Chemotherapy-Induced Nausea and Vomiting Mitigation With Music Interventions
Problem Identification: Despite three decades of studies examining music interventions as a mitigant of chemotherapy-induced nausea and vomiting (CINV), to date, no systematic review of this literature exists.
Literature Search: PubMed, Scopus, PsycInfo®, CINAHL®, Cochrane Library, and Google Scholar were searched. Keywords for all databases were music, chemotherapy, and nausea.
Data Evaluation: All studies were appraised for methodology and results.
Synthesis: 10 studies met inclusion criteria for review. Sample sizes were generally small and nonrandomized. Locus of control for music selection was more often with the investigator rather than the participant. Few studies controlled for the emetogenicity of the chemotherapy administered, nor for known patient-specific risk factors for CINV.
Implications for Research: The existing data have been largely generated by nurse scientists, and implications for nursing practice are many, because music interventions are low-cost, easily accessible, and without known adverse effects. However, this specific body of knowledge requires additional substantive inquiry to generate clinically relevant data.
Jump to a section
Chemotherapy-induced nausea and vomiting (CINV) is a potential adverse effect of cancer treatment. This phenomenon contains numerous subtypes (Navari & Aapro, 2016), and patients may experience each. Acute CINV occurs from chemotherapy administration (time 0) to 24 hours after administration. Delayed CINV is defined as occurring from 24 hours after chemotherapy to 120 hours (five days) after administration. Anticipatory CINV is a learned behavior resulting from experience with CINV and occurs prior to a subsequent chemotherapy cycle; it is the only form of CINV thought to be attributable to anxiety rather than chemotherapy. Breakthrough CINV occurs when antiemetic prophylaxis fails, and refractory CINV is considered when patients are unresponsive to antiemetic medications.
Multiple risk factors for the development of CINV are well documented in the literature. The dominant factor is the type of chemotherapy administered. The Hesketh scale is an evidence-based method of quantifying the emetogenicity of a chemotherapy regimen and guiding CINV prophylactic treatment (Hesketh et al., 1997). Additional, but less dominant, risk factors for CINV include female gender (Hesketh et al., 2006), age younger than 50 years (Roscoe et al., 2010), a history of motion sickness or pregnancy-induced nausea (Pirri et al., 2011), and little or no exposure to alcohol (Warr, Street, & Carides, 2011). Emerging evidence also suggests that some patients may have an above-average risk for CINV based on genetic polymorphisms affecting serotonin receptors, drug metabolism, and drug transport proteins (Kiernan, 2016).
CINV historically has been treated pharmaceutically, with less than perfect results. In 2016, Navari et al. published studies showing that the antipsychotic drug olanzapine significantly reduced CINV when coupled with standard-of-care prophylaxis, with strong effects for nausea control. In that study, total control of nausea (i.e., patients reporting no development of nausea) was 37% in the intervention group versus 22% in the placebo group (p = 0.002) (Navari et al., 2016). Although these data are encouraging, one of the most clinically significant findings from the study is that, despite a four-drug attempt at prophylaxis, most patients receiving highly emetogenic chemotherapy still will develop some degree of CINV. Effects for patients may include weight loss, malnutrition, electrolyte imbalance, and decline of performance status (National Comprehensive Cancer Network [NCCN], 2017), potentially affecting morbidity and mortality. Although data support a pharmacologic approach to CINV management, they also suggest that this modality has significant limitations; as such, complementary and alternative medicine (CAM) approaches to CINV management are worthy of inquiry. CAM approaches to CINV management are diverse, with research including acupressure, acupuncture, ginger, and relaxation (Greenlee et al., 2017).
Music is another potential complementary app-roach to addressing CINV. Since 1985, studies have been conducted examining the use of music as a mitigant of CINV. These music intervention studies include a variety of clinical settings, cancer types, and chemotherapy regimens. For anticipatory CINV specifically, data supporting music interventions are strong enough to be incorporated in NCCN’s (2017) antiemesis guidelines. Other subtypes of CINV, specifically acute and delayed CINV, have yet to reach such robust findings but contain a growing body of music-as-intervention literature. The objective of this review is to critically appraise music intervention studies specific to acute and delayed CINV.
Methods
All studies examining the relationship between a music intervention and its effect on acute and delayed CINV were considered eligible for review. No date limit was set. No limitations were placed on language or design (e.g., randomized, controlled trials only). PubMed, Scopus, PsycInfo®, CINAHL®, Cochrane Library, and Google Scholar databases were searched in January 2017. Keywords for all databases were music, chemotherapy, and nausea. Total return from all databases equaled 887 records. After removing duplicates, reviews, and eliminating studies not specific to acute or delayed CINV music interventions (e.g., music interventions for anticipatory CINV), 10 publications remained eligible for review. Of note, most bone marrow transplantation studies were not included because of the nausea and vomiting reported being outside the 120-hour (five-day) time frame defined for delayed CINV. One study using the Nevasic audio program was included, but it was unclear if the music used within the audio program was selected with the intention of reducing CINV independent of the embedded audio. A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)–based flow diagram depicts the study selection process (see Figure 1).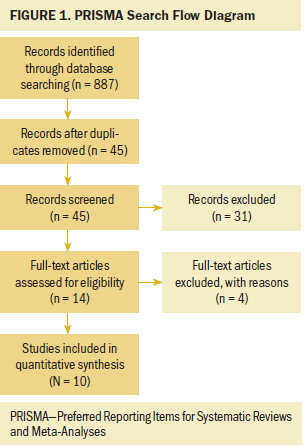
All studies reviewed used convenience sampling. Sample size was generally small, with only two studies recruiting more than 50 patients (Moradian et al., 2015; Sabo & Michael, 1996). Three studies limited their sample to a single cancer (Bozcuk et al., 2006; Madden, Mowry, Gao, Cullen, & Foreman, 2010; Moradian et al., 2015); however, no study reported on the cancer’s stage (extent of cancer spread within the body). Gender was limited to female patients only in two studies (Bozcuk et al., 2006; Moradian et al., 2015). Age was largely focused around middle to later adulthood; however, two studies specifically sampled pediatric patients (Hoseini, 2009; Madden et al., 2010). Patient-specific risk factors for CINV were largely unaddressed; they also were not factored into CINV risk analysis.
Design
Although all studies evaluated music as an adjunct to pharmacologic standard-of-care CINV treatment, this body of literature demonstrated a significant degree of design heterogeneity related to music interventions. For example, five studies were designed to evaluate music as the sole complementary intervention for patients with CINV (Bozcuk et al., 2006; Ezzone, Baker, Rosselet, & Terepka, 1998; Hoseini, 2009; Moradian et al., 2015; Standley, 1992), and others paired music with imagery (Frank, 1985; Gimeno, 2010; Karagozoglu, Tekyasar, & Yilmaz, 2013). Madden et al. (2010) included music as a component of creative arts therapy, which also included art and dance. Sabo and Michael (1996) added a physician’s spoken-word narrative to prerecorded harp music. The music used within the studies was largely administered without the guidance of a certified music therapist, except the two studies authored by music therapists (Gimeno, 2010; Standley, 1992).
Data Collection
Vomiting was recorded largely on a per-episode basis. Nausea, the more difficult symptom to measure, most commonly was self-reported using a 100 mm visual analog scale (Ezzone et al., 1998; Frank, 1985; Hoseini, 2009; Karagozoglu et al., 2013; Sabo & Michael, 1996). Bozcuk et al. (2006) and Gimeno (2010) used four-point Likert-type scales, and Moradian et al. (2015) and Standley (1992) used five-point Likert-type scales. A conceptual definition of nausea was not found within the studies.
Music Used
The music used in the studies contributed to the heterogeneity found among the studies’ designs. Patient-preferred music (self-selected music) was an option for participants in only three studies (Ezzone et al., 1998; Hoseini, 2009; Standley, 1992). Most often, music was chosen by the investigators. Karagozoglu et al. (2013) used music selected through consultation with a local conservatory in Sivas, Turkey. The genre of music typically was not reported. In the studies that did describe the music used, instrumental classical music (Bozcuk et al., 2006), New Age music (Gimeno, 2010), and relaxing Turkish music (Karagozoglu et al., 2013) were chosen for participants. One study, based in Iran, used Persian music (Hoseini, 2009), and Moradian et al. (2015) used the Nevasic audio program containing tones, frequencies, and pulses concealed by an over-layer of music. This study did not explicate whether the music over-layer was chosen for an anti-CINV effect, nor if the music had supporting evidence for use.
Timing of Music Intervention
Most studies were designed to administer the music intervention within the time encompassing acute CINV (from chemotherapy administration to 24 hours post-administration). Exceptions to this included two studies that recorded CINV symptoms in the delayed phase, occurring 24–120 hours after chemotherapy administration (Karagozoglu et al., 2013; Moradian et al., 2015). Two additional studies were designed to record CINV symptoms outside the acute phase; however, the additional data came from 7 and 14 days post–chemotherapy administration, outside the defined five-day (120-hour) boundary for CINV’s delayed phase.
Use of Controls
Six studies used a control group. Madden et al. (2010) had a repeated measures design in which participants served as their own control, and Moradian et al. (2015) employed a nonintervention control group, as well as a music intervention group distinct from the primary music intervention (the Nevasic audio program). All other studies with control groups used a standard-of-care, nonintervention control group (Ezzone et al., 1998; Sabo & Michael, 1996; Standley, 1992).
Randomization
Although no study employed random sampling, the researchers of two studies took their convenience samples and randomized participants once they had entered the study. Moradian et al. (2015) randomized participants to either an intervention group (Nevasic audio), a music group, or a nonintervention group. Madden et al. (2010) randomized participants to an intervention group led by a dance/movement therapist or to a group that received a volunteer’s attention.
Results
Of the studies that evaluated a music intervention on its own, only two studies produced statistically significant results (Ezzone et al., 1998; Hoseini, 2009). Statistical significance also was found with Frank (1985) and Karagozoglu et al. (2013), but these studies were not designed to detect if their results were because of the music intervention, the imagery, or both. Selected study characteristics and results are summarized in Table 1.
[[{"type":"media","view_mode":"media_original","fid":"38141","attributes":{"alt":"","class":"media-image","height":"957","typeof":"foaf:Image","width":"748"}}]]
Discussion
To date, the body of literature encompassing CINV music interventions offers contradictory findings. Small sample size, design heterogeneity, and minimal study controls make comparison among studies challenging. Adding to the difficulty is the wide span of time over which these studies were performed. CINV is more neurochemically understood today than when Frank (1985) and Standley (1992) first attempted to intervene with music. Likewise, antiemetic regimens have improved, and data regarding patient-specific CINV risk factors have emerged to guide clinicians and researchers. Based on the findings of this review, future investigation into CINV music interventions will be strengthened by addressing risk of bias, type of music intervention, timing of music intervention relative to chemotherapy, study controls, and standardization of the CINV experience.
Limitations
Each study contained multiple sources of bias. Common to all studies was the bias inherent to participant selection by convenience sampling. Incorporating random sampling into future research designs would take considerable design creativity but would greatly improve generalizability of findings. Although no study included blinding, future researchers may wish to include this in their study design. Participants in a music intervention trial cannot be blind to their intervention, but investigators may be blinded regarding which participants are in an intervention group versus a control group. In this way, bias introduced through the investigator may be minimized. Blinding also would be ideal for additional music intervention studies that include a music therapist within the study’s design, keeping the therapist from knowing which patients are contained within the study groups and reducing bias.
Patient-preferred music was used in only 30% of the CINV music intervention studies. However, evidence suggests that research participants should be given the opportunity to self-select the music used for their intervention (Chanda & Levitin, 2013). The counter-argument to use of patient-preferred music is that it sacrifices control within the research design. This is erroneous, and data support the use of patient-preferred music in a variety of clinical settings (Heiderscheit, Breckenridge, Chlan, & Savik, 2014; Liang et al., 2016; Mondanero et al., 2017). Therefore, designing additional studies that include patient-preferred music as a standardized intervention is logical and efficacious.
Acute and delayed CINV represent different levels of neurotransmitters producing the nausea effect. Therefore, it remains theoretically plausible that a music intervention that works as a mitigant for acute CINV may work differently in the delayed phase of CINV. The studies reviewed in the current article largely focused on acute CINV. Additional studies should acknowledge the timing of a participant’s chemotherapy and use that as a reference point from which to gauge what phase of CINV a music intervention is affecting. For example, for a patient who receives a music intervention for CINV 72 hours after chemotherapy administration, the intervention is influencing delayed CINV. This improvement to study design would allow additional studies to compare music’s effect between the two CINV phases and possibly hint at what neurotransmitter may show greater response to music.
Much of the literature reviewed lacked control at many levels. For example, some studies included a music intervention paired with one or two additional interventions, as previously described. However, none of these studies was designed to explain the findings in terms of the impact of each interventional variable. The easiest remedy for this would involve future research focusing on a standalone music intervention. Beyond that, multi-intervention studies should be designed so that each contributing variable may be assessed according to its individual impact on CINV. This kind of measurement is common within linear regression models, something not used in this body of research.
As previously mentioned, studies with control groups largely used a nonintervention, standard-of-care approach. Additional trials may increase study rigor by including control groups that use parallel, nonmusic experimental conditions (Chanda & Levitin, 2013). Having nonmusic participants who watch television or read a book would help identify if CINV changes are music-specific or related to any activity that provides distraction.
Only two studies reviewed used any form of randomization, as mentioned previously (Madden et al., 2010; Moradian et al., 2015). To enhance the rigor of this field of research, randomization should be used in future inquiry. The pressing question involves how to most appropriately use randomization to improve study quality but not make studies impossible to carry out. Random sampling may present logistic hurdles insurmountable in some settings. However, random assignment is easier and adds much to the overall quality of a study.
Additional studies involving music for CINV must account for multiple variables. These include the chemotherapy administered, female gender (Hesketh et al., 2006), age younger than 50 years (Roscoe et al., 2010), a history of motion sickness or pregnancy-induced nausea (Pirri et al., 2011), and little or no exposure to alcohol (Warr et al., 2011). Assessing for these covariates allows an investigator to build a statistical model that accounts for the variability in patients’ CINV and, therefore, a potential influence on their response to music.
The major determinant of CINV severity is the chemotherapy administered. Emetogenicity can be quantified using the classification schema published by Hesketh et al. (1997), and in so doing allows the risk of CINV to be considered during data analysis. Clinically, a group of patients receiving mildly nauseating chemotherapy may see better effects from music than a group of patients receiving highly nauseating chemotherapy.
Patient-specific risk factors for CINV also must be recorded and accounted for when evaluating music’s effect within a sample. A single study in the current review assessed for motion sickness history (Standley, 1992), but these minor contributors to CINV risk were largely ignored in the current body of literature. Researchers would be prudent to consider that a sample of young females may suffer more CINV than a sample containing older adult men with significant alcohol use and, therefore, should anticipate that the efficacy of a music intervention may be confounded by these variables.
Nausea, a subjective experience, presents a challenge to conceptualize and measure. However, a study that does not offer a conceptual definition of nausea risks measuring the phenomenon imprecisely. Therefore, a significant step toward standardization of CINV research rests on investigators explicitly stating how nausea is defined within the context of the study. In addition, participants must be clear regarding the study’s interpretation of nausea or risk-recording data on the severity of closely related but clinically distinct phenomena (e.g., dyspepsia).
Implications for Nursing
Even with the relative paucity of existing data, CINV music intervention research contains implications for nursing practice. For example, nurses may find benefit in sharing the developing knowledge of CINV music intervention studies, an area of research with which patients likely are unfamiliar. Here, the nurse plays a familiar role: patient advocate, patient educator, and research translator. Patient awareness of CINV music intervention research may encourage discovery of additional information involving music interventions in other areas of research. One such clinical scenario may be that of a patient with CINV and cancer-related pain. By introducing CINV music intervention research, this also may ignite interest in other related areas, such as music interventions within the setting of cancer pain and anxiety.
For patients who express an interest in use of music, nurses should consider education specifically for listening safety. In a systematic review of preferred listening levels, Jiang, Zhao, Guderley, and Manchaiah (2016) demonstrated that more than half of their participants used music in excess of the 100% daily dose limit for noise. This underscores a need for nurses to educate patients regarding safe listening practices, and this recommendation transcends into all aspects of music use, therapeutic and otherwise.
Patients who demonstrate an interest in CINV music interventions also may have an interest in learning about other forms of CAM. The Office of Cancer Complementary and Alternative Medicine (https://cam.cancer.gov) lists evidence-based CAM cancer adjunctive therapies, including acupuncture, exercise therapy, and mind–body intervention (e.g., hypnosis), as well as various diet and nutritional therapies. Here again, nurses play a familiar role of advocate and educator, using CINV music intervention information as a springboard to further explore a patient’s interest in CAM.
Finally, nurses may advise patients that music interventions for CINV, although still early in their evidence base, are unlikely to do harm. This contrasts sharply with nurses’ education to patients regarding pharmaceuticals, the explanation of which includes the intended effect of a drug as well as the adverse effects patients may experience. A pharmaceutical’s adverse effects outnumbering its therapeutic effects is not uncommon. By comparison, music interventions have no data suggesting they may worsen a condition, contribute to adverse effects, or otherwise negatively affect a patient’s quality of life. 
Conclusion
This systematic review was designed to summarize and appraise the existing data concerning music interventions as a mitigant for CINV. The studies performed used convenience sampling, had design heterogeneity, had little randomization, and had results that defy generalizability. Still, the growing body of data around the neurochemical modulatory effects of music suggests potential therapeutic value, and this information makes continued inquiry into CINV music interventions compelling and necessary. Future inquiry must seek to improve study design, as suggested previously. Convenience sampling may be difficult to avoid, but patients consenting to treatment can be randomized after recruitment. Additional studies will need to control for the chemotherapy administered and the other documented patient-specific CINV risk factors that currently exist. In addition, CINV music interventions should be designed to evaluate the acute and delayed phase of CINV. Patient-preferred music should be the standard music intervention, acknowledging prior data suggesting that this is ideal. In the best-case scenario, the advancement of knowledge in music neurochemistry will be complemented by parallel advancement of knowledge in translational research using music therapeutically in the clinical setting.
About the Author(s)
Jason M. Kiernan, RN, MSN, is a professor in the Faculty of Nursing at the University of Windsor in Ontario, Canada; Jody Conradi Stark, PhD, MT-BC, is a lecturer and music therapy clinical supervisor in the College of Arts and Sciences at Eastern Michigan University in Detroit; and April H. Vallerand, PhD, RN, FAAN, is an associate dean of research in the College of Nursing at Wayne State University in Detroit, MI. Vallerand has previously consulted for and has received provision of writing assistance from Astra Zeneca, and has received fees for participation in advisory or review activities from Shionogi. Kiernan and Vallerand contributed to the conceptualization and design. Kiernan completed the data collection and provided the analysis. All authors contributed to the manuscript preparation. Kiernan can be reached at jasonk@uwindsor.ca, with copy to ONFEditor@ons.org. (Submitted June 2017. Accepted July 26, 2017.)
References
Bozcuk, H., Artac, M., Kara, A., Ozdogan, M., Sualp, Y., Topcu, Z., . . . Savas, B. (2006). Does music exposure during chemotherapy improve quality of life in early breast cancer patients? A pilot study. Medical Science Monitor, 12(5), 200–205.
Chanda, M.L., & Levitin, D.J. (2013). The neurochemistry of music. Trends in Cognitive Sciences, 17(4), 179–190. https://doi.org/10.1016/j.tics.2013.02.007
Ezzone, S., Baker, C., Rosselet, R., & Terepka, E. (1998). Music as an adjunct to antiemetic therapy. Oncology Nursing Forum, 25, 1551–1556.
Frank, J.M. (1985). The effects of music therapy and guided visual imagery on chemotherapy induced nausea and vomiting. Oncology Nursing Forum, 12(5), 47–52.
Gimeno, M.M. (2010). The effect of music and imagery to induce relaxation and reduce nausea and emesis in patients with cancer undergoing chemotherapy treatment. Music and Medicine, 2, 174–181.
Greenlee, H., DuPont-Reyes, M.J., Balneaves, L.G., Carlson, L.E., Cohen, M.R., Deng, G., . . . Tripathy, D. (2017). Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA: A Cancer Journal for Clinicians, 67, 194–232. https://doi.org/10.3322/caac.21397
Heiderscheit, A., Breckenridge, S.J., Chlan, L.L., & Savik, K. (2014). Music preferences of mechanically ventilated patients participating in a randomized controlled trial. Music and Medicine, 6(2), 29–38.
Hesketh, P.J., Grunberg, S.M., Herrstedt, J., de Wit, R., Gralla, R.J., Carides, A.D., . . . Horgan, K.J. (2006). Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: Effect of gender on treatment response. Supportive Care in Cancer, 14, 354–360. https://doi.org/10.1007/s00520-005-0914-4
Hesketh, P.J., Kris, M.G., Grunberg, S.M., Beck, T., Hainsworth, J.D., Harker, G., . . . Lindley, C.M. (1997). Proposal for classifying the acute emetogenicity of cancer chemotherapy. Journal of Clinical Oncology, 15, 103–109. https://doi.org/10.1200/JCO.1997.15.1.103
Hoseini, A.S. (2009). Effect of music therapy on chemotherapy nausea and vomiting in children with malignancy. Journal of Hayat, 15(2), 5–14.
Jiang, W., Zhao, F., Guderley, N., & Manchaiah, V. (2016). Daily music exposure dose and hearing problems using personal listening devices in adolescents and young adults: A systematic review. International Journal of Audiology, 55(4), 197–205. https://doi.org/10.3109/14992027.2015.1122237
Karagozoglu, S., Tekyasar, F., & Yilmaz, F.A. (2013). Effects of music therapy and guided visual imagery on chemotherapy-induced anxiety and nausea-vomiting. Journal of Clinical Nursing, 22, 39–50. https://doi.org/10.1111/jocn.12030
Kiernan, J. (2016). Genetic influence on chemotherapy-induced nausea and vomiting: A narrative review. Oncology Nursing Forum, 43, 389–393. https://doi.org/10.1188/16.ONF.389-393
Liang, Z., Ren, D., Choi, J., Happ, M.B., Hravnak, M., & Hoffman, L.A. (2016). Music intervention during daily weaning trials—A 6 day prospective randomized crossover trial. Complementary Therapies in Medicine, 29, 72–77. https://doi.org/10.1016/j.ctim.2016.09.003
Madden, J.R., Mowry, P., Gao, D., Cullen, P.M., & Foreman, N.K. (2010). Creative arts therapy improves quality of life for pediatric brain tumor patients receiving outpatient chemotherapy. Journal of Pediatric Oncology Nursing, 27, 133–145. https://doi.org/10.1177/104345420955452
Mondanero, J.F., Homel, P., Lonner, B., Shepp, J., Lichtensztein, M., & Loewy, J.V. (2017). Music therapy increases comfort and reduces pain in patients recovering from spinal surgery. American Journal of Orthopedics, 46, E12–E22.
Moradian, S., Walshe, C., Shahidsales, S., Ghavam Nasiri, M.R., Pilling, M., & Molassiotis, A. (2015). Nevasic audio program for the prevention of chemotherapy induced nausea and vomiting: A feasibility study using a randomized controlled trial design. European Journal of Oncology Nursing, 19, 282–291. https://doi.org/10.1016/j.ejon.2014.10.016
National Comprehensive Cancer Network. (2017). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Antiemesis [v.2.2017]. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
Navari, R.M., & Aapro, M. (2016). Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. New England Journal of Medicine, 374, 1356–1367. https://doi.org/10.1056/NEJMra1515442
Navari, R.M., Qin, R., Ruddy, K.J., Lui, H., Powell, S.F., Bajaj, M., . . . Loprinzi, C.L. (2016). Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. New England Journal of Medicine, 375, 134–142. https://doi.org/10.1056/NEJMoa1515725
Pirri, C., Katris, P., Trotter, J., Bayliss, E., Bennett, R., & Drummond, P. (2011). Risk factors at pretreatment predicting treatment-induced nausea and vomiting in Australian cancer patients: A prospective, longitudinal, observational study. Supportive Care in Cancer, 19, 1549–1563. https://doi.org/10.1007/s00520-010-0982-y
Roscoe, J.A., Morrow, G.R., Colagiuri, B., Heckler, C.E., Pudlo, B.D., Colman, L., . . . Jacobs, A. (2010). Insight in the prediction of chemotherapy-induced nausea. Supportive Care in Cancer, 18, 869–876. https://doi.org/10.1007/s00520-009-0723-2
Sabo, C.E., & Michael, S.R. (1996). The influence of personal message with music on anxiety and side effects associated with chemotherapy. Cancer Nursing, 19, 283–289.
Standley, J.M. (1992). Clinical applications of music and chemotherapy: The effects on nausea and emesis 1. Music Therapy Perspectives, 10, 27–35. https://doi.org/10.1093/mtp/10.1.27
Warr, D.G., Street, J.C., & Carides, A.D. (2011). Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: Analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Supportive Care in Cancer, 19, 807–813. https://doi.org/10.1007/s00520-010-0899-5




