The Effects of Nonpharmacologic Interventions on Cognitive Function in Patients With Cancer: A Meta-Analysis
Problem Identification: To evaluate the effects of nonpharmacologic interventions on cognitive functioning in adult patients with cancer.
Literature Search: EMBASE, MEDLINE®, Cochrane Library CENTRAL, CINAHL®, and Korean databases.
Data Evaluation: Cochrane’s risk of bias for randomized studies and the RevMan, version 5.3, program of the Cochrane Library were used.
Synthesis: Fourteen controlled trials with a total of 977 participants met the inclusion criteria. Overall, nonpharmacologic interventions had beneficial effects on subjective cognitive functioning and memory, but not on attention, executive functioning, and verbal ability. In the subgroup analyses by approach type, psychological interventions had a significant effect on perceived cognitive function.
Conclusions: The findings suggest that nonpharmacologic interventions, particularly psychological interventions, may have a positive impact on perceived cognitive functioning and memory in patients with cancer. Additional research with adequate power is required to determine the effectiveness of behavioral intervention as a cognitive rehabilitation strategy.
Implications for Practice: Cognitive function would be most improved in patients with cancer when a multimodal intervention approach (education, retraining, and physical activity) is employed.
Jump to a section
Cognitive impairment is one of the most frequently reported adverse effects of chemotherapy (Hutchinson, Hosking, Kichenadasse, Mattiske, & Wilson, 2012). Data suggest that 15%–45% of patients who undergo chemotherapy experience loss of memory and attention problems, are unable to concentrate or multitask, or lose cognitive control, which negatively affects their quality of life (Hermelink et al., 2008; Matsuda et al., 2005; Schagen et al., 1999; Vardy & Tannock, 2007; Wefel & Schagen, 2012). Neuroimaging studies have shown reduced gray and white matter volume in multiple brain sites following chemotherapy, including the prefrontal, hippocampal, and parahippocampal areas (de Ruiter et al., 2011).
An increase in the awareness of chemotherapy-related cognitive impairments is reflected by the growth in the number of review articles focused on the structural and functional concomitants of chemotherapy in the human brain (Kaiser, Bledowski, & Dietrich, 2014). Five meta-analyses have suggested that cancer treatments are associated with cognitive deficits in patients compared with population norms and controls. These deficits are primarily in executive functioning, verbal ability, and visuospatial ability, albeit with a relatively small to medium effect size in each of these domains (Falleti, Sanfilippo, Maruff, Weih, & Phillips, 2005; Jansen, Miaskowski, Dodd, Dowling, & Kramer, 2005; Jim et al., 2012; Prabhu et al., 2014; Stewart, Bielajew, Collins, Parkinson, & Tomiak, 2006).
Two approaches to treat chemotherapy-related cognitive impairments have been evaluated in clinical trials: pharmacologic interventions and (neuro)psychological interventions, the latter of which refers to cognitive training programs aimed at either treating the cognitive deficits or providing education on how to manage them (Gehring, Roukema, & Sitskoorn, 2012). Cognitive training can be divided into the two most frequently used approaches, strategy training and retraining. Strategy training teaches patients to apply coping strategies to their cognitive impairment, which helps patients to focus on their rehabilitation by adjusting the certain environment (minimizing the distraction) and, therefore, be able to anticipate getting better. Retraining instructs patients to repetitively practice the same exercises (e.g., stimulation) to restore attention, memory, and executive functioning. Increasingly, retraining is being administered by computer-based programs (Gehring et al., 2012).
Although a growing body of literature exists on interventions to manage cognitive deficits, only two systematic reviews have investigated interventions for managing cognitive deficits in patients with cancer (Gehring et al., 2012; Gehring, Sitskoorn, Aaronson, & Taphoorn, 2008). These reviews reveal that cognitive training may improve self-reported cognitive symptoms. Cognitive training may be a more attractive option than pharmacologic treatments because it is less invasive. Several studies have found that nonpharmacologic interventions improve attention (Cimprich, 1993; Cimprich & Ronis, 2003; Gehring et al., 2009; Goedendorp, Knoop, Gielissen, Verhagen, & Bleijenberg, 2014; Oh et al., 2010; Von Ah et al., 2012) and increase well-being in patients with cancer (Locke et al., 2008; Milbury et al., 2013; Oh et al., 2010). However, other studies have not found an effect of nonpharmacologic interventions on cognitive function (Cherrier et al., 2013; Goedendorp et al., 2014; Kesler et al., 2013; Milbury et al., 2013; Poppelreuter, Weis, & Bartsch, 2009).
Meta-analysis is a recognized method for synthesizing results of controlled trials to estimate the overall effect size of an intervention and an ideal technique to help reconcile these conflicting data (Sheinfeld Gorin et al., 2012). Therefore, the goal of this study was to conduct a meta-analysis that examines cognitive function changes after nonpharmacologic interventions to determine whether the intervention can affect cognitive functioning in patients with cancer.
Although several previous meta-analyses have examined cognitive functioning in patients treated with chemotherapy, no meta-analysis has been conducted to determine the effectiveness of nonpharmacologic interventions on cognitive function in cancer survivors. Therefore, this review was conducted to identify the best available evidence regarding the effects of nonpharmacologic interventions on cognitive functioning in patients with cancer. Because intervention characteristics such as intervention type were the moderators of intervention efficacy (Faller et al., 2013), the authors have examined the treatment effect by performing subgroup analysis.
Methods
This meta-analysis is reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Liberati et al., 2009). The eligibility criteria are detailed in accordance with the PICOS (Participant, Intervention, Control, Outcome, Study design) framework.
Participants were adults aged 18 years or older who were diagnosed with cancer of any type and any tumor stage. Nonpharmacologic (psychological or behavioral) interventions for the management of cognitive deficits were considered. The authors defined nonpharmacologic interventions as any non-drug intervention administered with the intention of preventing or ameliorating cognitive deficits following chemotherapy. Psychological interventions included (but were not limited to) retraining, education, and compensation strategies. Behavioral interventions designed to prevent or ameliorate chemotherapy-related cognitive deficits also were included. Behavioral interventions included physical exercise and activities, such as walking or gentle movements. Studies that concomitantly administered pharmacologic and nonpharmacologic interventions were excluded. No treatment (e.g., standard care) and attention or placebo control conditions were considered.
The primary outcome was cognitive performance as assessed by neuropsychological tests and self-reported via questionnaires. The authors included general functioning—including mood/psychiatric symptoms, self-reported fatigue, and quality-of-life measurements—as the secondary outcomes. Both randomized, controlled trials (RCTs) and non-RCTs were considered. The authors included studies in which cognitive functioning was measured at baseline and following an intervention at any time point.
Search Strategy
To identify the relevant studies, the authors performed an electronic database search through EMBASE, MEDLINE®, Cochrane Library CENTRAL, CINAHL®, and Korean databases. In addition, the authors searched the Google Scholar® database and reference lists of screened studies. The main keywords used in the search indicated cognitive training intervention, people with cancer, and study design, combined. Searches were inclusive of studies in Korean or English from the earliest publication date available through November 2014.
Study Selection
All studies identified through the electronic searches were downloaded to RefWorks, a reference management database, and duplicates were removed. Two authors independently screened citations of studies, then assessed the full text of eligible citations for inclusion.
Studies were included if they (a) focused on adult patients with cancer (aged 18 years or older), (b) measured cognitive training interventions, (c) measured cognitive function, (d) were controlled trials (RCTs or non-RCTs), and (e) included sufficient data for the calculation of effect sizes between the treatment and control groups for the meta-analysis.
Data Extraction
The following information was extracted from each study based on a predesigned data extraction form: (a) article details (authors, year, country), (b) study design, (c) sample information (age, stage, cancer type), (d) description of the intervention (type, mode of delivery, provider, duration), (e) control conditions, (f) outcomes and instruments, and (g) results of cognitive functioning.
For continuous outcomes, the authors extracted the final value, the standard deviation, and the number of patients assessed at each endpoint for each treatment arm to estimate the mean difference and standard error between the treatment arms.
Risk of Bias Assessment
Studies were assessed for methodologic quality using a seven-item Risk of Bias scale, which was developed by the Cochrane Bias Method Group (Higgins & Green, 2011). In addition, the authors also assessed the monitoring procedures and the use of manuals of the intervention. These are considered to be crucial for the risk of bias assessment in nonpharmacologic intervention studies (Ranchor et al., 2012).
Pilot testing was performed on three studies by two independent reviewers before the independent assessment of study quality for all studies. Disagreements were resolved during meetings between the authors. Studies were assessed in relation to the five sources of bias. These include selection bias, performance bias, attrition bias, detection bias, and reporting bias (see Figure 1). The authors interpreted and reported all bias criteria as having a low, high, or unclear risk of bias. The authors reported an unclear risk of bias when insufficient information was provided or when uncertainty over the potential for bias was present.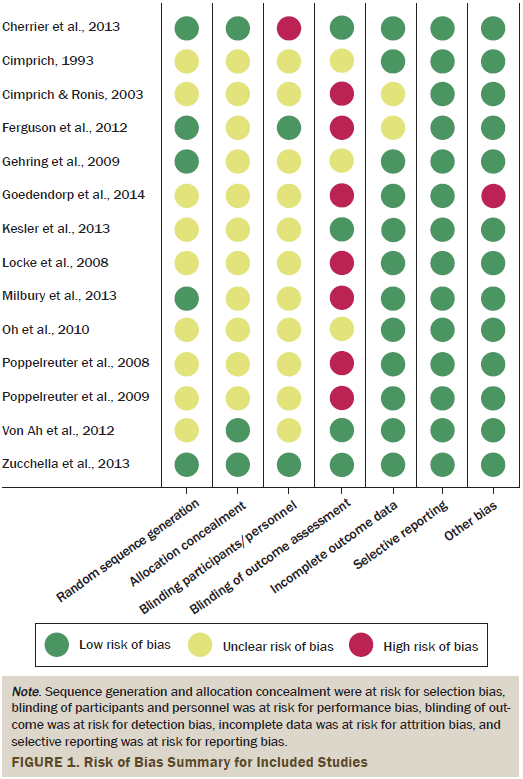
Measures of Treatment Effect
Meta-analyses were performed in RevMan, version 5.3. When calculating the mean effect size, each was weighted by its inverse variance, giving more weight to the studies with larger sample sizes (Higgins & Green, 2011). Random effect models were applied to calculate the effect sizes, which account for variance difference between the studies and participants within studies (Smedslund & Ringdal, 2004). Standardized mean difference greater than zero indicated a significant effect favoring the intervention. Heterogeneity was examined using the I2 statistic, which measured the percentages of total variation across studies determinable to heterogeneity rather than chance. An I2 of 25% indicated low heterogeneity, I2 of 50% was considered moderate, and I2 of 75% was considered high (Higgins & Green, 2011).
The authors used a funnel plot to assess the degree of publication bias. Effect sizes were plotted according to respective standard error, and the symmetry of these plots were evaluated. The authors considered publication bias to exist if there were no small studies without statistically significant effects (Higgins & Green, 2011). Egger’s test was then performed to examine for publication bias (Egger, Smith, Schneider, & Minder, 1997).
Results
After eliminating duplications, 4,360 studies were screened. The authors identified 38 potentially relevant studies from the screening of titles and abstracts and a full review was conducted on those 38 studies. Of the studies, 27 failed to meet the eligibility criteria: 9 studies did not provide sufficient data for the computation of the effect size, 8 studies had nonrelevant interventions, 6 studies were descriptive, and 4 studies had non-relevant outcomes. Three studies were retrieved by a manual search of the reference lists. A total of 14 English-language studies were selected for final inclusion: 11 studies were used for meta-analysis and 3 studies were included only for systematic review because of the insufficient statistical data.
Table 1 describes the characteristics of the 14 studies. More than half of the studies were conducted in the United States. All studies used an RCT design. The cancer types studied were breast (n = 7), brain (n = 3), hematologic (n = 1), and mixed types (n = 3). Of the 14 studies included in the analyses, three reports were studies of patients with brain tumors in which only some of the patients received chemotherapy. One study did not include a description of chemotherapy treatment or nontreatment. The remaining 10 studies described participants (n = 614) who received chemotherapy. Seven trials included patients with stage I–III cancer. Three trials included patients across all stages of cancer. The cancer stage of the patients in the remaining four trials was unclear. The mean age of the study participants was 53.1 years. The sample size across the 14 studies varied from 13–157 patients, and the total was 977 participants. An individual-based cognitive rehabilitation approach (n = 10) was the most frequent treatment format, and interventions were provided both in the patient’s home and at the clinic. The number of sessions varied from 4–36 (mean = 16.2 sessions). The time per session varied from 25–120 minutes (mean = 60.71 minutes). Nonpharmacologic interventions were divided into two approaches: psychological (n = 11) and behavioral (n = 3). Three studies used computer-based retraining programs to restore attention, memory, or executive functioning. Most cognitive rehabilitation programs were administered by a neuropsychologist (n = 6). Interventions were delivered by experienced occupational therapists in five studies and nurses in two studies. Among the 14 studies, the duration of the intervention ranged from two weeks to more than one year. All studies used standard care control groups.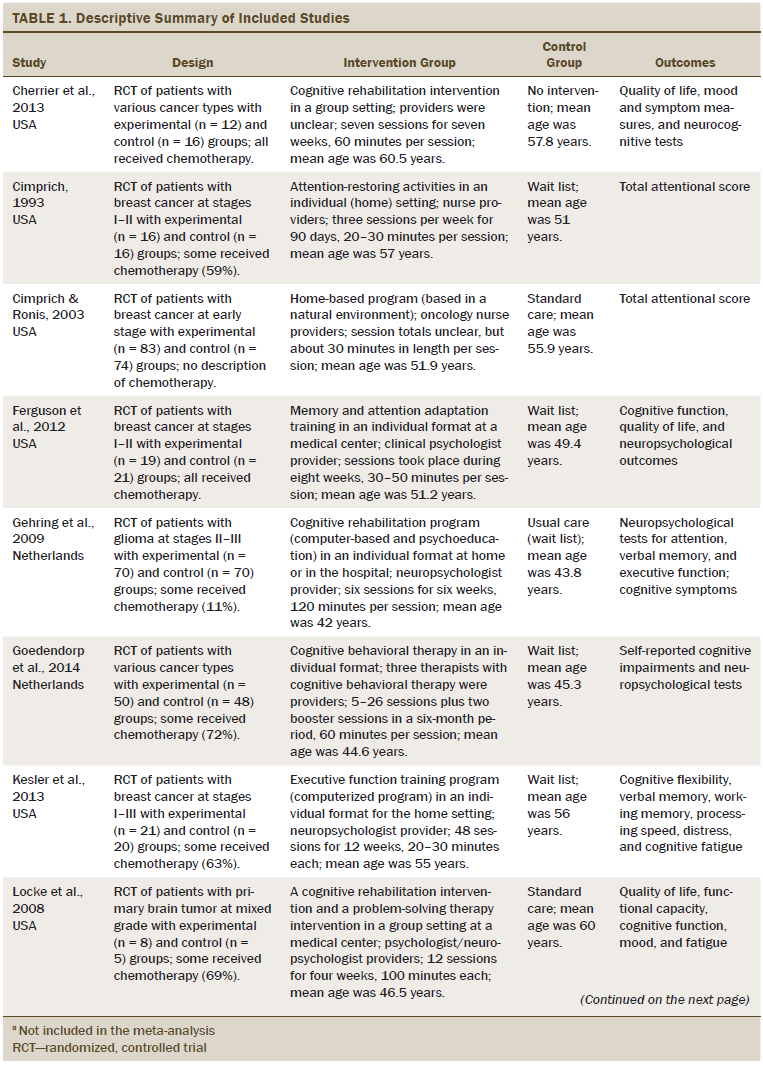
Outcome Measures
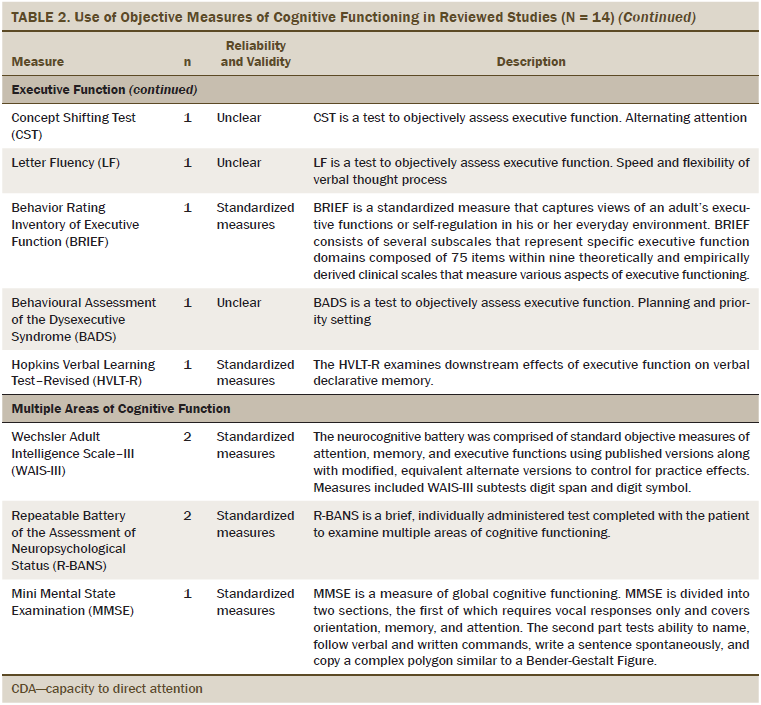
The primary outcome was cognitive functioning, which was evaluated as perceived cognitive functioning (n = 5), attention (n = 6), memory (n = 8), executive function (n = 7), verbal ability (n = 3), and multiple areas of cognitive function (n = 7). The most commonly used measures of perceived cognitive function were the Functional Assessment of Cancer Therapy–Cognitive and European Organisation for Research and Treatment of Cancer assessment tools. The objective measures of cognitive functioning are listed in Table 2, and the most commonly used tools to evaluate attention were the digit span (DS) test and Trail Making Test (TMT). Measures, such as the Rey Auditory Verbal Learning Test (RAVLT), Visual Verbal Learning Test (VVLT), and digit symbol test were used to test general memory. Category fluency was commonly used to evaluate executive functioning. For multiple areas of cognitive functioning, the Wechsler Adult Intelligence Scale–III (WAIS-III) and the Repeatable Battery of the Assessment of Neuropsychological Status (R-BANS) were used as the standardized assessment tools.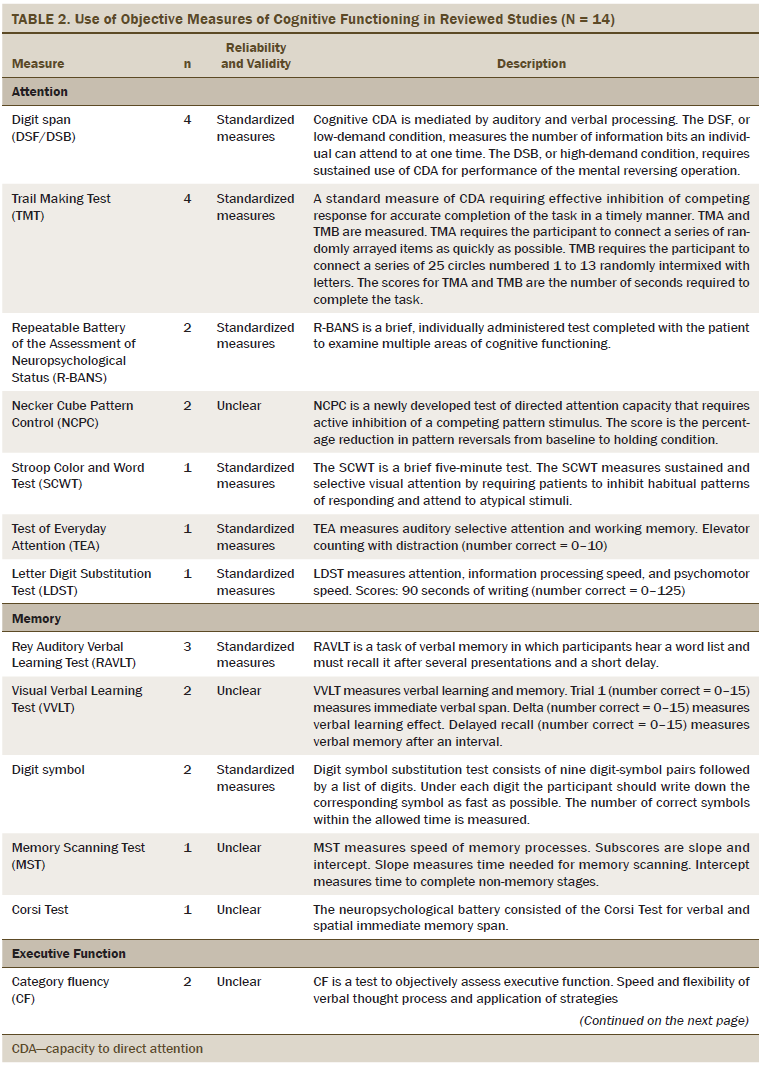
Study Quality
Of the 14 studies, only 5 reported adequate details on the randomization sequence. Nine studies with an unclear risk of bias reported the use of a randomization method, but the method could not be identified. Three of the 14 studies had a low risk of bias, but did not provide details about the allocation concealment. For 11 studies, it was unclear whether both the patients and intervention providers were blind to the intervention. Only two studies stated that the intervention provider or participant was blind to the group allocation (Ferguson et al., 2012; Zucchella et al., 2013), and one study was rated as high risk because the intervention providers were not blind to the intervention (Cherrier et al., 2013). Four studies (29%) reported that the individual conducting the outcome assessment was blind to condition (Cherrier et al., 2013; Kesler et al., 2013; Von Ah et al., 2012; Zucchella et al., 2013). Of the remaining 10 studies, seven were rated as high risk for bias and three were rated with an unclear risk of bias (Cimprich, 1993; Gehring et al., 2009; Oh et al., 2010). Twelve studies included reasons for participant dropout, which were unlikely to be related to outcomes. Therefore, the current authors evaluated participant dropout as low risk for attrition bias. For all studies, the prespecified expected outcome of interest was reported, and, therefore, all were judged to be low risk for reporting bias. The monitoring procedures and the use of manuals of the intervention are considered to be crucial for the risk of bias assessment in nonpharmacologic intervention studies (Ranchor et al., 2012). Thirteen studies provided an intervention manual and evaluated the intervention procedure. Therefore, the authors judged those 13 studies as low risk for other sources of bias; the remaining study was judged as high risk.
Meta- and Subgroup Analysis
The results of the analysis revealed effect sizes with 95% confidence. Statistical heterogeneity was observed between study estimates (I2 = 0%–68%). The current meta-analysis revealed significant treatment effects on memory and perceived cognitive functioning. The weighted average effect size for memory (n = 8) was 0.21 (95% confidence interval [CI] [0.04, 0.38], p = 0.02, I2 = 0%), indicating a small effect size. A significant small effect on perceived cognitive functioning (n = 5) also was observed (d = 0.41, 95% CI [0.2, 0.61], p < 0.001, I2 = 0%). No effects were noted on cognitive performance as measured by tests of attention, executive functioning, and verbal ability. The effect sizes of the funnel plot revealed a symmetry of outcomes. In addition, when Egger’s test was performed, the funnel plot asymmetry was not significant (p = 0.858). No significant effects were noted on quality of life (p = 0.58), fatigue (p = 0.24), depression (p = 0.61), or anxiety (p = 0.6). Some heterogeneity was observed between these study estimates (I2 = 37%–87%).
For subgroup analysis, the nonpharmacologic interventions were divided into two categories, psychological and behavioral. In this analysis, a significant effect of the psychological intervention on perceived cognitive function (n = 4) was observed (d = 0.35, 95% CI [0.13, 0.58], p = 0.002, I2 = 0%). No significant effect of behavioral interventions on cognitive functioning was observed (p = 0.05).
Discussion
The current meta-analysis synthesized data from 14 studies to examine the effects of cognitive rehabilitation programs on cognitive functioning in patients with cancer. Cognitive functioning in this meta-analysis included general neuropsychological outcomes, perceived cognitive functioning, memory, executive function, attention, and verbal ability as the primary outcomes. The results are consistent with those of previous systematic reviews, which have reported significant impairments in multiple domains of cognitive functioning, including motor function (Anderson-Hanley, Sherman, Riggs, Agocha, & Compas, 2003; Falleti et al., 2005; Stewart et al., 2006), memory (Anderson-Hanley et al., 2003; Jansen et al., 2005), executive functioning (Anderson-Hanley et al., 2003; Jansen et al., 2005), verbal ability (Falleti et al., 2005; Stewart et al., 2006), and visuospatial ability (Falleti et al., 2005; Stewart et al., 2006). The results of the meta-analyses indicated that cognitive rehabilitation programs significantly improved memory (d = 0.21) and perceived cognitive functioning (d = 0.41). When the four trials in which blinding of outcome assessment did not occur were excluded (Cimprich & Ronis, 2003; Ferguson et al., 2012; Locke et al., 2008; Milbury, 2013), no significant effects on memory were noted (d = 0.33, p = 0.06). The results for perceived cognitive functioning also remained unchanged after excluding three low-quality studies (Ferguson et al., 2012; Goedendorp et al., 2014; Milbury, 2013) (d = 0.51, p < 0.001). Therefore, additional well-designed RCTs with adequate sample sizes are necessary to enable appropriate conclusions.
No effects on cognitive performance as measured by tests of attention, executive functioning, and verbal ability were observed. Consequently, the current study suggests that patients with cancer with cognitive impairment can expect slight, focused improvement in memory ability and perceived cognitive function with nonpharmacologic interventions, whereas other cognitive domains remain unaffected. The improvement in self-reported cognitive symptoms in this study is consistent with the findings of previous review studies in this field (Gehring et al., 2012). These results also are consistent with two additional studies (Ferguson et al., 2007; Von Ah et al., 2012) that were not included in this meta-analysis because of insufficient statistical data.
Cognitive rehabilitation may be a more attractive option for treatment than pharmacologic interventions among patients with cancer because rehabilitation is less invasive (Gehring et al., 2012). However, the effect size cannot be considered to be robust because the fail-safe N for perceived cognitive functioning was 5.3, which did not exceed 5N+10. That is, 5.3 trials with no significant results were required for the mean effect to be nonsignificant.
According to the subgroup analyses in four trials with 316 participants, psychological interventions (e.g., meditation, cognitive behavioral therapy) had a significant effect on self-reported cognitive functioning among patients with cancer (d = 0.35, p = 0.002) with no statistical heterogeneity observed between study estimates (I2 = 0%). This result is consistent with the results of a meta-analysis on the well-being of patients with cancer (Zimmermann, Heinrichs, & Baucom, 2007). It has generally been observed that subjective cognitive function tends to correlate more highly with emotional distress and well-being than objective neuropsychological test performance (Cull et al., 1996; Hall, Isaac, & Harris, 2009; Middleton, Denney, Lynch, & Parmenter, 2006; Sawrie et al., 1999; Schagen et al., 2008). This finding may reflect the fact that subjective cognitive symptoms, fatigue, and mood disorders are more frequently improved than objectively assessed cognitive dysfunction, possibly related to the focused intervention on the patient’s perception of the problem (Gehring et al., 2012).
The psychological interventions reviewed in this study highlight the application of compensatory strategies to minimize the impact of cognitive deficits in daily life. Psychological interventions may help to improve or prevent cognitive dysfunction by retraining cognitive capacities or by introducing compensation strategies such as focusing education on memory and attention, self-awareness training, self-regulation training, and cognitive compensatory strategies training (Gehring et al., 2012). These interventions target plasticity of the brain via restoration or reorganization of function (Miotto et al., 2013; Mora, 2013). A previous systematic review of patients with brain injury also reported strong evidence supporting the use of external memory aids to compensate for functional memory problems (Rees, Marshall, Hartridge, Mackie, & Weiser, 2007) without necessarily improving underlying memory abilities (Kennedy et al., 2008). Therefore, cognitive rehabilitation is effective in helping patients to learn and apply compensation strategies for residual cognitive limitations. However, several studies suggest that intervention also may directly improve the underlying cognitive functions (Serino et al., 2007; Stablum, Umilta, Mazzoldi, Pastore, & Magon, 2007; Westerberg et al., 2007).
Three studies comprising eight psychological interventions in the current meta-analysis used computer-based programs for direct attention training, which was defined as the repeated stimulation of attention via graded exercises to improve the underlying neurocognitive system and attention functioning (Sohlberg et al., 2003). These studies did not observe a significant effect on cognitive functioning. A retraining program may, therefore, be more effective in mildly impaired patients when used in conjunction with external memory aids (Sohlberg et al., 2003).
In the current meta-analysis, cognitive rehabilitation programs had no effect on cognitive performance as measured by tests of attention, executive functioning, and verbal ability. This result is not consistent with the results of systematic reviews in patients with traumatic brain injury, which report substantial evidence supporting the positive effects of neuropsychological rehabilitation interventions on attention, memory, and executive function (Cicerone et al., 2011). Several reasons may exist as to why a significant effect was not observed in the current study. The overall recovery process after chemotherapy may have taken place, in terms of cognitive and physical condition. In addition, the low cutoff for the selection of patients with cognitive deficits may have contributed to the null finding (Poppelreuter et al., 2009). Seven reviewed studies did not report their selection criterion for patients with cognitive deficits. However, given that the studies were designed to treat patients with cognitive impairment, the presence of objective cognitive deficits would be considered the most important selection criterion for investigators to be able to measure the effect of an intervention (Gehring et al., 2012). In addition, it may be appropriate to screen for self-reported cognitive complaints, because the experience of cognitive symptoms may be crucial in motivating patients to adhere to time-consuming cognitive rehabilitation programs (Gehring et al., 2012).
The lack of long-term follow-up assessments also may have contributed to the null findings in this meta-analysis. It has been suggested that patients may require more time to integrate learned strategies into their daily routine. Six studies in this analysis included only one follow-up assessment after the intervention. Given that the possibility of a delayed intervention effect remains unknown, future studies may include such assessments to document information regarding the persistence of potentially beneficial intervention effects (Gehring et al., 2009; Winkens, Van Heugten, Wade, Habets, & Fasotti, 2009).
In the authors’ subgroup analyses, only three studies tested a behavioral intervention, and no significant effect on overall cognitive functioning was observed. The effect of physical activity on cognitive impairment in patients with cancer is a current area of interest, based on findings that physical exercise may have a positive effect on delaying or ameliorating cognitive deficits in older adults with or without cognitive decline (Day et al., 2014). Exercise has been associated with increased cerebral blood flow, hippocampal neurogenesis, changes in neurotransmitter release, increased arousal levels, and brain structures (Gligoroska & Manchevska, 2012). However, the physical activities described in this study do not necessarily represent physically demanding exercises, but rather activities such as walking in the natural environment, tending to plants, or engaging in gentle movements. When meta-analyses contain a small number of studies, the results and estimated effect sizes can be imprecise (Higgins & Green, 2011). Additional RCTs of physical exercise with adequate sample size are needed to enable the drawing of appropriate conclusions.
Limitations
In the current study, some relevant data could have been overlooked because the authors were not able to access international unpublished research and non-English based studies. Because nonpharmacologic interventions are so diverse, investigators had to provide specific information regarding (a) the degree of the patient’s cognitive impairment as well as the quality, focus, and long-term assessment of the intervention and (b) medical treatments and other interventions used during and after the intervention period. The methodologic quality of the included studies was another limitation of this meta-analysis. Blinding is a critical feature of the RCT methodology design, but because of the practicalities of psychological interventions, blinding was challenging to apply in these studies. Finally, the use of small sample sizes in many studies resulted in insufficient power to detect effects of the cognitive rehabilitation program on the outcomes.
Implications for Practice and Research
A significant number of patients with cancer who are treated with chemotherapy experience cognitive decline, which negatively affects their quality of life. Nurses need to be aware of the evidence-based interventions for this potentially debilitating side effect (Von Ah, Jansen, Allen, Schiavone, & Wulff, 2011). This meta-analysis was focused on nonpharmacologic interventions, which were divided into two intervention categories: psychological and behavioral. Psychological interventions included strategies as well as education and retraining, which was administered primarily via computer-based programs. The behavioral intervention method involves activities thought to be particularly helpful in ameliorating and restoring cognitive functioning (Cimprich, 1993), such as walking in the natural environment, tending to plants, or engaging in gentle movement. The findings of this meta-analysis support the positive effects of nonpharmacologic interventions on memory and self-reported cognitive functioning. However, the current review revealed a significant effect only on the psychological intervention when the intervention types were administered separately. These psychological interventions were more effective when strategies such as focusing on education regarding memory and attention, self-awareness training, self-regulation training, and cognitive retraining were applied using an integrated approach. Behavioral interventions included in this study were not physically demanding exercises, and there were relatively few studies with small sample sizes. Therefore, more research is needed to develop and refine behavioral interventions, such as physical exercise programs. To accomplish this, researchers will need to continue to investigate the underlying physiological mechanisms and treatment- or disease-related factors associated with cognitive impairment. Because the current study suggests that cognitive function would be most improved in patients with cancer when a mixed-intervention approach (compensation, education, retraining, and physical activity) is employed, additional research is needed.
Although the meta-analyses included studies with well-validated tools to test cognitive function, several questions remain. For example, the addition of findings from neuroimaging, such as magnetic resonance imaging, may provide a better understanding of the clinical benefits of nonpharmacologic interventions.
Conclusion
Despite some limitations, the current meta-analysis can tentatively conclude that nonpharmacologic interventions result in a small improvement in memory ability and perceived cognitive functioning among patients with cancer with cognitive impairment. However, most of the reviewed studies did not provide sufficient evidence for an effect of the cognitive rehabilitation program on cognitive performance as measured by objective tests of attention, executive functioning, and verbal ability. Therefore, additional studies on these cognitive outcomes are needed.
The authors gratefully acknowledge HyeYoun Han, SH, who provided data coding and organizational support at various stages of the study process.
References
Anderson-Hanley, C., Sherman, M.L., Riggs, R., Agocha, V.B., & Compas, B.E. (2003). Neuropsychological effects of treatments for adults with cancer: A meta-analysis and review of the literature. Journal of the International Neuropsychological Society, 9, 967–982. doi:10.1017/S1355617703970019
Cherrier, M.M., Anderson, K., David, D., Higano, C., Gray, H., Church, A., & Willis, S. (2013). A randomized trial of cognitive rehabilitation in cancer survivors. Life Sciences, 93, 617–622. doi:10.1016/j.lfs.2013.08.011
Cicerone, K.D., Langenbahn, D.M., Braden, C., Malec, J.F., Kalmar, K., Fraas, M., . . . Ashman, T. (2011). Evidence-based cognitive rehabilitation: Updated review of the literature from 2003 through 2008. Archives of Physical Medicine and Rehabilitation, 92, 519–530. doi:10.1016/j.apmr.2010.11.015
Cimprich, B. (1993). Developing an intervention to restore attention in cancer patients. Cancer Nursing, 16, 83–92. doi:10.1097/00002820-199304000-00001
Cimprich, B., & Ronis, D.L. (2003). An environmental intervention to restore attention in women with newly diagnosed breast cancer. Cancer Nursing, 26, 284–292. doi:10.1097/00002820-200308000-00005
Cull, A., Hay, C., Love, S.B., Mackie, M., Smets, E., & Stewart, M. (1996). What do cancer patients mean when they complain of concentration and memory problems? British Journal of Cancer, 74, 1674–1679. doi:10.1038/bjc.1996.608
Day, J., Zienius, K., Gehring, K., Grosshans, D., Taphoorn, M., Grant, R., . . . Brown, P.D. (2014). Interventions for preventing and ameliorating cognitive deficits in adults treated with cranial irradiation. Cochrane Database of Systematic Reviews, 12, CD011335. doi:10.1002/14651858.CD011335.pub2
de Ruiter, M.B., Reneman, L., Boogerd, W., Veltman, D.J., Caan, M., Douaud, G., . . . Schagen, S.B. (2011). Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Human Brain Mapping, 33, 2971–2983. doi:10.1002/hbm.21422
Egger, M., Smith, G.D., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple graphical test. BMJ, 315, 629–634. doi:10.1136/bmj.315.7109.629
Faller, H., Schuler, M., Richard, M., Heckl, U., Weis, J., & Kuffner, R. (2013). Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: Systematic review and meta-analysis. Journal of Clinical Oncology, 31, 782–793. doi:10.1200/JCO.2011.40.8922
Falleti, M.G., Sanfilippo, A., Maruff, P., Weih, L.A., & Phillips, K.A. (2005). The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: A meta-analysis of the current literature. Brain and Cognition, 59, 60–70. doi:10.1016/j.bandc.2005.05.001
Ferguson, R.J., Ahles, T.A. Saykin, A.J., McDonald, B.C., Furstenberg, C.T., Cole, B.F., & Mott, L.A. (2007). Cognitive-behavioral management of chemotherapy-related cognitive change. Psycho-Oncology, 16, 772–777. doi:10.1002/pon.1133
Ferguson, R.J., McDonald, B.C., Rocque, M.A., Furstenberg, C.T., Horrigan, S., Ahles, T.A., & Saykin, A.J. (2012). Development of CBT for chemotherapy-related cognitive change: Results of a waitlist control trial. Psycho-Oncology, 21, 176–186. doi:10.1002/pon.1878
Gehring, K., Roukema, J.A., & Sitskoorn, M.M. (2012). Review of recent studies on interventions for cognitive deficits for cognitive deficits in patients with cancer. Expert Review of Anticancer Therapy, 12, 255–269. doi:10.1586/era.11.202
Gehring, K., Sitskoorn, M.M., Aaronson, N.K., & Taphoorn, M.J. (2008). Interventions for cognitive deficits in adults with brain tumours. Lancet Neurology, 7, 548–560. doi:10.1016/S1474-4422 (08)70111-X
Gehring, K., Sitskoorn, M.M., Gundy, C.M., Sikkes, S.A., Klein, M., Postma, T.J., . . . Aaronson, N.K. (2009). Cognitive rehabilitation in patients with gliomas: A randomized, controlled trial. Journal of Clinical Oncology, 27, 3712–3722. doi:10.1200/JCO.2008.20.5765
Gligoroska, J.P., & Manchevska, S. (2012). The effect of physical activity on cognition—Physiological mechanisms. Mater Sociomed, 24, 198–202. doi:10.5455/msm.2012.24.198-202
Goedendorp, M.M., Knoop, H., Gielissen, M.F., Verhagen, C.A., & Bleijenberg, G. (2014). The effects of cognitive behavioral therapy for post-cancer fatigue on perceived cognitive disabilities and neuropsychological test performance. Journal of Pain and Symptom Management, 47, 35–44. doi:10.1016/j.jpainsymman
Hall, K.E., Isaac, C.L., & Harris, P. (2009). Memory complaints in epilepsy: An accurate reflection of memory impairment or an indicator of poor adjustment? A review of the literature. Clinical Psychology Review, 29, 354–367. doi:10.1016/j.cpr.2009.03.001
Hermelink, K., Henschel, V., Untch, M., Bauerfeind, I., Lux, M.P., & Munzel, K. (2008). Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: Results of a multicenter, prospective, longitudinal study. Cancer, 113, 2431–2439. doi:10.1002/cncr.23853
Higgins, J.P., & Green, S. (Eds.). (2011). Cochrane handbook for systematic reviews of interventions. Retrieved from http://www.cochrane-handbook.org
Hutchinson, A.D., Hosking, J.R., Kichenadasse, G., Mattiske, J.K., & Wilson, C. (2012). Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treatment Reviews, 38, 926–934. doi:10.1016/j.ctrv.2012.05.002
Jansen, C.E., Miaskowski, C., Dodd, M., Dowling. G., & Kramer, J. (2005). A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer, 104, 2222–2233. doi:10.1002/cncr.21469
Jim, H.S., Phillips, K.M., Chait, S., Faul, L.A., Popa, M.A., Lee, Y.H., . . . Small, B.J. (2012). Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. Journal of Clinical Oncology, 30, 3578–3587. doi:10.1200/JCO.2011.39.5640
Kaiser, J., Bledowski, C., & Dietrich, J. (2014). Neural correlates of chemotherapy-related cognitive impairment. Cortex, 54, 33–50. doi:10.1016/j.cortex.2014.01.010
Kennedy, M.R., Coelho, C., Turkstra, L., Ylvisaker, M., Moore, S.M., Yorkston, K., . . . Kan, P.F. (2008). Intervention for executive functions after traumatic brain injury: A systematic review, meta-analysis and clinical recommendations. Neuropsychological Rehabilitation, 18, 257–299. doi:10.1080/09602010701748644
Kesler, S., Hadi Hosseini, S.M., Heckler, C., Janelsins, M., Palesh, O., Mustian, K., . . . Morrow, G. (2013). Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clinical Breast Cancer, 13, 299–306. doi:10.1016/j.clbc.2013.02.004
Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gotzsche, P.C., Ioannidis, J.P., . . . Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine, 151, W65–W94.
Locke, D.E., Cerhan, J.H., Wu, W., Malec, J.F., Clark, M.M., Rummans, T.A., & Brown, P.D. (2008). Cognitive rehabilitation and problem-solving to improve quality of life of patients with primary brain tumors: A pilot study. Journal of Supportive Oncology, 6, 383–391.
Matsuda, T., Takayama, T., Tashiro, M., Nakamura, Y., Ohashi, Y., & Shimozuma, K. (2005). Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients—Evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer, 12, 279–287. doi:10.2325/jbcs.12.279
Middleton, L.S., Denney, D.R., Lynch, S.G., & Parmenter, B. (2006). The relationship between perceived and objective cognitive functioning in multiple sclerosis. Archives of Clinical Neuropsychology, 21, 487–494. doi:10.1016/j.acn.2006.06.008
Milbury, K., Chaoul, A., Biegler, K., Wangyal, T., Spelman, A., Meyers, C.A., . . . Cohen, L. (2013). Tibetan sound meditation for cognitive dysfunction: Results of a randomized controlled pilot trial. Psycho-Oncology, 22, 2354–2363. doi:10.1002/pon.3296
Miotto, E.C., Savage, C.R., Evans, J.J., Wilson, B.A., Martin, M.G., Balardin, J.B., . . . Amaro Junior, E. (2013). Semantic strategy training increases memory performance and brain activity in patients with prefrontal cortex lesions. Clinical Neurology and Neurosurgery, 115, 309–316. doi:10.1016/j.clineuro
Mora, F. (2013). Successful brain aging: Plasticity, environmental enrichment, and lifestyle. Dialogues in Clinical Neuroscience, 15, 45–52.
Oh, B., Butow, P.N., Mullan, B.A., Clarke, S.J., Beale, P.J., Pavlakis, N., . . . Vardy, J. (2010). Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: A randomized controlled trial. Supportive Care in Cancer, 20, 1235–1242. doi:10.1007/s00520-011-1209-6
Poppelreuter, M., Weis, J., & Bartsch, H.H. (2009). Effects of specific neuropsychological training programs for breast cancer patients after adjuvant chemotherapy. Journal of Psychosocial Oncology, 27, 274–296. doi:10.1080/07347330902776044
Poppelreuter, M., Weis, J., Mumm, A., Orth, H.B., & Bartsch, H.H. (2008). Rehabilitation of therapy-related cognitive deficits in patients after hematopoietic stem cell transplantation. Bone Marrow Transplantation, 41, 79–90. doi:10.1038/sj.bmt.1705884
Prabhu, R.S., Won, M., Shaw, E.G., Hu, C., Brachman, D.G., Buckner, J.C., . . . Mehta, M.P. (2014). Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: Secondary analysis of RTOG 98-02. Journal of Clinical Oncology, 32, 535–541. doi:10.1200/JCO.2013.53.1830
Ranchor, A.V., Fleer, J., Sanderman, R., Van der Ploeg, K.M., Coyne, J.C., & Schroevers, M. (2012). Psychological interventions for cancer survivors and cancer patients in the palliative phase. Cochrane Database of Systematic Reviews, 1, CD009511. doi:10.1002/14651858.CD009511
Rees, L., Marshall, S., Hartridge, C., Mackie, D., & Weiser, M. (2007). Cognitive interventions post acquired brain injury. Brain Injury, 21, 161–200. doi:10.1016/j.apmr.2010.11.015
Sawrie, S.M., Martin, R.C., Kuzniecky, R., Faught, E., Morawetz, R., Jamil, F., . . . Gilliam, F. (1999). Subjective versus objective memory change after temporal lobe epilepsy surgery. Neurology, 53, 1511–1517. doi:10.1212/WNL.53.7.1511
Schagen, S.B., Boogerd, W., Muller, M.J., Huinink, W.T., Moonen, L., Meinhardt, W., & Van Dam, F.S. (2008). Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncologica, 47, 63–70. doi:10.1080/02841860701518058
Schagen, S.B., van Dam, F.S., Muller, M.J., Boogerd, W., Lindeboom, J., & Bruning, P.F. (1999). Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer, 85, 640–650. doi:10.1002/(SICI)1097-0142(19990201)
Serino, A., Ciaramelli, E., Santantonio, A.D., Malagù, S., Servadei, F., & Làdavas, E. (2007). A pilot study for rehabilitation of central executive deficits after traumatic brain injury. Brain Injury, 21, 11–19. doi:10.1080/02699050601151811
Sheinfeld Gorin, S., Krebs, P., Badr, H., Janke, E.A., Spring, B., Mohr, D.C., . . . Jacobsen, P.B. (2012). Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. Journal of Clinical Oncology, 30, 539–547. doi:10.1200/jco.2011.37.0437
Smedslund, G., & Ringdal, G.I. (2004). Meta-analysis of the effects of psychosocial interventions on survival time in cancer patients. Journal of Psychosomatic Research, 57, 123–131. doi:10.1016/S0022-3999(03)00575-0
Sohlberg, M., Avery, J., Kennedy, M., Ylvisaker, M., Coelho, C., Turkstra, L., & Yorkston, K. (2003). Practice guidelines for direct attention training. Journal of Medical Speech-Language Pathology, 11, xix–xxxix.
Stablum, F., Umilta, C., Mazzoldi, M., Pastore, N., & Magon, S. (2007). Rehabilitation of endogenous task shift processes in closed head injury patients. Neuropsychology Rehabilitation, 17, 1–33. doi:10.1080/13506280500411111
Stewart, A., Bielajew, C., Collins, B., Parkinson, M., & Tomiak, E. (2006). A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clinical Neuropsychologist, 20, 76–89. doi:10.1080/138540491005875
Vardy, J., & Tannock, I. (2007). Cognitive function after chemotherapy in adults with solid tumours. Critical Reviews in Oncology Hematology, 63, 183–202. doi:10.1016/j.critrevonc .2007.06.001
Von Ah, D., Carpenter, J.S., Saykin, A., Monahan, P., Wu, J., Rebok, G., . . . Unverzagt, F. (2012). Advanced cognitive training for breast cancer survivors: A randomized controlled trial. Breast Cancer Research and Treatment, 135, 799–809.
Von Ah, D., Jansen, C., Allen, D.H., Schiavone, R.M., & Wulff, J. (2011). Putting Evidence Into Practice: Evidence-based interventions for cancer and cancer treatment-related cognitive impairment. Clinical Journal of Oncology Nursing, 15, 607–615. doi:10.1188/11.CJON.607-615
Wefel, J.S., & Schagen, S.B. (2012). Chemotherapy-related cognitive dysfunction. Current Neurology and Neuroscience Reports, 12, 267–275. doi:10.1007/s11910-012-0264-9
Westerberg, H., Jacobaeus, H., Hirvikoski, T., Clevberger, P., Östensson, M.L., Bartfai, A., & Klingberg, T. (2007). Computerized working memory training after stroke–A pilot study. Brain Injury, 21, 21–29. doi:10.1080/02699050601148726
Winkens, I., Van Heugten, C.M., Wade, D.T., Habets, E.J., & Fasotti, L. (2009). Efficacy of time pressure management in stroke patients with slowed information processing: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation, 90, 1672–1679. doi:10.1016/j.apmr.2009.04.016
Zimmermann, T., Heinrichs, N., & Baucom, D.H. (2007). “Does one size fit all?” Moderators in psychosocial interventions for breast cancer patients: A meta-analysis. Annals of Behavioral Medicine, 34, 225–239. doi:10.1007/bf02874548
Zucchella, C., Capone, A., Codella, V., De Nunzio, A.M., Vecchione, C., Sandrini, G., . . . Bartolo, M. (2013). Cognitive rehabilitation for early post-surgery inpatients affected by primary brain tumor: A randomized, controlled trial. Journal of Neuro-Oncology, 114, 93–100. doi:10.1007/s11060-013-1153-z
About the Author(s)
Oh is a professor in the Department of Nursing at Sahmyook University and Kim is a graduate student in the College of Nursing at Korea University, both in Seoul, South Korea. This research was funded by the National Research Foundation of Korea Basic Science Research Program through support from the Ministry of Education (2014RIAIA2053517). Oh contributed to the conceptualization and design and the manuscript preparation. Oh and Kim completed the data collection and provided statistical support and analysis. Kim can be reached at wocnkorea@gmail.com, with copy to editor at ONFEditor@ons.org. Submitted August 2015. Accepted for publication October 26, 2015.

