Self-Management Intervention for Adult Cancer Survivors After Treatment: A Systematic Review and Meta-Analysis
Problem Identification: This study aims to evaluate the effects of self-management interventions (SMIs) for cancer survivors who completed primary treatment.
Literature Search: Using PubMed, EMBASE, CINAHL®, PsycINFO®, and Cochrane Central Register of Controlled Trails (CENTRAL), the authors conducted a systematic search of randomized, controlled trials published in English from database conception through June 2016.
Data Evaluation: The meta-analysis was conducted with Cochrane Review Manager, version 5.3, and R program, version 3.3.1.
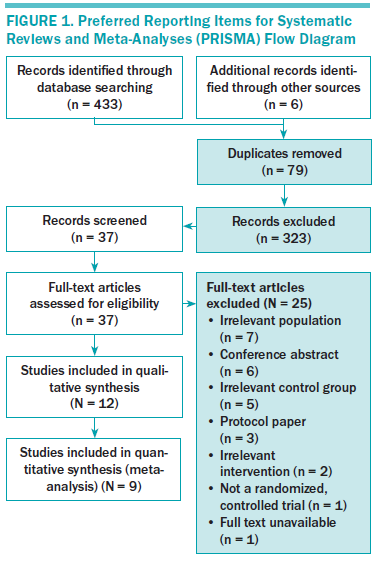
Synthesis: 12 studies were systematically reviewed for self-management content, mode of delivery, session composition, and type of self-management skills used. Then, a meta-analysis of nine randomized, controlled trials involving 2,804 participants was conducted comparing SMIs with usual care, attention control, and a waitlist group. Qualitative synthesis showed that (a) the major study population was comprised of breast cancer survivors; (b) SMIs focused on medical/behavioral and emotional management; (c) the most common mode of delivery was web-based; and (d) the most frequently evaluated outcomes were depression, self-efficacy, and health-related quality of life (HRQOL). Quantitative results demonstrated a significant medium effect on HRQOL and a large effect on fatigue of borderline significance. The effects on anxiety, depression, and self-efficacy were not statistically significant.
Conclusions: SMIs had a significant medium effect on HRQOL for cancer survivors post-treatment, but the findings should be interpreted with caution because of substantial heterogeneity. In addition, the small number of studies limits conclusions.
Implications for Nursing: SMI as a nursing intervention for improving HRQOL of cancer survivors can be recommended, but more research should be undertaken to determine the most effective SMI format in terms of type, mode of delivery, and session composition.
Jump to a section
Treatment completion does not signal the end of the cancer experience; many cancer survivors and their families continue to face problems associated with a complex chronic condition (Miller, 2008; Phillips & Currow, 2010). Long-term or late effects include fatigue (Kim et al., 2008; Pachman, Barton, Swetz, & Loprinzi, 2012), lymphedema (Paskett, Dean, Oliveri, & Harrop, 2012), anxiety and depression (Stanton, 2006), infertility (Ruddy & Partridge, 2012), sexual dysfunction (Bober & Varela, 2012), and cardiac complications (Lenihan & Cardinale, 2012), all of which negatively affect health-related quality of life (HRQOL) and increase medical costs (Hewitt, Greenfield, & Stovall, 2006). In addition, cancer survivors have elevated risks for additional malignancies and comorbid conditions, such as hypertension, diabetes, and osteoporosis (Rowland & Yancik, 2006; Schultz, Beck, Stava, & Vassilopoulou-Sellin, 2003; Wood et al., 2012). Therefore, long-term planning and preventive strategies are strongly recommended (Rowland & Yancik, 2006).
For cancer survivors, long-term planning requires an ongoing collaborative relationship between patients and healthcare providers rather than an acute, prescriptive relationship (McCorkle et al., 2011). These partnership relationships enable and empower patients to achieve their own care goals. Self-management may be a means of bridging the gap between survivors’ needs and the capacity of healthcare providers to meet those needs (Barlow, Wright, Sheasby, Turner, & Hainsworth, 2002).
Self-management in cancer survivorship has been defined as “awareness and active participation by the person in their recovery and rehabilitation to minimize the consequences of treatment and promote survival, health, and well-being” (Macmillan Cancer Support & NHS Improvement, 2010, p. 6). Self-management can empower patients with cancer, increase their confidence to manage problems associated with the disease and its treatment, and enhance HRQOL (Barlow, Bancroft, & Turner, 2005; Lorig, Sobel, Ritter, Laurent, & Hobbs, 2001).
Enthusiasm for self-management interventions (SMIs) for cancer survivors is growing as randomized, controlled trials (RCTs) provide evidence of its efficacy. SMIs significantly improve cancer knowledge (Gil et al., 2006; Mishel et al., 2005), cognitive reframing (Germino et al., 2013; Gil et al., 2006; Mishel et al., 2005), and self-efficacy (Lee et al., 2014; van den Berg et al., 2015; Yun et al., 2012); decrease psychological distress (e.g., anxiety, depression); and enhance HRQOL (Lee et al., 2014; Olesen et al., 2016; Yun et al., 2012). However, studies on the outcomes of self-management are not consistent, and some RCTs (Braamse et al., 2016; Foster et al., 2016; Owen et al., 2005) found no effects. Therefore, a need exists for a critical analysis of whether SMIs can improve physical or psychological outcomes among cancer survivors.
Four systematic reviews have contributed to the awareness of the importance of self-management in the area of cancer survivorship, but they did not focus on survivors who completed cancer treatment (Hammer et al., 2015; Kim & Park, 2015; McCorkle et al., 2011; Smith-Turchyn, Morgan, & Richardson, 2016). In the area of clinical practice and research, survivorship focuses on the health and life of a person with cancer post-treatment until the end of life (National Cancer Institute, 2017). A review focusing on people whose curative treatment has ended may contribute to the development of evidence-based SMIs for this population. In addition, two reviews evaluated the effects of only one mode of SMI delivery (i.e., web-based [Kim & Park, 2015] or group-based [Smith-Turchyn et al., 2016]), and the other two did not report quantitative synthesis (i.e., meta-analysis) (Hammer et al., 2015; McCorkle et al., 2011). Therefore, the current authors conducted a systematic review and meta-analysis of the effects of SMI for cancer survivors who completed their primary treatment.
Methods
This systematic review and meta-analysis was guided by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Moher, Liberati, Tetzlaff, & Altman, 2009).
Eligibility Criteria
The authors selected only RCTs published in English from peer-reviewed journals. Eligibility criteria followed the PICO (Participants, Interventions, Controls, and Outcomes) framework (Liberati et al., 2009). Participants were disease-free cancer survivors, aged 18 years or older, who completed curative primary cancer treatment (surgery, chemotherapy, radiation therapy, and/or adjuvant therapy). Participants diagnosed with cancer in childhood were excluded. SMI was defined as the systematic provision of education and supportive interventions with the purpose of promoting survivors’ skills in managing their condition, such as problem solving, decision making, resource use, forming partnerships, and taking action (Lorig & Holman, 2003). The authors included studies that applied at least one skill and compared them to the usual care group, attention control group, and waitlist group. Each eligible study included physical, psychological, cognitive, and/or behavioral outcomes measured with validated instruments.
Search Strategy
The authors conducted an extensive literature search in the electronic databases of PubMed, EMBASE, CINAHL®, PsycINFO®, and Cochrane Central Register of Controlled Trails (CENTRAL) from database conception through June 2016 using the following medical subject heading (MeSH) terms and keywords in the title or abstract: (“neoplasm” OR “cancer”) AND (“self-management” OR “self-care” OR “self-help” OR “self-administ*” OR “self-guided” OR “self-directed”) AND (“after care” OR “post treatment” OR “after treatment” OR “follow-up care” OR “survivorship”) AND (“randomized controlled trial” OR “randomised controlled trial” OR “randomiz*” OR “randomis*”). The authors then hand searched reference lists of the identified studies and relevant reviews.
Study Selection
Studies were selected following PRISMA guidelines (Moher et al., 2009). In the first step, one reviewer screened the titles and abstracts of all studies in a standardized manner and excluded those determined to be irrelevant. In the second step, the authors retrieved full-text articles of all potentially relevant studies, and two authors independently screened each one using defined inclusion criteria. Disagreements were resolved by consensus.
Data Extraction and Quality Assessment
Two reviewers independently extracted data using a standardized data extraction sheet developed by the authors. Disagreements were resolved by discussion. Data extracted from the study included authors, year of publication, country of origin, sample characteristics, intervention details (contents of SMI, mode of delivery, sessions, self-management skills included), control conditions, study outcomes, and measurement time points.
The same two reviewers independently assessed the methodologic quality of the identified studies using the Cochrane Collaboration’s tool for assessing risk of bias (Higgins et al., 2011), which evaluates random sequence generation, allocation concealment, blinding of participants and staff, blinding of outcome assessment, incomplete outcome data, and selective reporting. Each item was rated as having a low, unclear, or high risk of bias. For these six items, blinding of assessors was not considered because all studies used self-reported questionnaires for outcome assessment. A pilot test was conducted of three studies before the authors independently assessed study quality. Disagreement was resolved by discussion.
Statistical Analysis
The authors performed a meta-analysis when an outcome was reported in three or more studies and the study provided enough data to allow the calculation of effect sizes. If the study had multiple measuring points postintervention, effect sizes were primarily calculated with the first post-test value. The authors also examined long-term effects of SMIs if available.
The authors estimated between-group standardized mean difference (SMD) with 95% confidence interval as the summary measure of effect and used means and standard deviations of outcomes to calculate the SMD (Cohen’s d). The authors considered a Cohen’s d of 0.8 to be large, 0.5 to be medium, and 0.2 to be small (Cohen, 1962). Chi-square statistics were used to assess heterogeneity. Chi-square values higher than 50% were considered to indicate substantial heterogeneity, and a random-effects model was applied to analyze the data (Higgins et al., 2011). The authors did not evaluate publication bias. According to the guidelines, tests for funnel plot asymmetry should be used only when a meta-analysis includes at least 10 studies; with fewer studies, the power of the tests is too low to rule out chance in the observed asymmetry (Higgins et al., 2011). The authors conducted the meta-analysis with Cochrane Review Manager, version 5.3, and R program, version 3.3.1, and considered p values less than 0.05 to be statistically significant. All statistical tests were two-sided.
Results
Study Selection and Study Quality
Figure 1 depicts the study selection flow diagram. Twelve articles met the eligibility criteria and were included in the systematic review. Three of them, however, did not include the necessary data for calculating effect size. Therefore, the meta-analysis was conducted with nine articles.
All trials except for one (Gil et al., 2006) reported an adequate method of random sequence generation, but six did not report an adequate method of allocation concealment. Given the nature of the psychosocial intervention, no trials applied study participant blinding, which inevitably causes performance bias. Ten studies reported the dropout and attrition rate, providing sufficiently complete data. Bias from selective reporting was observed in five trials that did not publish a protocol paper or report partial outcomes.
Study Characteristics
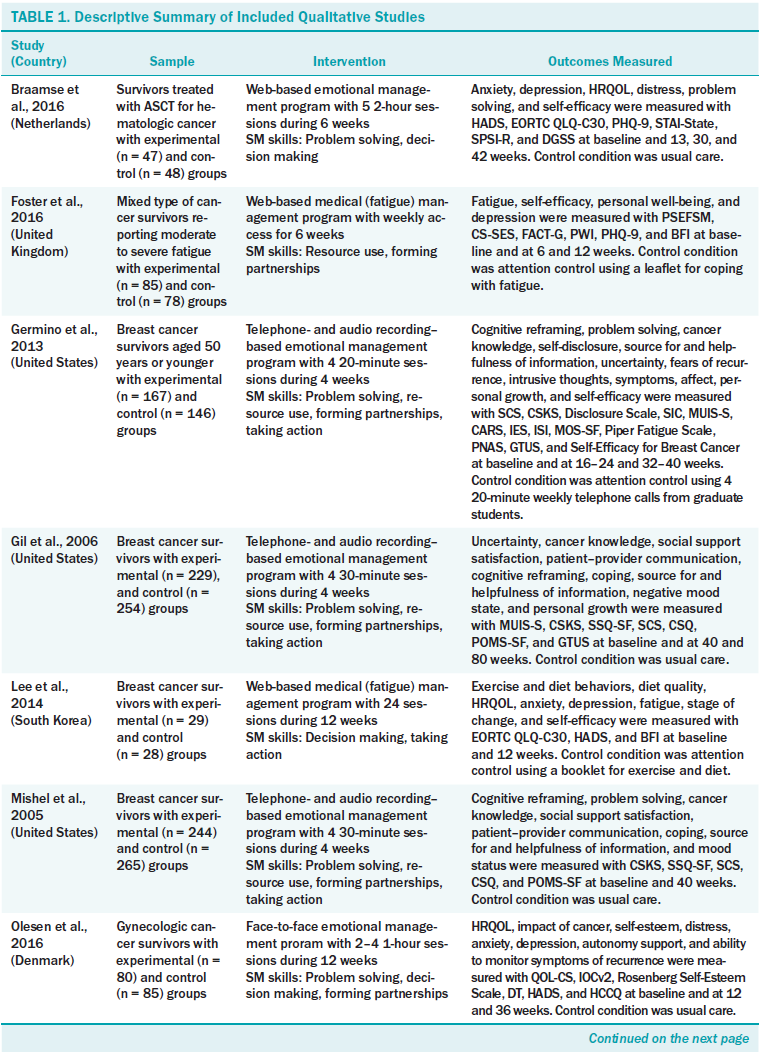
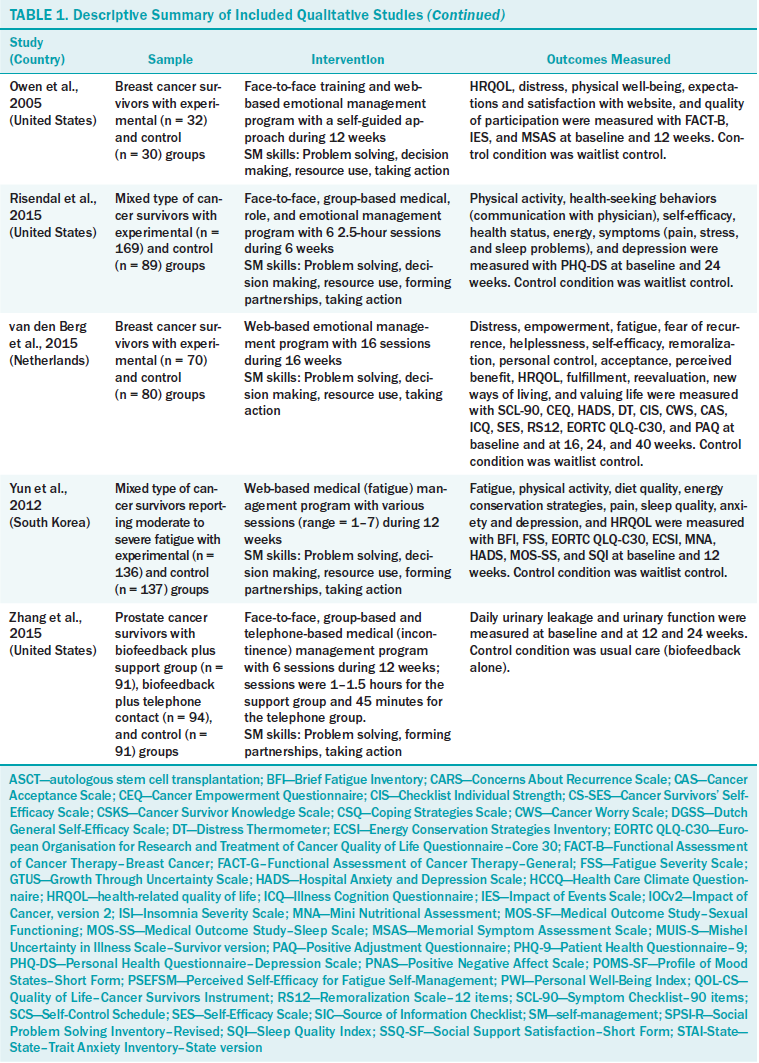
Table 1 summarizes the characteristics of the 12 studies. Six were conducted in the United States, two in the Netherlands, two in South Korea, one in Denmark, and one in the United Kingdom. The sample sizes varied from 57–509, with a total of 2,804 participants. The most common cancer type was breast cancer (six studies); other cancer types included in the studies were three mixed, one hematologic, one gynecologic, and one prostate.
Intervention and Control Conditions
The mean duration of an SMI was 8.8 weeks (range = 4–16). Based on Lorig and Holman’s (2003) model, the authors categorized the contents of the intervention as medical/behavioral management, role management, or emotional management. The majority of SMIs (n = 7) dealt with emotional management, including distress management (Braamse et al., 2016; van den Berg et al., 2015), uncertainty management (Germino et al., 2013; Gil et al., 2006; Mishel et al., 2005), empowerment (Olesen et al., 2016), and coping (Owen et al., 2005). Four trials targeted medical/behavioral management, such as fatigue management (Foster et al., 2016; Yun et al., 2012), incontinence management (Zhang et al., 2015), or exercise and diet (Lee et al., 2014). Only one trial (Risendal et al., 2015) applied a multicomponent intervention, combining medical, role, and emotional management. No study used role management alone.
The most common mode of delivery was web-based, either alone (n = 5) or combined with a face-to-face encounter (n = 1). Four trials were administered face-to-face (two alone, one combined with the Internet, and one via telephone) (Olesen et al., 2016; Owen et al., 2005; Risendal et al., 2015; Zhang et al., 2015). Three were administered via telephone combined with audio recording (Germino et al., 2013; Gil et al., 2006; Mishel et al., 2005). All studies conducted via telephone used only telecounseling intervention, not smartphone mobile applications. The number of sessions varied according to the mode of delivery. In the web-based trials, the duration of the intervention was clearly indicated (range = 6–16 weeks), but time per session was not limited in most studies (Foster et al., 2016; Lee et al., 2014; Owen et al., 2005; van den Berg et al., 2015; Yun et al., 2012). Researchers asked participants to access the research website regularly (one to two times per week) (Braamse et al., 2016; Foster et al., 2016; Lee et al., 2014; van den Berg et al., 2015) in a self-guided (Owen et al., 2005) or tailored (Yun et al., 2012) manner. In trials using a telephone (Germino et al., 2013; Gil et al., 2006; Mishel et al., 2005; Zhang et al., 2015), the duration of the intervention ranged from 4–12 weeks, and time per session ranged from 20–45 minutes (mean = 31.3 minutes).
The most frequently used self-management skill was problem solving (n = 10). Other skills included taking action (n = 9), resource use (n = 8), forming partnerships (n = 8), and decision making (n = 7). However, only two trials used the five skills together (Risendal et al., 2015; Yun et al., 2012).
The control groups were usual care (n = 6), attention control (n = 3), and waitlist (n = 3). The attention control group was provided with a leaflet (Foster et al., 2016) or a booklet (Lee et al., 2014) and telephone information (Germino et al., 2013).
Outcome Measures
All studies measured psychological outcomes with or without physical, social, or cognitive outcomes. Frequently measured outcomes were depression (n = 6), self-efficacy (n = 6), and HRQOL (n = 6), followed by fatigue (n = 5) and anxiety (n = 5). Fatigue as a physical outcome was measured using the Brief Fatigue Inventory (n = 3), the Piper Fatigue Scale (n = 1), or the Fatigue Severity Scale (n = 1). The authors of the examined studies commonly measured anxiety and depression with the Hospital Anxiety and Depression Scale (n = 5), and used the State–Trait Anxiety Inventory for anxiety and the Patient Health Questionnaire–9 for depression. Five of six studies of self-efficacy were for specific activities, such as exercise self-efficacy, diet self-efficacy, fatigue management self-efficacy, or breast cancer–specific self-efficacy. The most commonly used instrument for measuring HRQOL was the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 (n = 4). Others included the Functional Assessment of Cancer Therapy–Breast Cancer (n = 1), Functional Assessment of Cancer Therapy–General (n = 1), and Quality of Life–Cancer Survivors Instrument (n = 1).
Seven studies measured outcomes multiple times postintervention to examine long-term effects, with time points ranging from 12– 80 weeks. Five studies selected 36 weeks postintervention as the long-term evaluation point.
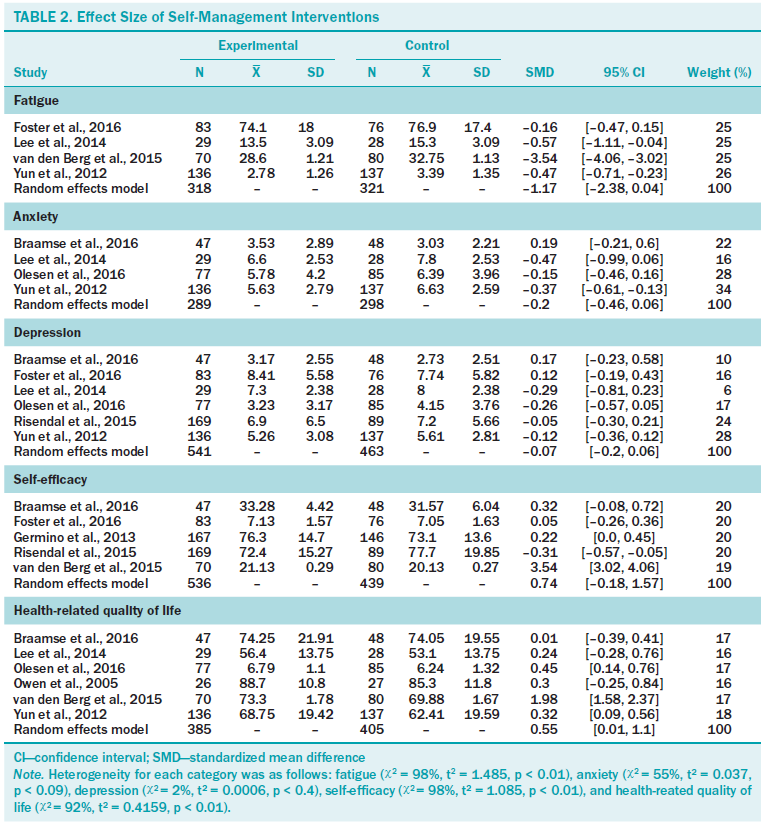
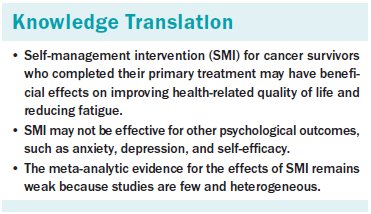
Table 2 demonstrates the effect sizes of selected outcomes. Regarding physical outcomes, the authors observed a large effect on fatigue of borderline statistical significance (d = –1.17, p = 0.058, chi-square = 98%). Among psychological outcomes, the authors observed a significant medium effect on HRQOL (d = 0.55, p = 0.046, chi-square = 92%). The authors found no significant effects on anxiety (d = –0.2, p = 0.132, chi-square = 55%), depression (d = –0.07, p = 0.284, chi-square = 2%), or self-efficacy (d = 0.73, p = 0.117, chi-square = 55%). Among five study outcomes, the authors could calculate long-term effect sizes for depression (n = 2) and self-efficacy (n = 3) only. A significant small effect of SMI on self-efficacy was found (d = 0.27, p = 0.021, chi-square = 44.9%), but no significant long-term effect on depression was found (d = –0.2, p = 0.477, chi-square = 51%) (data not shown).
Discussion
The finding of a significant medium effect of SMI (d = 0.55) in improving the HRQOL of cancer survivors is consistent with Kim and Park’s (2015) finding for a web-based SMI. Because one of the goals of survivorship care is to improve HRQOL, the finding is encouraging. Another important finding was the large effect of SMI on fatigue (d = –1.17), but the role of chance cannot be ruled out (p = 0.058). Cancer-related fatigue is one of the most frequent problems cancer survivors report and can persist for years after treatment is completed (Kim et al., 2008; Leak Bryant, Walton, & Phillips, 2015). Given the growing body of literature, survivors who complain of cancer-related fatigue may benefit from nonpharmacologic intervention, such as exercise and cognitive behavioral therapy (Leak Bryant et al., 2015; Mitchell et al., 2014), but these programs are not accessible to many and often require substantial resources (Foster et al., 2016). In this respect, SMI for fatigue management is promising.
The null findings for the effect of SMI on anxiety and depression were unexpected. One possible explanation may relate to mode of delivery. The majority of studies that tested these outcomes (three of four for anxiety and four of six for depression) administered SMI using the Internet. Web-based programs can reach a large population in a cost-effective way (Foster et al., 2016), but their use does not guarantee delivery of the planned intervention dose because of the nature of the intervention itself. In addition, except in the trial by Braamse et al. (2016), anxiety and depression were not primary outcomes and were not screened at baseline. Many participants included in these trials showed minimal to mild levels of anxiety and depression, leading to null findings because of the bottom effect. Finally, only two trials (Risendal et al., 2015; Yun et al., 2012) used all five self-management skills (problem solving, decision making, resource use, forming partnerships, and taking action) suggested by Lorig and Holman’s (2003) model. Additional trials need to develop SMI using diverse skills to intensify efficacy. Of note, the authors found no significant self-efficacy effect at the first post-test time point (n = 5) but did at a delayed time point (n = 3), perhaps because of less heterogeneity (chi-square = 98% versus 45%). Because of the small number of studies, further evaluation is required.
Qualitative synthesis revealed that program content focused only on medical/behavioral management (Foster et al., 2016; Lee et al., 2014; Yun et al., 2012; Zhang et al., 2015) and emotional management (Braamse et al., 2016; Germino et al., 2013; Gil et al., 2006; Mishel et al., 2005; Olesen et al., 2016; Owen et al., 2005; van den Berg et al., 2015). Healthcare professionals should be aware of the importance of role management, including in the family, at work, in communication, and in independent living. This is important in helping cancer survivors deal with their chronic condition in daily life.
The authors cannot draw firm conclusions about the effectiveness of single versus multiple content of SMI because the number of studies is small, but additional evaluations should fill in these details. In this study, only physical and psychological outcomes were reported. Three trials (Germino et al., 2013; Gil et al., 2006; Mishel et al., 2005) measured cognitive or social outcomes, such as cancer knowledge, cognitive reframing, and patient–provider communication, but the authors could not calculate the effect size for those because of insufficient data. Trials conducted with people with other chronic conditions have investigated SMI effects regarding cognitive or cost-effectiveness outcomes. Several reviews involving patients with type 2 diabetes or chronic obstructive pulmonary disease demonstrated that SMI had significant effects on improving disease knowledge and reducing hospital admissions and emergency department visits (Lian et al., 2017; Wang, Tan, Xiao, & Deng, 2017). Those outcomes warrant attention by clinicians and policy makers. Still, the majority of evidence comes from studies of breast cancer survivors, so additional trials are needed to expand the study population.
Limitations
The small number of studies (n = 9) included in the meta-analysis limits interpretation of the results. The authors could not perform moderator analysis (i.e., meta-regression), which could provide information about more effective SMI formats, and publication bias could not be evaluated. In the analysis of long-term SMI effects, the authors reported results of only two outcomes (depression and self-efficacy) because of insufficient data regarding other outcomes. Given the importance of long-term care planning for cancer survivors after treatment, long-term trials are needed to identify the sustained effects of SMI. In addition, considerable heterogeneity was found among the included studies for all outcomes except for depression. Therefore, the results must be interpreted with caution. Another important limitation is the high risk of bias from nonblinding. It is impossible to blind participants when administering psychosocial interventions. Alternatively, researchers can use an attention control group and blind participants to the study hypothesis. Lastly, although the authors tried to retrieve all potentially eligible articles, some may have been missed.
Implications for Nursing
Survivors are at risk not only for adverse treatment effects, but also for comorbid conditions (e.g., another cancer, cardiovascular disease, diabetes, osteoporosis) (Mayer, Nasso, & Earp, 2017; Rowland & Yancik, 2006; Wood et al., 2012). This population is likely to be highly motivated to promote post-treatment health (Demark-Wahnefried, Pinto, & Gritz, 2006). Within this context, cancer survivors are recognized as having a chronic condition similar to diabetes or arthritis, and self-management is an essential component of care (Knobf et al., 2015). However, this meta-analysis could not conclusively demonstrate SMI effects because the only significant effect observed was in HRQOL; evidence for other outcomes remains unclear.
Oncology nurses are optimally positioned to deliver support for cancer survivor self-management. For more effective implementation of SMI, oncology nurses should consider the following points. First, intervention is needed to promote delivery of the planned intervention dose, particularly when it is web-based. Second, interventions need to be adapted to the survivor’s needs. Several studies that did not screen for specific needs failed to prove the effectiveness of the intervention. Third, interventions should be developed by using various self-management skills, such as problem solving, forming partnerships, and taking action. Fourth, long-term follow-up is needed to identify sustained SMI effects. Finally, future studies should investigate neglected issues, such as targeting a population other than breast cancer survivors; using face-to-face delivery; and measuring cognitive, social, or cost-effectiveness outcomes.
Conclusion
SMI had a significant medium effect on improving HRQOL among cancer survivors and a large effect of borderline significance on reducing fatigue. The authors found no significant effect on other psychological outcomes (i.e., anxiety, depression, and self-efficacy). Still, because of the small number of included studies, no definite conclusion can be drawn about practical issues about a more effective SMI format in terms of content, delivery mode, or session composition. More trials are needed because SMI could be an important part of the management of the growing number of cancer survivors.
About the Author(s)
S. Kim is an associate professor in the Department of Nursing at Inha University in Incheon, South Korea; K. Kim is an associate professor in the College of Nursing at Chung-Ang University in Seoul, South Korea; and Mayer is a professor in the School of Nursing at the University of North Carolina and director of cancer survivorship at the University of North Carolina Lineberger Cancer Center in Chapel Hill. This research was funded by a Basic Science Research Program grant (2016R1D1A1B04932171) from the National Research Foundation of Korea through funding from the Ministry of Education. S. Kim and K. Kim completed the data collection and provided statistical support. K. Kim and Mayer provided the analysis. All authors contributed to the conceptualization and design and the manuscript preparation. K. Kim can be reached at kiskim@cau.ac.kr, with copy to editor at ONFEditor@ons.org. Submitted February 2017. Accepted for publication April 24, 2017.
References
Barlow, J., Wright, C., Sheasby, J., Turner, A., & Hainsworth, J. (2002). Self-management approaches for people with chronic conditions: A review. Patient Education and Counseling, 48, 177–187.
Barlow, J.H., Bancroft, G.V., & Turner, A.P. (2005). Self-management training for people with chronic disease: A shared learning experience. Journal of Health Psychology, 10, 863–872. https://doi.org/10.1177/1359105305057320
Bober, S.L., & Varela, V.S. (2012). Sexuality in adult cancer survivors: Challenges and intervention. Journal of Clinical Oncology, 30, 3712–3719. https://doi.org/10.1200/JCO.2012.41.7915
Braamse, A.M., van Meijel, B., Visser, O.J., Boenink, A.D., Cuijpers, P., Eeltink, C.E., . . . Dekker, J. (2016). A randomized clinical trial on the effectiveness of an intervention to treat psychological distress and improve quality of life after autologous stem cell transplantation. Annals of Hematology, 95, 105–114. https://doi.org/10.1007/s00277-015-2509-6
Cohen, J. (1962). The statistical power of abnormal-social psychological research: A review. Journal of Abnormal and Social Psychology, 65, 145–153.
Demark-Wahnefried, W., Pinto, B.M., & Gritz, E.R. (2006). Promoting health and physical function among cancer survivors: Potential for prevention and questions that remain. Journal of Clinical Oncology, 24, 5125–5131. https://doi.org/10.1200/JCO.2006.06.6175
Foster, C., Grimmett, C., May, C.M., Ewings, S., Myall, M., Hulme, C., . . . Richardson, A. (2016). A web-based intervention (RESTORE) to support self-management of cancer-related fatigue following primary cancer treatment: A multi-centre proof of concept randomised controlled trial. Supportive Care in Cancer, 24, 2445–2453. https://doi.org/10.1007/s00520-015-3044-7
Germino, B.B., Mishel, M.H., Crandell, J., Porter, L., Blyler, D., Jenerette, C., & Gil, K.M. (2013). Outcomes of an uncertainty management intervention in younger African American and Caucasian breast cancer survivors. Oncology Nursing Forum, 40, 82–92. https://doi.org/10.1188/13.ONF.82-92
Gil, K.M., Mishel, M.H., Belyea, M., Germino, B., Porter, L.S., & Clayton, M. (2006). Benefits of the uncertainty management intervention for African American and white older breast cancer survivors: 20-month outcomes. International Journal of Behavioral Medicine, 13, 286–294. https://doi.org/10.1207/s15327558ijbm1304_3
Hammer, M.J., Ercolano, E.A., Wright, F., Dickson, V.V., Chyun, D., & Melkus, G.D. (2015). Self-management for adult patients with cancer: An integrative review. Cancer Nursing, 38(2), E10–E26. https://doi.org/10.1097/NCC.0000000000000122
Hewitt, M., Greenfield, S., & Stovall, E. (Eds.). (2006). From cancer patient to cancer survivor: Lost in transition. Washington, DC: National Academies Press.
Higgins, J.P., Altman, D.G., Gotzsche, P.C., Jüni, P., Moher, D., Oxman, A.D., . . . Sterne, J.A. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343, d5928. https://doi.org/10.1136/bmj.d5928
Kim, A.R., & Park, H.A. (2015). Web-based self-management support interventions for cancer survivors: A systematic review and meta-analyses. Studies in Health Technology and Informatics, 216, 142–147.
Kim, S.H., Son, B.H., Hwang, S.Y., Han, W., Yang, J.H., Lee, S., & Yun, Y.H. (2008). Fatigue and depression in disease-free breast cancer survivors: Prevalence, correlates, and association with quality of life. Journal of Pain and Symptom Management, 35, 644–655. https://doi.org/10.1016/j.jpainsymman.2007.08.012
Knobf, M.T., Cooley, M.E., Duffy, S., Doorenbos, A., Eaton, L., Given, B., . . . Mallory, G. (2015). The 2014-2018 Oncology Nursing Society research agenda. Oncology Nursing Forum, 42, 450–465. https://doi.org/10.1188/15.ONF.450-465
Leak Bryant, A., Walton, A.L., & Phillips, B. (2015). Cancer-related fatigue: Scientific progress has been made in 40 years. Clinical Journal of Oncology Nursing, 19, 137–139. https://doi.org/10.1188/15.CJON.137-139
Lee, M.K., Yun, Y.H., Park, H.A., Lee, E.S., Jung, K.H., & Noh, D.Y. (2014). A web-based self-management exercise and diet intervention for breast cancer survivors: Pilot randomized controlled trial. International Journal of Nursing Studies, 51, 1557–1567. https://doi.org/10.1016/j.ijnurstu.2014.04.012
Lenihan, D.J., & Cardinale, D.M. (2012). Late cardiac effects of cancer treatment. Journal of Clinical Oncology, 30, 3657–3664. https://doi.org/10.1200/JCO.2012.45.2938
Lian, J.X., McGhee, S.M., Chau, J., Wong, C.K.H., Lam, C.L.K., & Wong, W.C.W. (2017). Systematic review on the cost-effectiveness of self-management education programme for type 2 diabetes mellitus. Diabetes Research and Clinical Practice, 127, 21-34. https://doi.org/10.1016/j.diabres.2017.02.021
Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gøtzsche, P.C., Ioannidis, J.P., . . . Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine, 151(4), W65–W94.
Lorig, K.R., & Holman, H. (2003). Self-management education: History, definition, outcomes, and mechanisms. Annals of Behavioral Medicine, 26, 1–7.
Lorig, K.R., Sobel, D.S., Ritter, P.L., Laurent, D., & Hobbs, M. (2001). Effect of a self-management program on patients with chronic disease. Effective Clinical Practice, 4(6), 256–262.
Macmillan Cancer Support & NHS Improvement. (2010). The national cancer survivorship initiative vision. Retrieved from http://webarchive.nationalarchives.gov.uk/20100809113601/http://www.imp…
Mayer, D.K., Nasso, S.F., & Earp, J.A. (2017). Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncology, 18(1), e11–e18. https://doi.org/10.1016/S1470-2045(16)30573-3
McCorkle, R., Ercolano, E., Lazenby, M., Schulman-Green, D., Schilling, L.S., Lorig, K., & Wagner, E.H. (2011). Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA: A Cancer Journal for Clinicians, 61, 50–62. https://doi.org/10.3322/caac.20093
Miller, R. (2008). Implementing a survivorship care plan for patients with breast cancer. Clinical Journal of Oncology Nursing, 12, 479–487. https://doi.org/10.1188/08.CJON.479-487
Mishel, M.H., Germino, B.B., Gil, K.M., Belyea, M., Laney, I.C., Stewart, J., . . . Clayton, M. (2005). Benefits from an uncertainty management intervention for African-American and Caucasian older long-term breast cancer survivors. Psycho-Oncology, 14, 962–978. https://doi.org/10.1002/pon.909
Mitchell, S.A., Hoffman, A.J., Clark, J.C., DeGennaro, R.M., Poirier, P., Robinson, C.B., & Weisbrod, B.L. (2014). Putting Evidence Into Practice: An update of evidence-based interventions for cancer-related fatigue during and following treatment. Clinical Journal of Oncology Nursing, 18(Suppl.), S38–S58. https://doi.org/10.1188/14.CJON.S3.38-58
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D.G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology, 62, 1006–1012. https://doi.org/10.1016/j.jclinepi.2009.06.005
National Cancer Institute. (2017). NCI dictionary of cancer terms: survivorship. Retrieved from https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=445…
Olesen, M.L., Duun-Henriksen, A.K., Hansson, H., Ottesen, B., Andersen, K.K., & Zoffmann, V. (2016). A person-centered intervention targeting the psychosocial needs of gynecological cancer survivors: A randomized clinical trial. Journal of Cancer Survivorship, 10, 832–841. https://doi.org/10.1007/s11764-016-0528-5
Owen, J.E., Klapow, J.C., Roth, D.L., Shuster, J.L., Jr., Bellis, J., Meredith, R., & Tucker, D.C. (2005). Randomized pilot of a self-guided internet coping group for women with early-stage breast cancer. Annals of Behavioral Medicine, 30, 54–64. https://doi.org/10.1207/s15324796abm3001_7
Pachman, D.R., Barton, D.L., Swetz, K.M., & Loprinzi, C.L. (2012). Troublesome symptoms in cancer survivors: Fatigue, insomnia, neuropathy, and pain. Journal of Clinical Oncology, 30, 3687–3696. https://doi.org/10.1200/JCO.2012.41.7238
Paskett, E.D., Dean, J.A., Oliveri, J.M., & Harrop, J.P. (2012). Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: A review. Journal of Clinical Oncology, 30, 3726–3733. https://doi.org/10.1200/JCO.2012.41.8574
Phillips, J.L., & Currow, D.C. (2010). Cancer as a chronic disease. Collegian, 17(2), 47–50.
Risendal, B.C., Dwyer, A., Seidel, R.W., Lorig, K., Coombs, L., & Ory, M.G. (2015). Meeting the challenge of cancer survivorship in public health: Results from the evaluation of the chronic disease self-management program for cancer survivors. Frontiers in Public Health, 2, 214. https://doi.org/10.3389/fpubh.2014.00214
Rowland, J.H., & Yancik, R. (2006). Cancer survivorship: The interface of aging, comorbidity, and quality care. Journal of the National Cancer Institute, 98, 504–505. https://doi.org/10.1093/jnci/djj154
Ruddy, K.J., & Partridge, A.H. (2012). Fertility (male and female) and menopause. Journal of Clinical Oncology, 30, 3705–3711. https://doi.org/10.1200/JCO.2012.42.1966
Schultz, P.N., Beck, M.L., Stava, C., & Vassilopoulou-Sellin, R. (2003). Health profiles in 5836 long-term cancer survivors. International Journal of Cancer, 104, 488–495. https://doi.org/10.1002/ijc.10981
Smith-Turchyn, J., Morgan, A., & Richardson, J. (2016). The effectiveness of group-based self-management programmes to improve physical and psychological outcomes in patients with cancer: A systematic review and meta-analysis of randomised controlled trials. Clinical Oncology, 28(5), 292–305. https://doi.org/10.1016/j.clon.2015.10.003
Stanton, A.L. (2006). Psychosocial concerns and interventions for cancer survivors. Journal of Clinical Oncology, 24, 5132–5137. https://doi.org/10.1200/JCO.2006.06.8775
van den Berg, S.W., Gielissen, M.F., Custers, J.A., van der Graaf, W.T., Ottevanger, P.B., & Prins, J.B. (2015). BREATH: Web-based self-management for psychological adjustment after primary breast cancer—Results of a multicenter randomized controlled trial. Journal of Clinical Oncology, 33, 2763–2771. https://doi.org/10.1200/JCO.2013.54.9386
Wang, T., Tan, J.Y., Xiao, L.D., & Deng, R. (2017). Effectiveness of disease-specific self-management education on health outcomes in patients with chronic obstructive pulmonary disease: An updated systematic review and meta-analysis. Patient Education and Counseling, 100, 1432–1446. https://doi.org/10.1016/j.pec.2017.02.026
Wood, M.E., Vogel, V., Ng, A., Foxhall, L., Goodwin, P., & Travis, L.B. (2012). Second malignant neoplasms: Assessment and strategies for risk reduction. Journal of Clinical Oncology, 30, 3734–3745. https://doi.org/10.1200/JCO.2012.41.8681
Yun, Y.H., Lee, K.S., Kim, Y.W., Park, S.Y., Lee, E.S., Noh, D.Y., . . . Park, S. (2012). Web-based tailored education program for disease-free cancer survivors with cancer-related fatigue: A randomized controlled trial. Journal of Clinical Oncology, 30, 1296–1303. https://doi.org/10.1200/JCO.2011.37.2979
Zhang, A.Y., Bodner, D.R., Fu, A.Z., Gunzler, D.D., Klein, E., Kresevic, D., . . . Zhu, H. (2015). Effects of patient centered interventions on persistent urinary incontinence after prostate cancer treatment: A randomized, controlled trial. Journal of Urology, 194, 1675–1681. https://doi.org/10.1016/j.juro.2015.07.090

