Development of an Instrument to Examine Nursing Attitudes Toward Fertility Preservation in Oncology
Purpose/Objectives: To develop an instrument to measure staff nurse perceptions of the barriers to and benefits of addressing fertility preservation (FP) with patients newly diagnosed with cancer.
Design: A prospective, nonrandomized instrument development approach.
Setting: Harold C. Simmons Comprehensive Cancer Center at the University of Texas Southwestern Medical Center in Dallas.
Sample: 224 RNs who care for patients with cancer.
Methods: The instrument was developed with content experts and field-tested with oncology staff nurses. Responses to a web-based survey were used in exploratory factor analysis. After refining the instrument, the authors conducted a confirmatory factor analysis with 230 web-based survey responses.
Main Research Variables: Self-perceived barriers to providing FP options to patients newly diagnosed with cancer.
Findings: The results supported a 15-item instrument with five domains: (a) confidence, (b) self-awareness, (c) external barriers, (d) time barriers, and (e) perceived treatment barriers.
Conclusions: This instrument can be used to explore oncology nurses’ attitudes toward FP in newly diagnosed people with cancer in their reproductive years.
Implications for Nursing: A more comprehensive understanding of attitudes and barriers related to FP will guide the building of optimal systems that support effective FP options, resources, and programs for individuals with cancer.
Jump to a section
The American Cancer Society (2017) estimated that about 1.7 million people would be newly diagnosed with cancer in 2017. In 2015, an estimated 70,000 of those diagnosed with cancer were adolescents and young adults aged 15–39 years, still of childbearing age (National Cancer Institute, 2015). Individuals in their childbearing years have reported that fertility is of high concern and, at times, trivialized by clinicians (Peate, Meiser, Hickey, & Friedlander, 2009). Many cancer treatments reduce fertility, and some eliminate its possibility. A diagnosis of cancer is unexpected and life-changing. Healthcare providers fully understand the implications of treating the malignancy as soon as possible, but many overlook the options related to survivorship when treatments are effective (Ethics Committee of the American Society for Reproductive Medicine, 2005; King et al., 2008; Peate et al., 2009). Knowing that time to treatment is a factor in survival, therapy, including surgical resection, chemotherapy, systemic therapies, and radiation, is often started as soon as possible. The delivery of information about fertility may also be confounded by the type of cancer, insurance provider, and socioeconomic resources available to the patient and clinic (Loren et al., 2013).
In meeting the goal of initiating treatment as soon as possible, critical components like fertility preservation (FP) may be overlooked or minimized (Nobel Murray, Chrisler, & Robbins, 2016; Peate et al., 2009). In addition, individuals have reported that they desire additional information on FP in the early stages of cancer. The preferred method of delivery of this information is a trained clinician (Quinn, Vadaparampil, Bell-Ellison, Gwede, & Albrecht, 2008). Quinn et al. (2008) identified physician familiarity with fertility options, data, and statistics, as well as physician comfort level, as potential barriers to facilitation of FP consultations with people diagnosed with cancer.
FP requires dedicated staff to counsel patients and ensure they are fully informed regarding treatment implications, including all of their potential choices. Systems also have to be in place to support people newly diagnosed with cancer through discussion of these options. People in their childbearing years have reported that they would like information about fertility options initially and on a regular basis throughout their treatment because their options and treatment courses may change and affect fertility (Peate et al., 2009). Comprehensive cancer care requires a team approach to ensure that the full range of resources is available during optimal periods, such as pretreatment or new diagnosis, to optimize care.
Many cancer treatments involving surgery, chemotherapy, or radiation lead to reduced fertility or infertility. Men with cancer aged 18–45 years can experience the toxic effects of systemic therapies and radiation directed at the gonads or the hypothalamus and/or pituitary gland, which lead to infertility 30%–75% of the time (Ginsberg, 2012). Women’s infertility risk varies considerably based on multiple variables, including treatment age, modality, and dosing (Levine, Kelvin, Quinn, & Gracia, 2015; Winkler-Crepaz, Ayuandari, Ziehr, Hofer, & Wildt, 2015). Cancer survivors of childbearing age have reported that one of their greatest concerns is the effect of treatment on their ability to have a biologic child (Benedict, Shuk, & Ford, 2016; Ellis, Wakefield, McLoone, Robertson, & Cohn, 2016).
Survivors who were not fully informed or given the choice to consult a reproductive endocrinologist or fertility specialist in their pretreatment phase of care often experience resentment toward or distrust of the medical community (Deshpande, Braun, & Meyer, 2015). Individuals who did not receive a fertility consultation reported higher levels of depressive symptomatology and lower quality of life (Letourneau et al., 2012). Most women receiving FP counseling reported that the possibility of FP was instrumental to improved coping. Receiving FP counseling reduced long-term regret and dissatisfaction concerning fertility and was associated with improved physical quality of life and trends toward improved psychological quality of life (Deshpande et al., 2015).
National oncology organizations have championed FP as a critical component of comprehensive cancer care. Often, certification criteria incorporate FP as a core standard. The 2006 American Society of Clinical Oncology’s Quality Oncology Practice Initiative guidelines stated that infertility risks should be discussed prior to initiating fertility-reducing therapies for individuals in their reproductive years (Lee et al., 2006). Clinicians agree that FP is an important topic for men and women diagnosed with cancer, yet they do not always address it (Klosky et al., 2015). Despite this guideline, national compliance with FP counseling remains low (Blayney et al., 2014). A review of 69 studies revealed that individuals with cancer often lack adequate information and are often not informed about FP options (Flink, Sheeder, & Kondapalli, 2017). Oncology nurses are key clinical staff who often play a pivotal role in ensuring adherence to quality guidelines, based on evidence-based practice and national recommendations for optimal care (Benedict et al., 2016; Ellis et al., 2016).
Because clinicians (e.g., physicians, hospital administrators, leadership teams) rely on nurses to fully educate and support patients through the cancer journey, understanding what nurses need regarding FP advocacy becomes critical. However, no validated instruments address nursing attitudes toward FP counseling. The purpose of this study is to develop a tool to assess the potential barriers encountered by oncology nurses to recommending FP as an option for patients with newly diagnosed cancer.
Methods
This was a multiphase instrument development study. The study, which received institutional review board approval from the University of Texas Southwestern Medical Center in Dallas, was completed in four phases. In phase 1, clinical experts developed items considered to be important in evaluating oncology nurses’ attitudes toward addressing FP with a patient following a new diagnosis of cancer. Phase 2 involved developing and refining a survey, and included field-testing of the items with nurse clinicians who provide direct care in adult oncology units. Phase 3 involved distribution of the survey in a web-based format to 67 oncology staff nurses at a single institution. Exploratory factor analysis was used to refine the instrument. Factor analysis can be used to help determine the number of items and factors (elements) to retain when developing a new instrument (DeVellis, 2012; Pett, Lackey, & Sullivan, 2003). Phase 4 included confirmatory factor analysis with 230 participants from one region who provide direct care to individuals receiving cancer treatments.
This study was developed and initiated at the Harold C. Simmons Comprehensive Cancer Center at the University of Texas Southwestern Medical Center in Dallas. The cancer center houses three outpatient clinics and two inpatient units. The research team recruited nurses from the greater Dallas–Fort Worth metroplex by engaging nursing societies and events aimed at oncology nurses.
Two subsets of participants were included in this study. The first subset sample, involved in phase 3, were the 67 nurses employed at the collaborating site. These nurses had regular contact with adults in their reproductive years diagnosed with cancer. Nurses were recruited via email and in person at staff meetings. The second subset sample, involved in phase 4, were the 230 oncology nurses employed within the greater Dallas–Fort Worth metroplex who had regular contact with individuals in their reproductive years diagnosed with cancer.
Phase 1 of the current study involved engaging clinical experts and conducting a literature review of previous research studies and instruments addressing FP in oncology. The investigators met biweekly to discuss findings, gaps in the literature, and evidence-based practices to address FP. Statements were developed with Likert-type scale responses. Phase 2 began when consensus was reached on the list of items. Content experts were consulted and provided feedback on each item for readability, face validity, structure, and response options. Next, the items were entered into a web-based platform (www.proprofs.com), and three staff nurse champions and two nurse leaders were asked to take the survey and provide feedback on the structure and face validity. The content experts and nurse champions provided unstructured feedback, which was used for critical revisions (i.e., structure and visual clarity). The web-based platform allowed participants to take the survey anonymously, avoiding additional risks of loss of confidentiality.
During phase 3, participants from the local institution were invited to complete the web-based survey, consisting of 16 items. Invitations occurred via staff meetings, daily staff five-minute huddle meetings, face-to-face invitations, and group email announcements. Staff opting to participate were given a link to the ProProfs website. Data were downloaded from the ProProf platform as tab-delimited, entered into an electronic spreadsheet, and uploaded to SAS®, version 9.4, for statistical analyses. Standard measures of central tendency were computed for all variables (mean, median, interquartile range). Exploratory factor analysis with orthogonal rotation was used to explore the results of phase 3. Scree plot analysis examining eigenvalues indicated five factors: confidence, self-awareness, external barriers, time barriers, and perceived treatment barriers. The instrument was further refined by examining the individual factor loadings. The variable with the highest association for each factor was assigned. By deleting one item, the survey was reduced to a 15-item tool.
Phase 4 involved sending the refined (15-item) web-based survey to staff nurses who provide direct care to individuals with cancer in north Texas. Recruitment of nurses occurred at local meetings, conferences, and events for oncology nurses. Research staff members provided participants with electronic devices (i.e., a laptop or tablet) that opened directly to the web-based survey. During phase 4, inverse scoring was applied for items 6, 19, and 20 (based on exploratory factor analysis in phase 3). Data were downloaded from the web-based platform. All data were anonymous and therefore de-identified. Data were stored in Excel on secure drives on computers. First, scree plots of the eigenvalues were examined and noted, indicating a five-factor tool; all factor loadings were greater than 0.35. PROC CALIS using SAS, version 9.4, was used for confirmatory factor analysis.
Results
The 67 nurse respondents in phase 2 had an average of 14.2 (SD = 5.9) years of clinical experience and 11.1 (SD = 6) years of oncology experience. Exploratory factor analysis revealed a five-factor, 15-item instrument (see Table 1). Factor 1, confidence, consisted of four items, two with positive loading (0.87, 0.74) and two with negative loading (–0.8, –0.48). Factor 2, self-awareness, included five items, four with positive loading (0.8, 0.66, 0.63, 0.6) and one with negative loading (–0.36). Factor 3, external barriers, included two items with positive loading (0.68, 0.65). Factor 4, time barriers, included two items with positive loading (0.68, 0.65). The time barriers were intended to capture workload time barriers experienced by the nurses. Factor 5, perceived treatment barriers, included two items with positive loading (0.68, 0.64). The 15 items all were included in the survey distributed in phase 4. 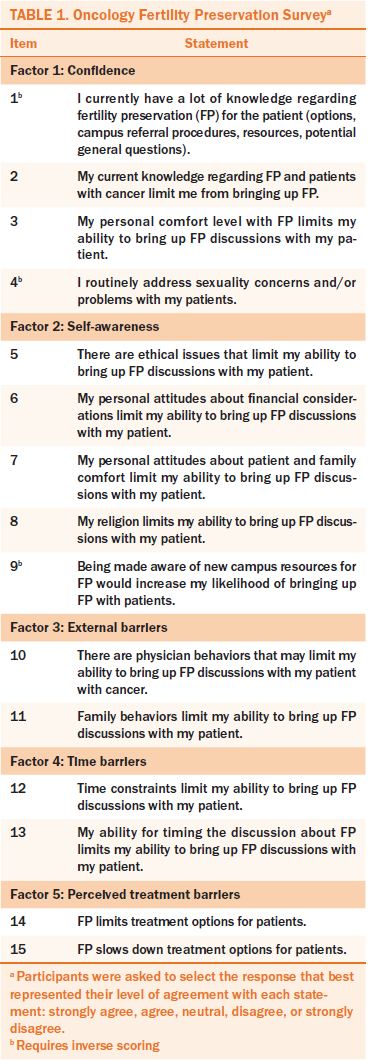
Phase 4 (confirmatory factor analysis) initially included 230 surveys from nurses with clinical and oncology experience. Of these, six respondents omitted two or more items, which were dropped from the final analysis. A broad range of responses existed for each item. Confirmatory factor analysis met convergence criteria for five factors (c2 =164.8, p < 0.0001, Bentler’s comparative fit index = 0.9). Almost every item received the full range of possible responses, from “strongly agree” to “strongly disagree.” The exceptions were items 6, 7, and 14, with which no respondents strongly agreed (see Table 2). 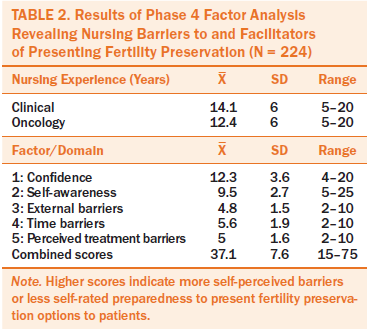
Discussion
FP is important to most individuals of reproductive age (Yee, Abrol, McDonald, Tonelli, & Liu, 2012). In attempts to improve FP counseling for adolescents and young adults with cancer, interventions tend to exclusively target nurses through educational offerings (Nobel Murray et al., 2016; Vadaparampil et al., 2016). Patient education is generally offered in the form of an informational pamphlet or brief in-office counseling (Peate et al., 2009). The content of these educational interventions is general, not tailored to the patient. These efforts could be improved if more was known of patients’ specific needs. Despite an extensive literature search, the authors of the current study were not able to find an established instrument to measure learner readiness or barriers to FP counseling in oncology (Vadaparampil, Hutchins, & Quinn, 2013).
The results support the use of a 15-item instrument to measure five distinct domains of nurse preparedness to address FP. The tool may be used to assess the barriers to delivery of FP education by nurses in oncology settings. This instrument can be distributed on paper or on the web to determine the current overall preparedness of oncology nursing staff to address FP. This survey may also be used with oncology nurses from specific departments of all skill levels to create targeted interventions within defined subgroups. The survey is designed to provide a future potential treatment pathway for individuals with cancer during their reproductive years.
Limitations
Although the setting of the study, Dallas–Fort Worth metroplex, represents a diverse cohort, the need for and acceptance of FP counseling may vary by region. This survey should be used in other settings with more diverse samples. In addition, this survey did not ask nurses to consider the age of individuals with cancer, which may be important considering the trend toward family planning later in life (e.g., age 35–50 years) and how this affects nurses’ perceived barriers to FP counseling. Future surveys may want to consider age as a factor in FP. Also, the research team did not account for nurses’ previous education or counseling regarding FP. For example, at one recruiting hospital, all healthcare providers are prompted by the electronic health record to assess an individual’s level of interest in FP counseling. Although this may present a bias, it is also helpful when considering the generalizability of the tool for future studies; additional questions may need to be added to consider such bias. This survey was completed by self-report, which may not reflect the nurses’ actual knowledge of FP resources and referral options. Instead, it may indicate the nurses’ perceived understanding of FP in their oncology population.
Implications for Nursing
The final instrument can be used by staff, educators, and researchers to develop and test quality initiatives designed to improve the use of FP counseling. Staff nurses can use the instrument for self-assessment, as well as to determine which domain should be addressed first to have the greatest impact. Educators can use the instrument to facilitate a needs assessment tailored to their practice environment.
Nurses engaged in research can use the instrument to evaluate the impact of practice change interventions. For example, one site had incorporated a comprehensive resource and referral option within the electronic health record with individualized education and referral, as well as education within a continuing education offer and new nurse orientation. However, based on survey responses, this did not increase all of the nurses’ confidence levels or perceived time constraints. Therefore, the survey results were critical to the development of a new, targeted education plan for nurses. Future plans for this work include testing nursing interventions targeted at specific domains associated with real or perceived barriers to promoting FP counseling. 
Conclusion
This 15-item instrument can be broadly disseminated to nursing staff who provide direct care to people with cancer. The findings support the initial validity of the survey to gain insight into the barriers nurses face in discussing FP measures with individuals with cancer in their reproductive years. Additional research is warranted to confirm the validity of this instrument in a larger and more diverse cohort.
About the Author(s)
Grabowski is a program manager and Spitzer is an RN and nurse educator, both in the Harold C. Simmons Comprehensive Cancer Center; and Stutzman is a clinical research manager and Olson is an associate professor, both in the Department of Neurology and Neurotherapeutics, all at the University of Texas Southwestern Medical Center in Dallas. No financial relationships to disclose. Grabowski and Spitzer completed the data collection. Olson provided statistical support. Grabowski, Stutzman, and Olson provided the analysis. All authors contributed to the conceptualization and design and the manuscript preparation. Grabowski can be reached at maria.grabowski@utsouthwestern.edu, with copy to editor at ONFEditor@ons.org. Submitted July 2016. Accepted for publication November 21, 2016.
References
American Cancer Society. (2017). Cancer facts and figures, 2017. Retrieved from https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and…
Benedict, C., Shuk, E., & Ford, J.S. (2016). Fertility issues in adolescent and young adult cancer survivors. Journal of Adolescent and Young Adult Oncology, 5, 48–57. doi:10.1089/jayao.2015.0024
Blayney, D.W., McNiff, K., Eisenberg, P.D., Gilmore, T., Jacobsen, P.B., Jacobson, J.O., . . . Simone, J. (2014). Development and future of the American Society of Clinical Oncology’s Quality Oncology Practice Initiative. Journal of Clinical Oncology, 32, 3907–3913. doi:10.1200/JCO.2014.56.8899
Deshpande, N.A., Braun, I.M., & Meyer, F.L. (2015). Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: A systematic review. Cancer, 121, 3938–3947. doi:10.1002/cncr.29637
DeVellis, R.F. (2012). Scale development: Theory and applications (3rd ed). London: Sage.
Ellis, S.J., Wakefield, C.E., McLoone, J.K., Robertson, E.G., & Cohn, R.J. (2016). Fertility concerns among child and adolescent cancer survivors and their parents: A qualitative analysis. Journal of Psychosocial Oncology, 34, 347–362. doi:10.1080/07347332.2016.1196806
Ethics Committee of the American Society for Reproductive Medicine. (2005). Fertility preservation and reproduction in cancer patients. Fertility and Sterility, 83, 1622–1628. doi:10.1016/j.fertnstert.2005.03.013
Flink, D.M., Sheeder, J., & Kondapalli, L.A. (2017). A review of the oncology patient’s challenges for utilizing fertility preservation services. Journal of Adolescent and Young Adult Oncology, 6, 31–44. doi:10.1089/jayao.2015.0065
Ginsberg, J. P. (2012). Gonadotoxicity of cancer therapies in pediatric and reproductive-age males. In C. Gracia & T.K. Woodruff (Eds.), Oncofertility medical practice: Clinical issues and implementation (pp. 15–23). New York, NY: Springer.
King, L., Quinn, G.P., Vadaparampil, S.T., Gwede, C.K., Miree, C.A., Wilson, C., . . . Perrin, K. (2008). Oncology nurses’ perceptions of barriers to discussion of fertility preservation with patients with cancer. Clinical Journal of Oncology Nursing, 12, 467–476. doi:10.1188/08.CJON.467-476
Klosky, J.L., Simmons, J.L., Russell, K.M., Foster, R.H., Sabbatini, G.M., Canavera, K.E., . . . McDermott, M.J. (2015). Fertility as a priority among at-risk adolescent males newly diagnosed with cancer and their parents. Supportive Care in Cancer, 23, 333–341. doi:10.1007/s00520-014-2366-1
Lee, S.J., Schover, L.R., Partridge, A.H., Patrizio, P., Wallace, W.H., Hagerty, K., . . . Oktay, K. (2006). American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. Journal of Clinical Oncology, 24, 2917–2931. doi:10.1200/JCO.2006.06.5888
Letourneau, J.M., Ebbel, E.E., Katz, P.P., Katz, A., Ai, W.Z., Chien, A.J., . . . Rosen, M.P. (2012). Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer, 118, 1710–1717. doi:10.1002/cncr.26459
Levine, J.M., Kelvin, J.F., Quinn, G.P., & Gracia, C.R. (2015). Infertility in reproductive-age female cancer survivors. Cancer, 121, 1532–1539. doi:10.1002/cncr.29181
Loren, A.W., Mangu, P.B., Beck, L.N., Brennan, L., Magdalinski, A.J., Partridge, A.H., . . . Oktay, K. (2013). Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology, 31, 2500–2510. doi:10.1200/jco.2013.49.2678
National Cancer Institute. (2015). Adolescents and young adults with cancer. Retrieved from http://www.cancer.gov/types/aya
Nobel Murray, A., Chrisler, J.C., & Robbins, M.L. (2016). Adolescents and young adults with cancer: Oncology nurses report attitudes and barriers to discussing fertility preservation [Online exclusive]. Clinical Journal of Oncology Nursing, 20, E93–E99. doi:10.1188/16.CJON.E93-E99
Peate, M., Meiser, B., Hickey, M., & Friedlander, M. (2009). The fertility-related concerns, needs and preferences of younger women with breast cancer: A systematic review. Breast Cancer Research and Treatment, 116, 215–223. doi:10.1007/s10549-009-0401-6
Pett, M.A., Lackey, N.R., & Sullivan, J.J. (2003). Making sense of factor analysis: The use of factor analysis for instrument development in health care research. Thousand Oaks, CA: Sage.
Quinn, G.P., Vadaparampil, S.T., Bell-Ellison, B.A., Gwede, C.K., & Albrecht, T.L. (2008). Patient–physician communication barriers regarding fertility preservation among newly diagnosed cancer patients. Social Science and Medicine, 66, 784–789. doi:10.1016/j.socscimed.2007.09.013
Vadaparampil, S.T., Gwede, C.K., Meade, C., Kelvin, J., Reich, R.R., Reinecke, J., . . . Quinn, G.P. (2016). ENRICH: A promising oncology nurse training program to implement ASCO clinical practice guidelines on fertility for AYA cancer patients. Patient Education and Counseling, 99, 1907–1910. doi:10.1016/j.pec.2016.05.013
Vadaparampil, S.T., Hutchins, N.M., & Quinn, G.P. (2013). Reproductive health in the adolescent and young adult cancer patient: An innovative training program for oncology nurses. Journal of Cancer Education, 28, 197–208. doi:10.1007/s13187-012-0435-z
Winkler-Crepaz, K., Ayuandari, S., Ziehr, S.C., Hofer, S., & Wildt, L. (2015). Fertility preservation in cancer survivors. Minerva Endocrinologica, 40, 105–118.
Yee, S., Abrol, K., McDonald, M., Tonelli, M., & Liu, K.E. (2012). Addressing oncofertility needs: Views of female cancer patients in fertility preservation. Journal of Psychosocial Oncology, 30, 331–346. doi:10.1080/07347332.2012.664257

