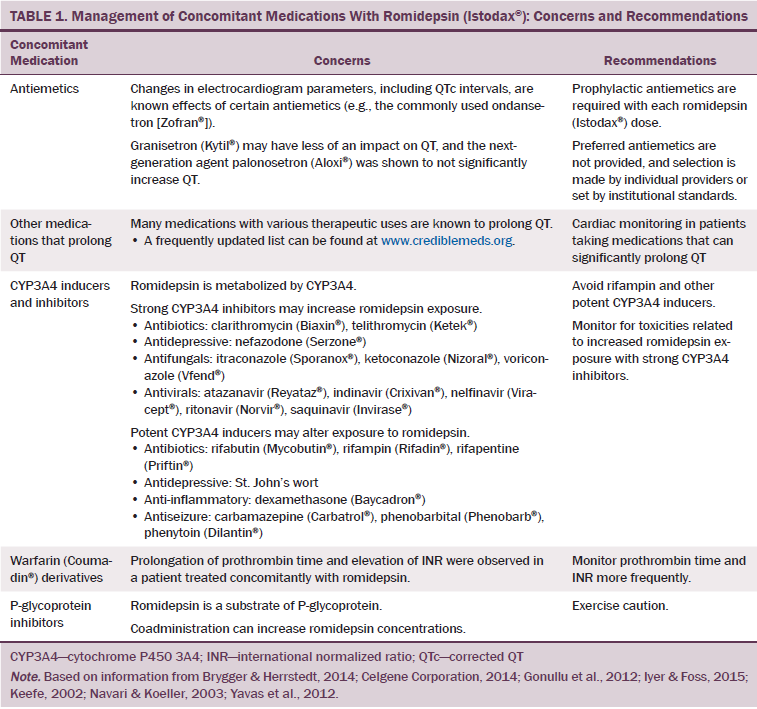
As a result of early concerns, patients with significant cardiac abnormalities were excluded from phase II trials, and more rigorous cardiac monitoring was incorporated (Coiffier et al., 2012; Piekarz et al., 2006; Whittaker et al., 2010). Protocols also required that potassium and magnesium be maintained in the high to normal range because of the association of hypokalemia and hypomagnesemia with ECG abnormalities (El-Sherif & Turitto, 2011; Napolitano et al., 1994; Piekarz et al., 2006). Patients receiving drugs that can significantly prolong QTc or inhibit cytochrome P450 3A4 (CYP3A4), which may increase romidepsin exposure (Celgene Corporation, 2014), were also excluded from phase II studies. Because these precautions were implemented, emerging data have suggested no additional cardiac safety concerns. However, as a postmarketing requirement, the FDA requested additional evaluation of the potential for romidepsin to prolong QTc.
Cardiac Data Reported in Phase II Studies
As a result of clinical activity of romidepsin in patients with PTCL and CTCL in phase I studies and determination of the MTD, several phase II studies were initiated. Table 2 lists the romidepsin dosing and ECG assessments in key studies. In the pivotal study in R/R PTCL (N = 131), no clinically significant changes in QTc were observed when assessed during the first four treatment cycles (Coiffier et al., 2012). ECG abnormalities were reported as AEs in eight patients. Three patients had mild to moderate (grade 1–2) QTc prolongation, and only one patient had severe (grade 3) QTc prolongation. None of the patients had concurrent symptoms of syncope or other cardiac AEs. 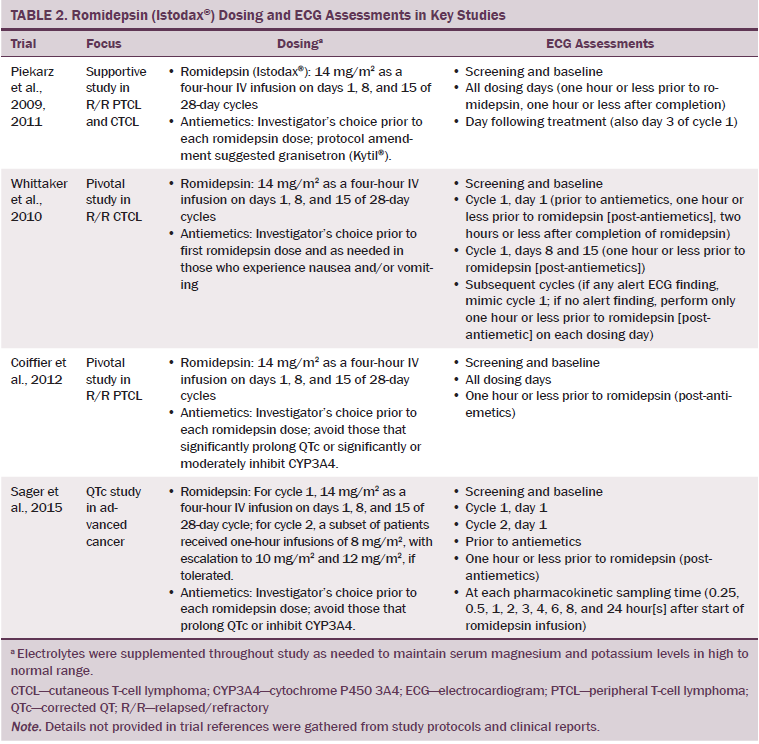
In the pivotal study in R/R CTCL (N = 96), no Fridericia’s QTc (QTcF) greater than 480 ms or change from baseline of greater than 60 ms was reported (Whittaker et al., 2010). Prolonged QTc was reported as an AE in two patients. ECG changes returned to baseline within 24 hours and were not symptomatic or associated with changes in cardiac function. In the CTCL study, QTcF was also measured at preantiemetic and postantiemetic/pre-romidepsin baselines because certain antiemetics (including the commonly used ondansetron) are known to prolong the QTc interval (Brygger & Herrstedt, 2014; Keefe, 2002; Navari & Koeller, 2003). At two hours post-romidepsin, the mean changes in QTcF were 4.6 ms from preanti-emetic baseline and 1.3 ms from postantiemetic/pre-romidepsin baseline, indicating a contribution of antiemetics to changes in QTcF.
FDA approvals were supported by a National Cancer Institute (NCI) study in patients with R/R PTCL (n = 47) and CTCL (n = 71) (Piekarz et al., 2009, 2011). In this study, asymptomatic low-grade ECG T-wave or ST changes were reported during cycle 1 of treatment in patients with CTCL (grade 1–2 T-wave or ST changes = 71%/9%; grade 1 QTc prolongation = 9%) and PTCL (grade 1–2 T-wave changes = 53%/11%). No ECG changes greater than or equal to grade 3 were reported. However, in this study, any ECG change was reported as an AE, regardless of clinical significance, leading to elevated AE rates compared to other studies. Patients who received romidepsin therapy for 18 months or longer did not have evidence of cumulative cardiac toxicity (Piekarz et al., 2011), and intensive cardiac monitoring of the first 42 patients in the study showed no association with myocardial damage, no impaired cardiac function, and no significant changes in LVEF (Piekarz et al., 2006). The median change in QTc was 14.4 ms, and all 42 patients had premedication with the antiemetic ondansetron. No cases of treatment-related sustained or systematic arrhythmia were reported. A later analysis including more patients (n for PTCL = 47; n for CTCL = 84) examined changes in heart rates and supported the need for electrolyte replacement in patients with TCL (Noonan et al., 2013). In that analysis, heart rate increased by a mean of 11 bpm following romidepsin, with no evidence of increased dysrhythmia. Replacement of potassium and/or magnesium was required prior to 55% of romidepsin doses, and only 10 patients (8%) never required electrolyte supplementation during the trial.
Maintaining Electrolytes
Nurses caring for patients on romidepsin should be aware of the importance of maintaining electrolytes in the high to normal range throughout treatment. For routine monitoring, including the measurement of magnesium in the comprehensive metabolic panel is important. Although cutoffs for low potassium and magnesium can vary and are set by institutional standards, triggers for supplementation in the romidepsin clinical studies ranged from 3.5–4 mmol/L for potassium and from 0.8–0.85 mmol/L for magnesium. Retesting potassium and magnesium levels of patients after supplementation to ensure that they have reached target levels should be considered. In the romidepsin clinical trials, patient electrolyte levels were retested after supplementation and prior to administration of romidepsin.
Electrolyte imbalances are common in patients with TCL and other malignancies. In addition to the need for electrolyte supplementation that was observed in the supportive NCI study of romidepsin in R/R PTCL and CTCL, a retrospective case series of patients with CTCL also showed that most patients had low levels of magnesium and/or calcium (Morgan, Maloney, & Duvic, 2002; Noonan et al., 2013). Low electrolytes are associated with ECG abnormalities and are known risk factors for cardiac arrhythmia and sudden cardiac death (Del Gobbo et al., 2013; El-Sherif & Turitto, 2011; Osadchii, 2010; Peacock et al., 2010; Santoro et al., 2008). Symptoms of low potassium and/or magnesium are common in patients with cancer and may include weakness or fatigue, muscle cramps, constipation, loss of appetite, nausea, and vomiting.
Postmarketing Cardiac Study
For the postmarketing cardiac study, data were gathered from a phase I bioavailability study in patients with advanced malignancies (Sager et al., 2015). During cycle 1 of treatment, patients received romidepsin at the approved dose (14 mg/m2 as four-hour infusions). The protocol excluded patients with significant cardiac abnormalities and asked for avoidance of concomitant medications that prolong QTc or inhibit CYP3A4; of 26 evaluable patients, 18 had a history of ongoing cardiovascular abnormalities and 18 received antiemetic premedication with 24 mg ondansetron. Triplicate ECGs were done at preantiemetic and postantiemetic/pre-romidepsin baselines, with the mean QTcF increasing 9.7 ms, consistent with known effects of certain antiemetics (including ondansetron) on the QTc interval (Brygger & Herrstedt, 2014; Keefe, 2002; Navari & Koeller, 2003).
Although use of the preantiemetic baseline ECGs exaggerates the QTc effects of romidepsin, it is clinically relevant because romidepsin is routinely given with antiemetics. The maximal mean change in QTcF from the preantiemetic baseline was 10.1 ms (upper bound of two-sided 90% confidence interval [CI], 14.5 ms), which was below the 20 ms threshold for meaningful clinical relevance for patients receiving nonadjuvant cancer treatment (Sarapa & Britto, 2008). Use of the upper bound of a two-sided 90% CI is equivalent to the upper bound of a one-sided 95% CI, which is consistent with FDA (2005) guidelines (Sarapa & Britto, 2008).
As expected, QTcF was somewhat dependent on heart rate (particularly at more rapid heart rates) because the Fridericia formula may result in overcorrection and inflation of QTcF increases independent of repolarization in the setting of considerable heart rate increases (Sager, 2008). Mean heart rate began to increase at the 2-hour time point, was at its maximum at the 6-hour time point (increase of about 20 bpm from both baselines), and returned near baseline at the 24-hour time point (Sager et al., 2015).
During cycle 2 of the study, 14 of the patients received romidepsin 8, 10, or 12 mg/m2 as one-hour infusions, resulting in supratherapeutic romidepsin concentrations (median maximum concentration 1.4-, 1.9-, and 2.7-fold higher than with the approved dose, respectively). Changes in QTcF did not show trends across dose levels, with maximal mean changes in QTcF from the postantiemetic/pre-romidepsin baseline of 7.6 ms, 3.1 ms, and 4.5 ms for 8, 10, and 12 mg/m2, respectively (only 2 of 14 patients had assessments at the preantiemetic baseline). Changes in heart rate with supratherapeutic dosing were similar to those with approved dosing, with maximal mean changes of 19.2 ms, 16.5 ms, and 18.2 ms for 8, 10, and 12 mg/m2, respectively. Only one patient in the study had a change in QTcF greater than 60 ms from the preantiemetic baseline (none from postantiemetic/pre-romidepsin baseline), and no patients had an absolute QTcF value greater than 450 ms. Data showed that supratherapeutic romidepsin concentrations did lead to increased changes in QTc (Sager et al., 2015).
Balancing Risks and Benefits
Cardiovascular complications with effective cancer therapies aimed at improving survival and quality of life can profoundly affect patient health. These complications are more common among patients with underlying cardiac conditions, which are frequent in older adult patients with cancer. However, excessive concern regarding potential cardiac toxicity may result in undertreatment of patients and poor oncologic outcomes. The threshold for regulatory concern for increased QTc is greater than 10 ms upper bound of the 90% CI, which correlates with negligible risk of drug-induced proarrythmia (FDA, 2005). This threshold is not appropriate for risk–benefit assessment of oncology agents, which may provide life-saving benefits. A risk–benefit threshold for meaningful clinical relevance of 20 ms for change in QTc in patients receiving nonadjuvant cancer treatment has been proposed (Sarapa & Britto, 2008). In the postmarketing romidepsin study, the maximal mean change in QTcF from the preantiemetic baseline was 10.1 ms (90% upper CI, 14.5 ms) at 0.25 hour following initiation of romidepsin.
Implications for Nursing
Although the data as a whole demonstrate the cardiac safety of romidepsin, risk factors must be managed properly by nurses to ensure that patients realize maximum benefit from treatment. Concomitant medications, including antiemetics and other medications known to prolong QTc, as well as medications that can potentially alter exposure to romidepsin, should be appropriately evaluated for risk–benefit. Electrolytes should be monitored routinely and maintained within the high to normal range. Retesting may be needed after electrolyte supplementation and before administration of romidepsin to ensure that electrolyte levels have reached target levels.
For patients with existing cardiac issues, drug selection, as well as optimization of the clinical picture, are important to maximize cardiac safety. Romidepsin is associated with transient heart rate increases, which should be taken into consideration during patient selection (Celgene Corporation, 2014). With romidepsin use, appropriate cardiac monitoring, including baseline and periodic ECGs, should be considered in patients with congenital long QT syndrome and a history of significant cardiovascular disease, as well as in those receiving concomitant medications that can significantly prolong QT (Celgene Corporation, 2014). Nurses can facilitate improved patient outcomes by recognizing factors that influence cardiac safety during treatment with romidepsin, identifying risks and reporting them, and recommending appropriate interventions.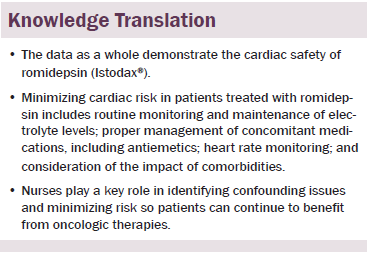
Conclusion
Romidepsin is not associated with clinically meaningful changes in QTc or other ECG abnormalities and does not cause myocardial damage or impair cardiac function. Treatment-emergent ECG changes are exaggerated by transient increases in heart rate and administration of QT-prolonging antiemetics. As a whole, data have demonstrated the cardiac safety of romidepsin. However, other factors within the overall clinical situation can lead to cardiac concerns if not properly managed.
With romidepsin, minimizing cardiac risk includes routinely assessing electrolytes to maintain normal serum potassium and magnesium levels, using caution when administering antiemetics or other medications associated with ECG abnormalities, carefully monitoring all concomitant medications along with heart rate, and considering the impact of patient comorbidities. Although the data presented in this article are specific to romidepsin, many of these issues are common for a multitude of patients with cancer. Nurses and other support staff play crucial roles in identifying confounding issues specific to patients and treatments, which, ultimately, minimizes risk and enables patients to derive maximum benefit from oncologic therapies.
References
Benoit, S.R., Mendelsohn, A.B., Nourjah, P., Staffa, J.A., & Graham, D.J. (2005). Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. European Journal of Cardiovascular Prevention and Rehabilitation, 12, 363–368. doi:10.1097/01.hjr.0000173110.21851.a9
Bolden, J.E., Peart, M.J., & Johnstone, R.W. (2006). Anticancer activities of histone deacetylase inhibitors. Nature Reviews Drug Discovery, 5, 769–784. doi:10.1038/nrd2133
Bradner, J.E., West, N., Grachan, M.L., Greenberg, E.F., Haggarty, S.J., Warnow, T., & Mazitschek, R. (2010). Chemical phylogenetics of histone deacetylases. Nature Chemical Biology, 6, 238–243. doi:10.1038/nchembio.313
Brygger, L., & Herrstedt, J. (2014). 5-Hydroxytryptamine3 receptor antagonists and cardiac side effects. Expert Opinion on Drug Safety, 13, 1407–1422. doi:10.1517/14740338.2014.954546
Celgene Corporation. (2014). Istodax® (romidepsin) [Package insert]. Summit, NJ: Author.
Coiffier, B., Pro, B., Prince, H.M., Foss, F., Sokol, L., Greenwood, M., . . . Horwitz, S. (2012). Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. Journal of Clinical Oncology, 30, 631–636. doi:10.1200/JCO.2011.37.4223
Coiffier, B., Pro, B., Prince, H.M., Foss, F., Sokol, L., Greenwood, M., . . . Horwitz, S. (2014). Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: Pivotal study update demonstrates durable responses. Journal of Hematology and Oncology, 7, 11. doi:10.1186/1756-8722-7-11
Del Gobbo, L.C., Imamura, F., Wu, J.H., de Oliveira Otto, M.C., Chiuve, S.E., & Mozaffarian, D. (2013). Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. American Journal of Clinical Nutrition, 98, 160–173. doi:10.3945/ajcn.112.053132
El-Sherif, N., & Turitto, G. (2011). Electrolyte disorders and arrhythmogenesis. Cardiology Journal, 18, 233–245.
Gonullu, G., Demircan, S., Demirag, M.K., Erdem, D., & Yucel, I. (2012). Electrocardiographic findings of palonosetron in cancer patients. Supportive Care in Cancer, 20, 1435–1439. doi:10.1007/s00520-011-1226-5
Iyer, S.P., & Foss, F.F. (2015). Romidepsin for the treatment of peripheral T-cell lymphoma. Oncologist, 20, 1084–1091. doi:10.1634/theoncologist.2015-0043
Jin, Y., Sun, W., Gu, D., Yang, J., Xu, Z., & Chen, J. (2013). Comparative efficacy and safety of palonosetron with the first 5-HT3 receptor antagonists for the chemotherapy-induced nausea and vomiting: A meta-analysis. European Journal of Cancer Care, 22, 41–50. doi:10.1111/j.1365-2354.2012.01353.x
Keefe, D.L. (2002). The cardiotoxic potential of the 5-HT(3) receptor antagonist antiemetics: Is there cause for concern? Oncologist, 7, 65–72. doi:10.1634/theoncologist.7-1-65
Khan, O., & La Thangue, N.B. (2012). HDAC inhibitors in cancer biology: Emerging mechanisms and clinical applications. Immunology and Cell Biology, 90, 85–94. doi:10.1038/icb.2011.100
Kristeleit, R., Fong, P., Aherne, G.W., & de Bono, J. (2005). Histone deacetylase inhibitors: Emerging anticancer therapeutic agents? Clinical Lung Cancer, 7(Suppl. 1), S19–S30. doi:10.3816/clc.2005.s.004
Marshall, J.L., Rizvi, N., Kauh, J., Dahut, W., Figuera, M., Kang, M.H., . . . Hawkins, M.J. (2002). A phase I trial of depsipeptide (FR901228) in patients with advanced cancer. Journal of Experimental Therapeutics and Oncology, 2, 325–332. doi:10.1046/j.1359-4117.2002.01039.x
Marsoni, S., Damia, G., & Camboni, G. (2008). A work in progress: The clinical development of histone deacetylase inhibitors. Epigenetics, 3, 164–171. doi:10.4161/epi.3.3.6253
Mayo Clinic Staff. (2015). Long QT syndrome: Symptoms. Retrieved from http://www.mayoclinic.org/diseases-conditions/long-qt-syndrome/basics/s…
Merck & Co, Inc. (2013). Zolinza® (vorinostat) [Package insert]. Whitehouse Station, NJ: Author.
Mizusawa, Y., Horie, M., & Wilde, A.A. (2014). Genetic and clinical advances in congenital long QT syndrome. Circulation Journal, 78, 2827–2833. doi:10.1253/circj.cj-14-0905
Molife, R., Fong, P., Scurr, M., Judson, I., Kaye, S., & de Bono, J. (2007). HDAC inhibitors and cardiac safety. Clinical Cancer Research, 13, 1068. doi:10.1158/1078-0432.ccr-06-2380
Morgan, M., Maloney, D., & Duvic, M. (2002). Hypomagnesemia and hypocalcemia in mycosis fungoides: A retrospective case series. Leukemia and Lymphoma, 43, 1297–1302. doi:10.1080/10428190290026367
Napolitano, C., Priori, S.G., & Schwartz, P.J. (1994). Torsade de pointes. Mechanisms and management. Drugs, 47, 51–65. doi:10.2165/00003495-199447010-00004
Navari, R.M., & Koeller, J.M. (2003). Electrocardiographic and cardiovascular effects of the 5-hydroxytryptamine3 receptor antagonists. Annals of Pharmacotherapy, 37, 1276–1286. doi:10.1345/aph.1c510
New, M., Olzscha, H., & La Thangue, N.B. (2012). HDAC inhibitor-based therapies: Can we interpret the code? Molecular Oncology, 6, 637–656. doi:10.1016/j.molonc.2012.09.003
Noonan, A.M., Eisch, R.A., Liewehr, D.J., Sissung, T.M., Venzon, D.J., Flagg, T.P., . . . Bates, S.E. (2013). Electrocardiographic studies of romidepsin demonstrate its safety and identify a potential role for KATP channel. Clinical Cancer Research, 19, 3095–3104. doi:10.1158/1078-0432.CCR-13-0109
Novartis Pharmaceuticals Corporation. (2015). Farydak® (panobinostat) [Package insert]. East Hanover, NJ: Author.
Osadchii, O.E. (2010). Mechanisms of hypokalemia-induced ventricular arrhythmogenicity. Fundamental and Clinical Pharmacology, 24, 547–559. doi:10.1111/j.1472-8206.2010.00835.x
Peacock, J.M., Ohira, T., Post, W., Sotoodehnia, N., Rosamond, W., & Folsom, A.R. (2010). Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) study. American Heart Journal, 160, 464–470. doi:10.1016/j.ahj.2010.06.012
Piekarz, R.L., Frye, A.R., Wright, J.J., Steinberg, S.M., Liewehr, D.J., Rosing, D.R., . . . Bates, S.E. (2006). Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clinical Cancer Research, 12, 3762–3773. doi:10.1158/1078-0432.ccr-05-2095
Piekarz, R.L., Frye, R., Prince, H.M., Kirschbaum, M.H., Zain, J., Allen, S.L., . . . Bates, S.E. (2011). Phase II trial of romidepsin in patients with peripheral T-cell lymphoma. Blood, 117, 5827–5834. doi:10.1182/blood-2010-10-312603
Piekarz, R.L., Frye, R., Turner, M., Wright, J.J., Allen, S.L., Kirschbaum, M.H., . . . Bates, S.E. (2009). Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. Journal of Clinical Oncology, 27, 5410–5417. doi:10.1200/JCO.2008.21.6150
Rawlinson, A., Kitchingham, N., Hart, C., McMahon, G., Ong, S.L., & Khanna, A. (2012). Mechanisms of reducing postoperative pain, nausea and vomiting: A systematic review of current techniques. Evidence-Based Medicine, 17, 75–80. doi:10.1136/ebmed-2011-100265
Roden, D.M. (1997). A practical approach to torsade de pointes. Clinical Cardiology, 20, 285–290. doi:10.1002/clc.4960200318
Sager, P.T. (2008). Key clinical considerations for demonstrating the utility of preclinical models to predict clinical drug-induced torsades de pointes. British Journal of Pharmacology, 154, 1544–1549. doi:10.1038/bjp.2008.222
Sager, P.T., Balser, B., Wolfson, J., Nichols, J., Pilot, R., Jones, S., & Burris, H.A. (2015). Electrocardiographic effects of class 1 selective histone deacetylase inhibitor romidepsin. Cancer Medicine, 4, 1178–1185. doi:10.1002/cam4.467
Salvo, N., Doble, B., Khan, L., Amirthevasar, G., Dennis, K., Pasetka, M., . . . Chow, E. (2012). Prophylaxis of radiation-induced nausea and vomiting using 5-hydroxytryptamine-3 serotonin receptor antagonists: A systematic review of randomized trials. International Journal of Radiation Oncology, Biology, Physics, 82, 408–417. doi:10.1016/j.ijrobp.2010.08.060
Sandor, V., Bakke, S., Robey, R.W., Kang, M.H., Blagosklonny, M.V., Bender, J., . . . Bates, S.E. (2002). Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clinical Cancer Research, 8, 718–728.
Santoro, A., Mancini, E., London, G., Mercadal, L., Fessy, H., Perrone, B., . . . Cavalcanti, S. (2008). Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrology, Dialysis, Transplantation, 23, 1415–1421. doi:10.1093/ndt/gfm730
Sarapa, N., & Britto, M.R. (2008). Challenges of characterizing proarrhythmic risk due to QTc prolongation induced by nonadjuvant anticancer agents. Expert Opinion on Drug Safety, 7, 305–318. doi:10.1517/14740338.7.3.305
Shah, M.H., Binkley, P., Chan, K., Xiao, J., Arbogast, D., Collamore, M., . . . Grever, M. (2006). Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clinical Cancer Research, 12, 3997–4003. doi:10.1158/1078-0432.ccr-05-2689
Spectrum Pharmaceuticals. (2014). Beleodaq® (belinostat) [Package insert]. Irvine, CA: Author.
Stadler, W.M., Margolin, K., Ferber, S., McCulloch, W., & Thompson, J.A. (2006). A phase II study of depsipeptide in refractory metastatic renal cell cancer. Clinical Genitourinary Cancer, 5, 57–60. doi:10.3816/cgc.2006.n.018
Tan, J., Cang, S., Ma, Y., Petrillo, R.L., & Liu, D. (2010). Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. Journal of Hematology and Oncology, 3, 5. doi:10.1186/1756-8722-3-5
Tang, D.H., & Malone, D.C. (2012). A network meta-analysis on the efficacy of serotonin type 3 receptor antagonists used in adults during the first 24 hours for postoperative nausea and vomiting prophylaxis. Clinical Therapeutics, 34, 282–294. doi:10.1016/j.clinthera.2012.01.007
U.S. Food and Drug Administration. (2005). Guidance for industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Retrieved from http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformat…
U.S. Food and Drug Administration. (2016). FDA drug safety communication: Updated information on 32 mg intravenous ondansetron (Zofran) dose and pre-mixed ondansetron products. Retrieved from http://www.fda.gov/Drugs/DrugSafety/ucm330049.htm
Whittaker, S.J., Demierre, M.F., Kim, E.J., Rook, A.H., Lerner, A., Duvic, M., . . . Kim, Y.H. (2010). Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. Journal of Clinical Oncology, 28, 4485–4491. doi:10.1200/JCO.2010.28.9066
Yavas, C., Dogan, U., Yavas, G., Araz, M., & Ata, O.Y. (2012). Acute effect of palonosetron on electrocardiographic parameters in cancer patients: A prospective study. Supportive Care in Cancer, 20, 2343–2347. doi:10.1007/s00520-011-1348-9
About the Author(s)
Hronek is a nurse practitioner and team lead, and Lehner Reed is a nurse practitioner, both in the Sarah Cannon Research Institute at Tennessee Oncology in Nashville. Medical editorial assistance was provided by Stacey Rose, PhD, and William Ho, PhD, through support from Celgene Corporation. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society. Hronek and Lehner Reed both contributed to the conception of the work, analysis, data intepretation, and manuscript preparation.Hronek can be reached at jhronek@tnonc.com, with copy to editor at ONFEditor@ons.org. Submitted October 2015. Accepted for publication December 16, 2015.

