Symptom Clusters in Patients With Pancreatic Cancer Undergoing Surgical Resection: Part I
Objectives: To describe patient-reported symptoms and symptom clusters in patients with pancreatic cancer (PC) undergoing surgical resection.
Sample & Setting: 143 patients with stage II PC undergoing surgical resection alone or with subsequent adjuvant chemoradiation or chemotherapy were recruited to participate in a nested, longitudinal, exploratory study through convenience sampling techniques from Thomas Jefferson University Hospital, a National Cancer Institute–designated cancer center.
Methods & Variables: The Functional Assessment in Cancer Therapy–Hepatobiliary questionnaire was used to assess 17 PC symptoms preoperatively and at three, six, and nine months postoperatively. Exploratory and confirmatory factor analyses were used to identify symptom clusters.
Results: Fatigue, trouble sleeping, poor appetite, trouble digesting food, and weight loss were consistently reported as the most prevalent and severe symptoms. Sixteen distinct symptom clusters were identified within nine months of surgery. Four core symptom clusters persisted over time: affective, gastrointestinal, gustatory, and discomfort.
Implications for Nursing: Findings may be used to provide anticipatory patient and family guidance and to inform clinical assessments of symptoms and symptom clusters in this population.
Jump to a section
About 55,440 new cases of pancreatic cancer (PC) will be diagnosed in the United States in 2018 (Siegel, Miller, & Jemal, 2018). The five-year survival rate for all stages of PC in the United States has improved from 6% in 2013 to 8% in 2018 (Siegel et al., 2018; Siegel, Naishadham, & Jemal, 2013), and improved survival rates have made concerns about managing symptoms increasingly important. Some common concerns among patients with PC undergoing surgical resection include fatigue, pain, weakness, anxiety, depression, weight loss, insomnia, gastrointestinal disturbances, and symptoms of diabetes (Huang et al., 2000; Scheingraber, Scheingraber, Brauckhoff, & Dralle, 2005; Yeo et al., 2012). Evidence suggests that patients with cancer do not experience symptoms in isolation, but rather as multiple, concurrent symptoms or symptom clusters (SCs). Although the presence of SCs has been documented in many cancer types, little is known about SCs in patients with or without surgically resected PC.
SCs are defined as the simultaneous presence of two or more symptoms, which may or may not share etiology and are more strongly related to one another than other symptoms (Dodd, Miaskowski, & Lee, 2004; Kim, McGuire, Tulman, & Barsevick, 2005). SCs have been identified in individuals with lung (Franceschini, Jardim, Fernandes, Jamnik, & Santoro, 2013), ovarian (Huang et al., 2016), prostate (Dirksen, Belyea, Wong, & Epstein, 2016), and breast cancers (Starkweather et al., 2013) and are associated with decreased functional status (Kim, Barsevick, Beck, & Dudley, 2012), poor quality of life (QOL) (Franceschini et al., 2013), and reduced survival (Wikman, Johar, & Lagergren, 2014). Given the negative impact that SCs have on clinical outcomes, identifying and creating a classification of SCs and developing interventions to manage SCs have become priorities for nursing research (Knobf et al., 2015).
SCs are a concern for patients with cancer and their family members, who must assume the daily responsibility of managing them. Understanding patients’ perception of SCs over time is critical to ensure appropriate counseling, education, and symptom management (Dodd, Miaskowski, & Paul, 2001). Greater understanding of SCs may enable oncology professionals to tailor assessments to address SCs rather than individual symptoms. This may allow nursing professionals to not only more accurately identify and monitor multiple symptom concerns, but also to allow the most appropriate SC counseling, education, and symptom management strategies to be implemented at a particular point in time during the cancer trajectory (Nho, Reul Kim, & Nam, 2017). Through such applications, SC findings have the potential to have a positive effect on patient- and family-reported outcomes.
To date, only four researchers have examined SCs in patients with PC (see Table 1). In a longitudinal study, Reyes-Gibby et al. (2007) found that fatigue and anorexia formed a distinct SC over time in patients with unresectable, locally advanced PC who were undergoing chemoradiation. Noquez (2008) identified a SC of anxiety, depression, somatization, pain, and fatigue in patients with several cancer types, including PC. Laird et al. (2011) described a SC of fatigue, pain, and depression in patients with advanced gastrointestinal cancer, lung cancer, or PC. Yeo et al. (2012) explored SCs in patients with pancreatic and periampullary cancer (PPC) (i.e., ampullary, duodenal, and bile duct) three months postoperatively. The SCs identified were (a) fatigue, bodily pain, depression, weakness, and anxiety; (b) fatigue and shortness of breath; and (c) fatigue, diarrhea, pain, anxiety, and difficulty sleeping. Yeo et al. (2012) concluded that additional research was needed to determine the stability of SCs over time. 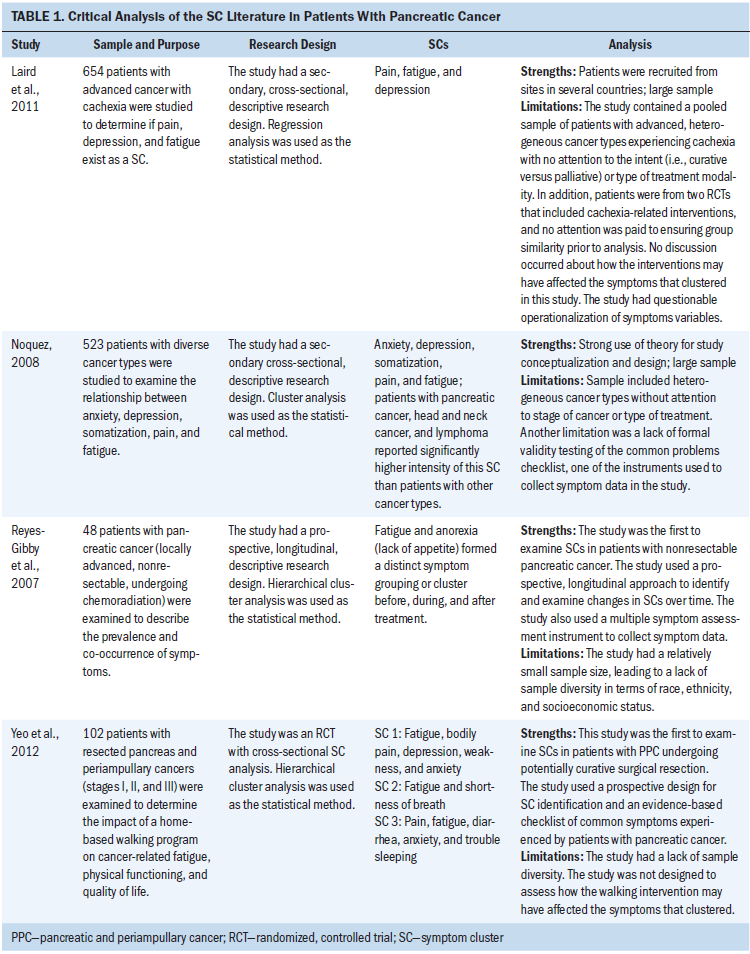
Research has not been conducted on SCs, longitudinally, in a cohort of patients with stage II PC undergoing surgical resection. The purpose of this article is to describe patient-reported symptom profiles and to identify SCs in patients with stage II PC prior to surgery and three, six, and nine months after surgery.
Theoretical Framework
The conceptualization and design of the current study was guided by the Theory of Unpleasant Symptoms (TOUS) (Lenz, Pugh, Milligan, Gift, & Suppe, 1997). TOUS is comprised of three major components: (a) symptoms; (b) physiological, psychological, and situational factors that influence the perception of symptoms; and (c) performance (i.e., the impact of symptoms on clinical outcomes). Although the concept of a SC is not explicitly included in the TOUS, the theory addresses the presence and interaction of multiple concurrent symptoms, which is congruent with the concept of SCs (Lenz & Pugh, 2008). The TOUS framework was used to guide the selection, definition, and operationalization of key study variables to gain a better understanding of symptoms and SCs experienced by patients with PC undergoing surgical resection throughout the perioperative period.
Methods
Design, Sample, and Setting
This study was conducted as a nested, longitudinal, exploratory survey within a randomized, controlled trial (i.e., the parent study). The parent study (Lavu et al., 2015) evaluated the effectiveness of an intraoperative alcohol celiac nerve block (CNB) in reducing pain in patients with PPC undergoing surgery. Informed consent was obtained prior to parent study enrollment and after the purposes, methods, anticipated benefits, alternatives to participation, and potential risks were explained. A convenience sample of 485 patients participated in the parent study at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania.
The current study analyzed a sub-sample of patients from the parent study, specifically patients with American Joint Committee on Cancer (2009) stage IIa or IIb PC who underwent potentially curative surgery alone or with adjuvant therapy. Patients with stage I, III, or IV PPC, stage II periampullary cancer, and unresectable PC and those who did not return at least one preoperative and postoperative symptom questionnaire were excluded. The most common stage of PC in patients undergoing potentially curative resection is stage II (Yeo et al., 2012). In addition, researchers have found that patients with periampullary cancer have better survival than patients with PC (Sommerville et al., 2009), and stage of cancer is associated with increased number or severity of SCs (Karabulut, Erci, Ozer, & Ozdemir, 2010; Wang, O’Conner, Xu, & Liu, 2012), which has the potential to affect the disease trajectory and symptoms that cluster. Accordingly, the decision was made to ensure a homogenous sample by limiting participants to patients with stage II PC to enhance the clinical meaningfulness and use of SC findings from this study.
Experimental and placebo groups from the parent study were evaluated to determine (a) if the CNB was a confounding variable that could alter the pain-related SCs, and (b) if participants were equally distributed and could be combined into one sample for this study. No significant differences were observed among parent study groups on any clinical, demographic, or pain-related variables over time (all p > 0.05). Therefore, the authors determined that the CNB was not a confounding variable and the study groups could be combined for this investigation. Institutional review board approval from Thomas Jefferson University Hospital and Villanova University was obtained specifically for this analysis as a nested study within the parent study.
Measures and Variables
Symptoms were assessed with the Functional Assessment in Cancer Therapy–Hepatobiliary (FACT-Hep) questionnaire (Heffernan et al., 2002), which measures 20 symptom concerns and QOL in patients with hepatobiliary cancers. The FACT-Hep instrument has been translated into more than 40 different languages, and it has been widely used to measure QOL and symptom concerns in patients with PC (Crippa et al., 2008; Okada et al., 2017; Serrano et al., 2014; Sun et al., 2008, 2016). The FACT-Hep has demonstrated good internal consistency (Cronbach alpha range = 0.72–0.94) and test-retest reliability (Spearman correlation range = 0.84–0.91) within one week of baseline assessment (Heffernan et al., 2002). All symptoms, except constipation and jaundice, are assessed on a five-point severity scale ranging from 0 (not at all) to 4 (very much). Constipation and jaundice are measured in terms of bother. Two FACT-Hep symptoms, fever and chills, were excluded because they are atypical in postoperative patients with PC (Huang et al., 2000; Yeo et al., 2012). Fatigue and abdominal pain are measured by two FACT-Hep items; therefore, the decision was made to collapse these variables into fatigue and abdominal pain/cramping variables by retaining the highest score recorded. Although three pain items are included on the FACT-Hep questionnaire (back pain, abdominal pain/cramping, and general pain), the authors excluded the general pain item because it is not specific to PC. Seventeen symptoms were included in the current study.
Data Collection
Symptom data were collected preoperatively (T1) and at three (T2), six (T3), and nine (T4) months postoperatively by trained personnel. Patients were mailed questionnaires at the appropriate times. If a questionnaire was not returned within two weeks, the patient was called and reminded to complete the questionnaire and was given the option to complete the questionnaire by phone. Demographic and clinical data were obtained through a review of electronic health records. All data for this study were obtained from a review of the parent study folders.
Statistical Analysis
Data were analyzed using IBM SPSS Statistics, version 22.0; Mplus, version 6.0; and SAS, version 9.3 and 9.4. Descriptive statistics were used to describe demographic, clinical, and symptom prevalence and severity data. Exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) were used to identify SCs at each time point (Sass & Schmitt, 2010). In EFA, the number of factors is specified, but otherwise no structure is specified a priori. In CFA, results of the EFA are used to specify the number of factors/latent variables, as well as which observed symptoms are likely to be related to those variables. Symptoms that did not achieve at least 10% prevalence at each study time point were excluded from the EFA and CFA (Kim, Barsevick, Tulman, & McDermott, 2008).
Weighted least squares with orthogonal geomin rotation was used to conduct EFA (Bryant & Yarnold, 1995; Sass & Schmitt, 2010). The number of factors was determined using the chi-square test of model fit and Kaiser-Guttman rule (Bryant & Yarnold, 1995). To determine the reliability of identified SCs, a CFA using the Bayesian method (Muthén & Asparouhov, 2012) was conducted for each study time point using all patients with complete symptom data (n = 55). In the CFA models, loadings for symptoms related to factors from the EFA were assigned noninformative prior distributions with large variances (expressing uncertainty about the association of the symptom with the latent factors and allowing the data to drive the estimates), whereas symptoms thought to be unrelated to factors were given highly informative prior distributions centered at 0 (indicating no association) with little variance (expressing confidence that no association existed between the symptom and the factor).
Final factor models for each study time point were determined by the following criteria: (a) at least two symptoms with absolute factor loadings of 0.4 or greater (Kim et al., 2008) and (b) congruence between the EFA factor and CFA factor structures. Two or more symptoms with salient loadings on a factor were considered a SC. If the same symptom loaded on two factors, both symptoms were retained to enhance the clinical meaningfulness of findings.
Sample Size Estimation
Subject-to-variable guidelines requiring at least five subjects for each variable were followed to determine the study sample size (Gorsuch, 1983). A sample size of 85 was deemed adequate to conduct a reliable factor analysis. 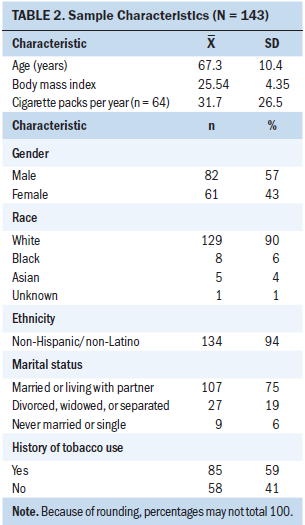
Results
Sample Characteristics
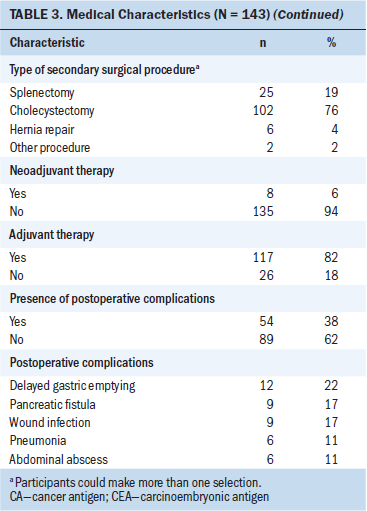
Survey response rates were 76% (n = 109) at three months, 64% (n = 92) at six months, and 62% (n = 89) at nine months postoperatively. The majority of participants were male, White (non-Hispanic/Latino), and married, with a mean age of 67.3 (SD = 10.4) years (see Table 2). Ninety-eight percent of patients (n = 120) reported one or more comorbid conditions. Twenty-two percent of patients reported having a mental health disorder (n = 31), most often including a past medical history of anxiety or depression. All patients underwent surgical resection, with the most common surgical procedure being the pylorus-preserving pancreaticoduodenectomy. Pathological histology confirmed the diagnosis of stage IIa (n = 28) or IIb (n = 115) pancreatic ductal adenocarcinoma in all patients. Following surgery, 82% of patients (n = 117) received adjuvant chemotherapy or combined therapy (see Table 3). 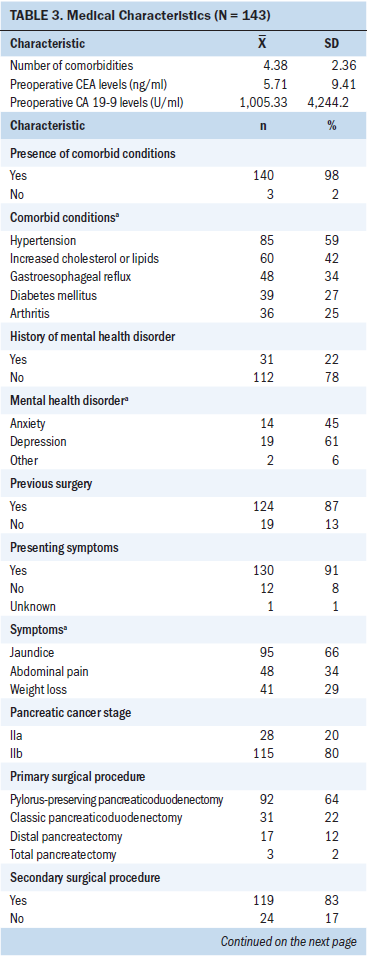
Symptoms
Prevalence rates of all 17 symptoms were relatively high across all time points, with the exception of jaundice, which fell dramatically after surgery (see Table 4). Six symptoms were commonly reported at all study time points: fatigue, trouble sleeping, poor appetite, trouble digesting food, weight loss, and abdominal pain/cramping. Fatigue was the most consistently found symptom, with occurrence rates ranging from 92% (n = 131) at T1 to 90% (n = 83) at T3. The mean symptom severity scores were relatively mild to moderate, with all severity scores being below 2.15 (2 indicates “somewhat”) on a 0–4 scale (see Table 5). The five most severe symptoms reported at all study time points were fatigue, trouble sleeping, poor appetite, trouble digesting food, and weight loss. The most severe symptom was fatigue, which ranged from a mean score of 1.92 (SD = 1.13) at T1 to a mean score of 2.14 (SD = 1.17) at T2. The mean number of concurrent symptoms was 9.62 (SD = 3.35) at T1, 8.75 (SD = 3.68) at T2, 8.66 (SD = 3.45) at T3, and 8.98 (SD = 3.55) at T4. 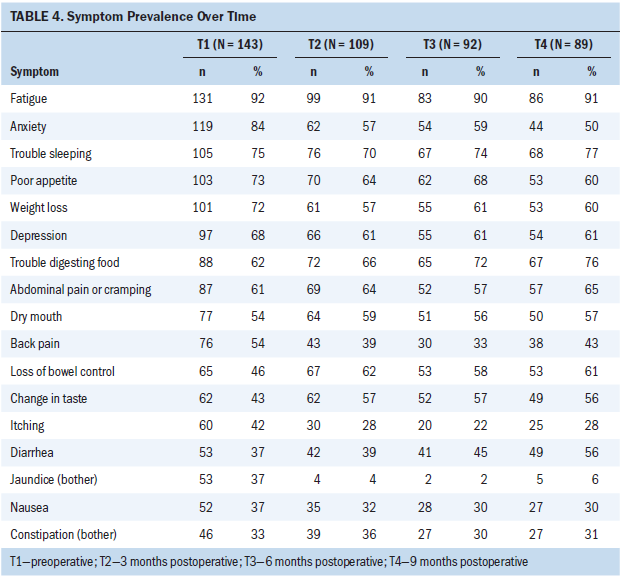
Preoperative Symptom Clusters
Seventeen symptoms were included in the EFA and CFA models preoperatively (see Table 6). A five-factor solution was a good fit with the EFA (chi-square61 = 62.7; p = 0.42) and CFA models. Trouble sleeping and diarrhea did not cluster (load of 0.4 or greater) at T1. 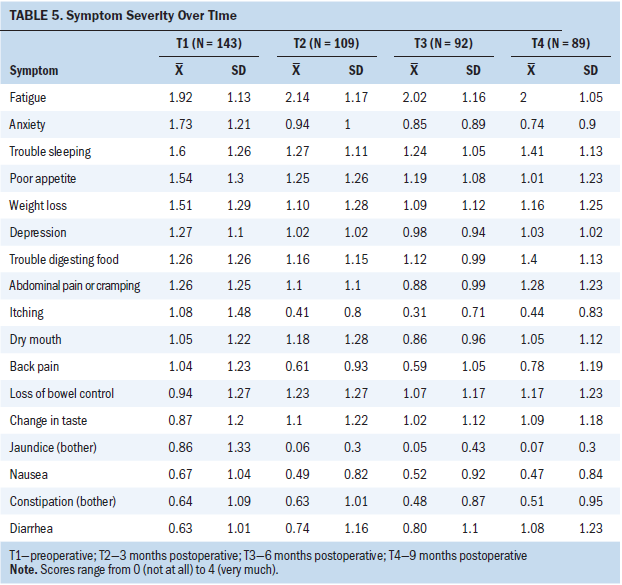
Pain–gastrointestinal symptom cluster: In the EFA and CFA (p < 0.05) models, nausea, trouble digesting food, poor appetite, back pain, constipation, and abdominal pain/cramping loaded (clustered) on factor 1 at T1.
Mood symptom cluster: Anxiety and depression loaded on preoperative factor 2 in both the EFA and CFA (p < 0.05) models.
Digestive problems symptom cluster: In the EFA and CFA (p < 0.05) models, loss of bowel control and trouble digesting food loaded on factor 3 at T1.
Fatigue–nutritional problems symptom cluster: In the EFA model, weight loss, fatigue, itching, change in taste, and dry mouth loaded on preoperative factor 4. In the CFA model, weight loss, fatigue, change in taste, and dry mouth significantly loaded on factor 4 (p < 0.05). Given the variability in loading of itching between the models, itching was determined to be unstable and was not included in final factor 4 structure at T1.
Jaundice symptom cluster: Nausea and jaundice loaded on preoperative factor 5 in the EFA model. In the CFA model, nausea, jaundice, itching, fatigue, change in taste, and dry mouth loaded with p < 0.05. Only symptoms that loaded on both models were retained; therefore, only jaundice and itching were included in the final factor 5 structure at T1. 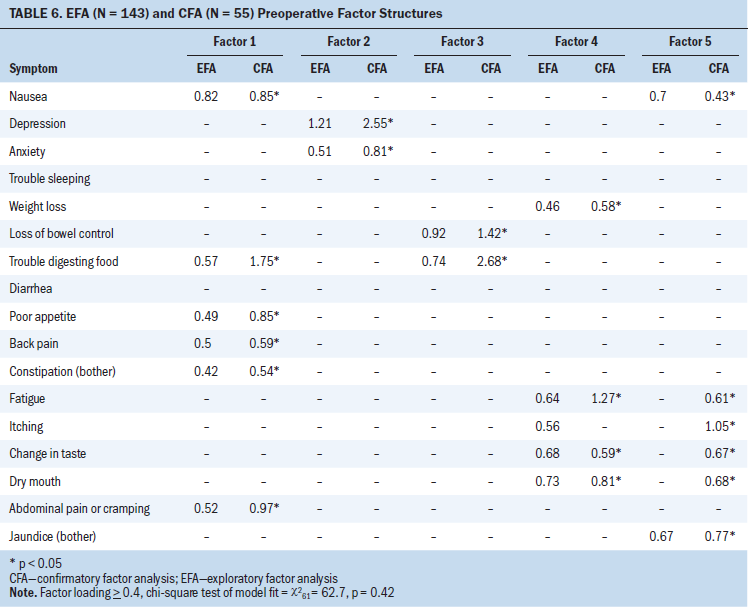
Postoperative Symptom Clusters at Three Months
Sixteen symptoms were included in the EFA and CFA models at three, six, and nine months postoperatively (jaundice was excluded because it did not meet the 10% prevalence criteria). At 3 months after surgery, a four-factor solution was a good fit with data in the EFA (chi-square62 = 74.2; p = 0.14) and CFA models. Constipation did not cluster (load at 0.4 or greater) at T2 (see Table 7).
Mood–pain–anorexia–fatigue symptom cluster: In the EFA and CFA (p < 0.05) models, nausea, depression, anxiety, poor appetite, back pain, fatigue, and abdominal pain/cramping loaded on factor 1 at T2.
Insomnia–digestive problems symptom cluster: Trouble sleeping, loss of bowel control, and trouble digesting food loaded on factor 2 at T2 in the EFA and CFA (p < 0.05) models.
Gastrointestinal sickness symptom cluster: In the EFA model, nausea, diarrhea and itching loaded on factor 3 at T2. These same symptoms loaded in the CFA model; however, significance was not achieved (p = 0.1), suggesting this clustering of symptoms is less reliable.
Nutritional problems symptom cluster: Weight loss, itching, change in taste, and dry mouth loaded on factor 4 at T2 in the EFA and CFA (p < 0.05) models. 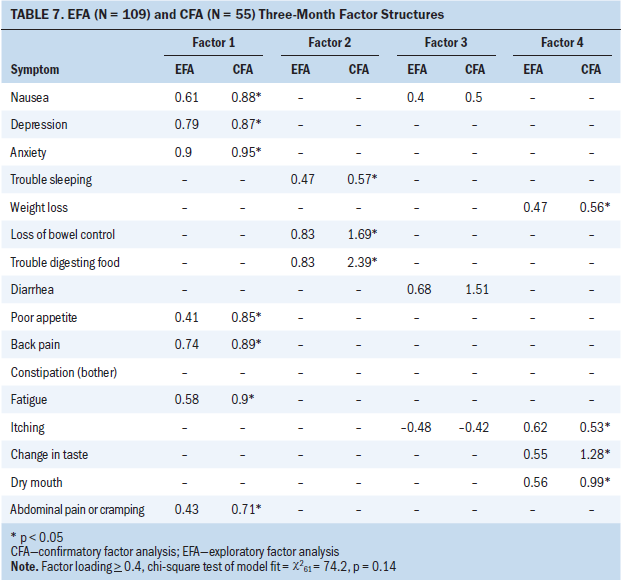
Postoperative Symptom Clusters at Six Months
As shown in Table 8, 16 symptoms were included in the EFA and CFA models at 6 months after surgery. A four-factor solution was a good fit with data in the EFA (chi-square62 = 66.7; p = 0.32) and CFA models. Nausea did not cluster at T3. 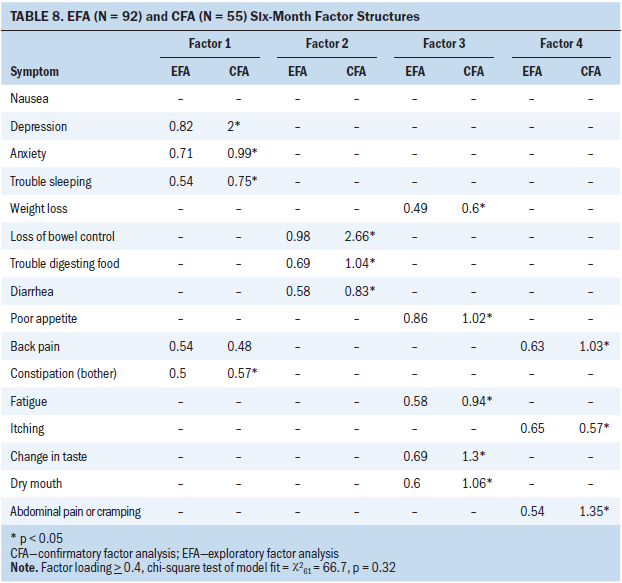
Mood–pain–insomnia-gastrointestinal symptom cluster: Depression, anxiety, trouble sleeping, back pain, and constipation loaded on factor 1 in the EFA and CFA models at T3. Although depression, anxiety, trouble sleeping, and constipation all loaded (p < 0.05), back pain failed to achieve significance in the CFA model (p = 0.1). This suggests that the inclusion of back pain as a component of SCs is less reliable.
Bowel–digestive problems symptom cluster: Loss of bowel control, trouble digesting food, and diarrhea loaded on factor 2 at T3 in the EFA and CFA (p < 0.05) models.
Fatigue–anorexia–nutritional problems symptom cluster: Fatigue, poor appetite, weight loss, change in taste, and dry mouth loaded on factor 3 at T3 in the EFA and CFA (p < 0.05) models.
Pain–itching symptom cluster: In the EFA and CFA (p < 0.05) models, itching, abdominal pain/cramping, and back pain loaded on factor 4 at T3.
Postoperative Symptom Clusters at Nine Months
Sixteen symptoms were included in the EFA and CFA models at nine months after surgery (see Table 9). A four-factor solution was a good fit with data in the EFA (chi-square62 = 69.9; p = 0.23) and CFA models. Only one symptom (itching) loaded on factor 4 in this model; therefore, it did not meet the definition of a SC. Poor appetite failed to cluster at T4. 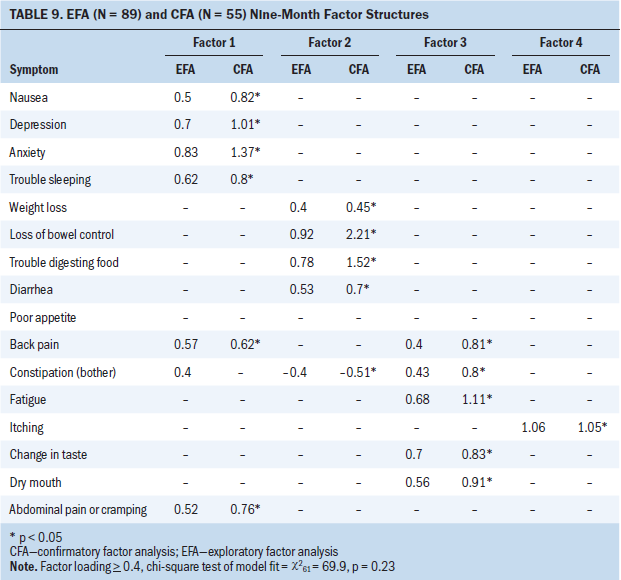
Mood–insomnia–pain–nausea symptom cluster: In the EFA, depression, anxiety, nausea, trouble sleeping, constipation, back pain, and abdominal pain/cramping loaded on factor 1 at T4. In the CFA model, only depression, anxiety, nausea, trouble sleeping, back pain, and abdominal pain/cramping significantly loaded (p < 0.05). Constipation failed to load in the CFA model and was not included in final factor 1 structure.
Digestive–weight loss–bowel problems symptom cluster: In the EFA and CFA (p < 0.05) models, loss of bowel control, trouble digesting food, diarrhea, constipation, and weight loss loaded on factor 2 at T4.
Fatigue–pain–nutritional problems symptom cluster: In the EFA and CFA (p < 0.05) models, fatigue, change in taste, dry mouth, back pain, and constipation loaded on factor 3 at T4.
Core Symptom Clusters
Although the symptoms that clustered over time were not identical, four consistent core SCs were identified. The affective core SC included the symptoms of anxiety and depression, which consistently clustered at each study time point. The gastrointestinal core SC was present at all study time points and included the symptoms of trouble digesting food and the loss of bowel control. The gustatory core SC, which included a change in taste and dry mouth, also consistently clustered at all study time points. The symptoms of nausea, back pain, and abdominal pain/cramping demonstrated a relatively consistent relationship by clustering together at T1, T2, and T4, and were termed the discomfort core SC.
Discussion
Symptoms
The prevalence of the 17 symptoms experienced by patients with PC prior to and after surgical resection was relatively high, except for jaundice, which fell below 10% occurrence after surgery. Fatigue had the highest prevalence, which is consistent with the often-reported finding of it being the most common and distressing symptom for patients with advanced cancers (Butt et al., 2008) and specifically in a cohort of patients with PPC (Yeo et al., 2012). The most prevalent symptoms in this sample varied somewhat over time, but fatigue, trouble sleeping, poor appetite, trouble digesting food, and weight loss were consistently the most prevalent and severe symptoms at all four time points. Overall, the mean symptom severity was mild to moderate, with all severity scores below 2.15 on a 0–4 scale. These findings suggest that patients with PC undergoing surgical resection experience a wide variety of symptoms concerns, although the most prevalent symptoms tend to be similar throughout the perioperative period.
Patients with PC reported a median of 10 concurrent symptoms preoperatively and 9 concurrent symptoms postoperatively at T2, T3, and T4. Eighty-two percent of patients (n = 117) received postresection adjuvant therapy. Although recovery from surgery typically occurs within six months, the side effects of neoadjuvant and adjuvant treatment may account for the persistent concurrent symptoms. Similarly, Chang, Hwang, Feuerman, and Kasimis (2000) surveyed 240 patients with cancer undergoing active treatment and found that they experienced a median of eight concurrent symptoms.
Symptom Clusters
The findings from the current study provide the first data-driven evidence of 16 distinct SCs in surgically resected patients with PC within nine months of surgery. Although the SCs identified over time were not identical, four core SCs persisted over time: the affective, gastrointestinal, gustatory, and discomfort SCs.
Affective core symptom cluster: Anxiety and depression clustered at each study time point. A distinct anxiety and depression SC has not been previously identified in this population, although anxiety and depression have been long reported by patients with PC. Green and Austin (1993) examined 20 studies (n = 21) and found that 48% (n = 10) of patients with PC experienced anxiety and 71% (n = 15) experienced symptoms of depression. Although anxiety and depression are common affective reactions to cancer throughout the disease trajectory, patients with PC have been found to experience some of the highest levels of anxiety and depression when compared to patients with other cancer types (Carlson et al., 2004; Noquez, 2008; Zabora, BrintzenhofeSzoc, Curbow, Hooker, & Piantadosi, 2001). Therefore, it was expected that these symptoms would cluster in surgically resected patients with PC. The reason that high levels of anxiety and depression occur in patients with PC remains largely unknown, but some evidence suggests that pathologic changes associated with the tumor itself may account for high rates of some affective reactions, such as depression, in this population (Musselman et al., 2001).
The high rates of anxiety and depression found in previous studies (Carlson et al., 2004; Noquez, 2008; Zabora et al., 2001) are consistent with the current findings that 84% (n = 119) of patients reported anxiety and 68% (n = 97) reported depression at T1. SCs of anxiety and depression have also been reported in patients with breast (Bender, Ergÿn, Rosenzweig, Cohen, & Sereika, 2005), brain (Gleason et al., 2007), and advanced cancers (Cheung, Le, Gagliese, & Zimmerman, 2011; Chow et al., 2008). In the PC literature, anxiety and depression were found to cluster with several other symptoms. Noquez (2008) found anxiety and depression to be clustered with pain, somatization, and fatigue in a large sample of patients with mixed cancer types, including PC. Fatigue, bodily pain, anxiety, depression, and weakness clustered in a study of postoperative patients with PPC (Yeo et al., 2012).
Gastrointestinal core symptom cluster: Trouble digesting food and the loss of bowel control clustered at all study time points. The symptoms of trouble digesting food and loss of bowel control have not been previously reported to cluster in the SC literature. Digestive problems, including the inability to digest food accompanied by diarrhea (steatorrhea) that may be associated with incontinence is likely related to pancreatic enzyme insufficiency (Coleman, 2010), a common physiologic occurrence in patients with PC before and after potential curative surgical resection.
Preoperatively, pancreatic enzyme insufficiency occurs when the pancreas fails to produce enough digestive enzymes as a result of malignant changes or an obstruction of the biliary or pancreatic ducts that prevent digestive enzymes from reaching the duodenum to allow for proper digestion of carbohydrates, proteins, and fats (Coleman, 2010; Hodgins, 2011). Trouble digesting food and the loss of bowel control are also concerns after resection, which may be related to (a) removal of a portion of the pancreas, (b) resection of a portion of the stomach, or (c) resection of part of the duodenum, which may reduce mucosal surface area in the duodenum that is needed for absorptive processes (Mackay, Hayes, & Yeo, 2006; Sohn & Yeo, 2002). In addition, these gastrointestinal symptoms may also be caused by neoadjuvant chemotherapy or postoperative adjuvant therapy (Coleman, 2010).
Gustatory core symptom cluster: Changes in taste and dry mouth consistently clustered at all four time points. Although this SC has not been previously identified in patients with PC, the relationship among these symptoms has been established in the literature. Neoadjuvant and adjuvant chemotherapy and/or radiation therapy have been associated with a wide range of gastrointestinal symptoms in patients with PC, including nausea, vomiting, changes in taste or smell, loss of appetite, and bowel changes (Coleman, 2010), which may account for the clustering of dry mouth and changes in taste in this study. Dry mouth is one of the most common side effects of many pain medications prescribed during the perioperative period. An association between the symptoms of changes in taste and dry mouth has also been identified as a part of a larger SC and was found in patients with breast (Roiland & Heidrich, 2011), head and neck (Xiao et al., 2012), esophageal (Wikman et al., 2014), and advanced cancers (Aktas, Walsh, & Rybicki, 2012).
Discomfort core symptom cluster: Nausea, back pain, and abdominal pain/cramping demonstrated a relatively consistent relationship by clustering at T1, T2, and T4. Although this clustering of symptoms was not previously reported in PC populations, a relationship between pain and poor appetite has been reported as part of a larger SC in patients with lung cancer (Brown, Cooley, Chernecky, & Sarna, 2011), patients undergoing adjuvant therapy (Skerman, Yates, & Battistutta, 2012), and patients undergoing stem cell transplantation (Jarden, Nelausen, Hovgaard, Boesen, & Adamsen, 2009). Pain associated with PC is often described by patients as a dull, intermittent, and diffuse pain located in the abdomen or back (Hodgins, 2011). In the current investigation, 57% (n = 52) of patients reported abdominal pain/cramping and 33% (n = 30) of patients reported back pain throughout the perioperative period. Nausea is a common PC symptom that is associated with alterations in gastrointestinal functioning from the tumor itself, surgical resection, and neoadjuvant/adjuvant therapy (Coleman, 2010; Hodgins, 2011; Mackay et al., 2006; Sohn & Yeo, 2002). Therefore, it was not surprising that abdominal and/or back pain would cluster with nausea in patients with PC undergoing surgical resection.
Limitations
Several limitations exist for this study. Convenience sampling of patients from a single, high-volume PC center and inclusion of a small homogenous sample with limited racial and ethnic diversity limit the generalizability of the authors’ findings. In addition, the current sample included a cohort of patients with stage II PC, the majority of whom underwent surgical resection via a pylorus-preserving pancreaticoduodenectomy. A systematic review suggested that patients undergoing a potentially curative resection via a classic pancreaticoduodenectomy may experience significantly higher rates of delayed gastric emptying postoperatively when compared to patients undergoing a pylorus-preserving pancreaticoduodenectomy (Hüttner et al., 2016), which may have affected how associated symptoms, such as nausea and vomiting, clustered in this study. Similarly, although depression and anxiety are common occurrences in patients with PC (Carlson et al., 2004; Noquez, 2008; Zabora et al., 2001) and have been found to be present months (as many as 42 months) prior to the time of diagnosis (Fras, Litin, & Pearson, 1967), 22% of patients with PC in this study (n = 31) reported a history of a mental health disorder, the vast majority of whom reported a mood disorder, including 61% depression (n = 19) and 45% anxiety (n = 14) which, to reiterate, may have affected the clustering of symptoms.
The use of the FACT-Hep instrument and failure to capture specific data related to neoadjuvant and adjuvant treatments are also limitations of this study. Although the FACT-Hep tool is a valid and reliable symptom and QOL measure, it was not designed to conduct a multidimensional symptom assessment. The FACT-Hep tool measured most symptoms in terms of severity; however, two symptoms were measured in terms of bother (jaundice and constipation). This may have affected the symptoms that clustered in this analysis. Neoadjuvant and adjuvant treatment data were self-reported by patients, and details regarding specific chemotherapy agents, radiation therapy treatment, and the timing of adjuvant therapy were only sporadically reported, which precluded more detailed data analysis in this study.
Implications for Nursing
The TOUS (Lenz et al., 1997) served as a helpful framework to guide this study. The first step toward effective symptom management is to gain a thorough understanding of the symptoms or SCs (Dodd et al., 2001). Findings from this study provide valuable insight into the presence and severity of symptoms and SCs experienced by surgically resected patients with PC preoperatively and as many as nine months postoperatively, which has not been previously reported. In addition, the TOUS would be a helpful framework to guide future interprofessional SC research in patients with PC. More specifically, the TOUS provides an ideal method to conceptualize (i.e., variable selection and identification of relationships to examine) and conduct research focused on gaining a better understanding of the factors that influence the presence or severity of SCs and consequences that SCs may have on important performance outcomes, such as functional status, QOL, and survival.
Implications for Nursing Practice
Results of this study may be used in oncology nursing practice to increase understanding of symptoms and SCs experienced by patients with PC undergoing surgical resection, which may inform assessments and monitoring, counseling and education, and the anticipatory guidance provided to patients and their families about what symptoms to expect after PC surgery. The findings may be used to target assessments to address SCs, rather than individual symptoms, which is consistent with the experience of postoperative patients with PC. The variability in the symptoms that clustered over time in the current study also underscores the need to complete regular assessments to capture changes in SCs.
Knowledge of the differences in distinct SC patterns may be used to not only enhance the accuracy of SC assessments and monitoring to allow for the earlier identification of existing SCs, but also to predict what symptoms are likely to cluster in patients with PC undergoing surgical resection throughout the perioperative period. More accurate and regular SC assessments and monitoring may allow oncology nurses to implement earlier counseling, education, and symptom management strategies as indicated throughout the cancer trajectory (Nho et al., 2017) to mitigate the presence or severity of SCs or prevent the occurrence of SCs in their entirety. As such, SC findings from this study have the potential to have a positive impact on important patient- and family-reported outcomes.
Current oncology practice may also use several specific core SC findings to enhance the nursing and collaborative care provided to patients with PC during the perioperative period. For example, the affective core SC was present throughout the perioperative period, suggesting that oncology nursing professionals must be vigilant in assessing patients for the presence of anxiety and depression. It is well documented in the literature that high levels of anxiety and depression have been associated with negative impact on patients’ adherence to cancer treatment and clinical outcomes, such as QOL and even survival (Arrieta et al., 2013; Frick, Tyroller, & Panzer, 2007; Vodermaier, Lucas, Linden, & Olson, 2017). Given the detrimental impact that anxiety and depression may have on clinical outcomes, oncology nurses must advocate for patients with PC to ensure they are receiving appropriate evidence-based symptom management strategies and referrals to mental health professionals, as indicated.
Similarly, the gastrointestinal core SC, consisting of trouble digesting food and loss of bowel control, is likely associated with preoperative or postoperative pancreatic enzyme insufficiency, and is currently managed in clinical practice by the use of oral pancreatic enzyme replacement with meals and snacks (Coleman, 2010). For optimal management, oral pancreatic enzyme supplements are titrated until stools achieve a desirable consistency, which requires patient and family education and counseling. Oncology nursing professionals are in a prime role to teach patients and families about the proper use of pancreatic enzyme supplements, coordinate with the oncology team to ensure proper enzyme replacement titration, and advocate for referrals to a registered dietitian when more intensive nutritional management is required.
Implications for Nursing Research
Larger, multicenter studies with greater racial and ethnic diversity are warranted to confirm the presence of the SCs identified in this study in patients with stage II PC and to generalize findings to patients with other stages of PC. Given the large number of SCs identified in this study, researchers should focus their future attention on confirming the presence of the four core SCs that persisted over time: affective, gastrointestinal, gustatory, and discomfort. Future research should be conducted using a systematic, regular interval approach to SC assessments using a well-validated and reliable comprehensive symptom assessment tool to ensure the capture of cancer and treatment-related symptoms in a consistent manner, with attention to important symptom qualities (i.e., timing, severity, interference, and distress). In addition, several symptoms loaded on two different factors (clustered with two symptom groupings) over time in the EFA and CFA models. Although these findings may be a product of the relatively small sample size, such findings may also suggest that there is a shared underlying causative mechanism that warrants additional study. 
Conclusion
This study is the first to explore symptoms and identify SCs over time in patients with stage II PC undergoing surgical resection. Sixteen SCs were identified within the first nine months of surgery alone or along with adjuvant therapy. Although the symptoms that clustered were not identical at all times, four persistent core SCs were identified. Findings from this study may be used to provide anticipatory guidance and inform clinical assessments of symptoms and SCs in this population. Additional research is warranted to (a) confirm the presence of the identified SCs in large, multicenter studies with adequate racial and ethnic diversity; (b) generalize the identified SCs to patients with all stages of PC undergoing surgery; and (c) identify underlying biologic mechanisms that contribute to the development of SCs in this population.
About the Author(s)
Sherry A. Burrell, PhD, RN, CNE, is an assistant professor in the M. Louise Fitzpatrick College of Nursing at Villanova University in Pennsylvania; Theresa P. Yeo, PhD, MPH, AOCNP-BC®, is co-director of the Jefferson Pancreas Tumor Registry, a nurse practitioner in the Department of Surgery at Thomas Jefferson University Hospital, and an adjunct professor in the Jefferson College of Nursing at Thomas Jefferson University in Philadelphia, PA; Suzanne C. Smeltzer, EdD, RN, ANEF, FAAN, is a professor in the M. Louise Fitzpatrick College of Nursing and Director of the Center for Nursing Research at Villanova University; Benjamin E. Leiby, PhD, BA, is an associate professor and director of the Division of Biostatistics at Thomas Jefferson University; Harish Lavu, MD, is the section chief of hepatopancreatobiliary surgery in the Department of Surgery at Thomas Jefferson University Hospital and an associate professor in the Sidney Kimmel Medical College at Thomas Jefferson University; Eugene P. Kennedy, MD, is the chief medical officer at NewLink Genetics in Philadelphia; and Charles J. Yeo, MD, is a professor in the Sidney Kimmel Medical College at Thomas Jefferson University and chair of the Department of Surgery at Thomas Jefferson University Hospital. This research was funded by a doctoral degree scholarship in cancer nursing awarded to Burrell from the American Cancer Society (DSCN 11-195-01). Burrell has previously received honorarium from the Oncology Nursing Society and Wolters Kluwer for review activities. Leiby has previously consulted for Bayer HealthCare. Burrell, T. Yeo, and Lavu completed the data collection. Leiby provided statistical support. Burrell, T. Yeo, Leiby, Lavu, and C. Yeo provided the analysis. All authors contributed to the conceptualization and design and the manuscript preparation. Burrell can be reached at sherry.burrell@villanova.edu, with copy to ONFEditor@ons.org. (Submitted November 2017. Accepted February 8, 2018.)
References
Aktas, A., Walsh, D., & Rybicki, L. (2012). Symptom clusters and prognosis in advanced cancer. Supportive Care in Cancer, 20, 2837–2843. https://doi.org/10.1007/s00520-012-1408-9
American Joint Committee on Cancer. (2009). Pancreas cancer staging (7th ed.). Retrieved from https://cancerstaging.org/references-tools/quickreferences/Documents/Pa…
Arrieta, O., Angulo, L.P., Núñez-Valencia, C., Dorantes-Gallareta, Y., Macedo, E.O., Martínez-López, D., . . . Oñate-Ocaña, L.F. (2013). Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Annals of Surgical Oncology, 20, 1941–1948. https://doi.org/10.1245/s10434-012-2793-5
Bender, C.M., Ergÿn, F.S., Rosenzweig, M.Q., Cohen, S.M., & Sereika, S.M. (2005). Symptom clusters in breast cancer across 3 phases of the disease. Cancer Nursing, 28, 219–225.
Brown, J.K., Cooley, M.E., Chernecky, C., & Sarna, L. (2011). A symptom cluster and sentinel symptom experienced by women with lung cancer [Online exclusive]. Oncology Nursing Forum, 38, E425–E435. https://doi.org/10.1188/11.ONF.E425-E435
Bryant, F.B., & Yarnold, P.R. (1995). Principal-components analysis and exploratory and confirmatory factor analysis. In L.G. Grimm and P.R. Yarnold (Eds.), Reading and understanding multivariate statistics (pp. 99–136). Washington, DC: American Psychological Association.
Butt, Z., Rosenbloom, S.K., Abernethy, A.P., Beaumont, J.L., Paul, D., Hampton, D., . . . Cella, D. (2008). Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. Journal of the National Comprehensive Cancer Network, 6, 448–455.
Carlson, L.E., Angen, M., Cullum, J., Goodey, E., Koopmans, J., Lamont, L., . . . Bultz, B.D. (2004). High levels of untreated distress and fatigue in cancer patients. British Journal of Cancer, 90, 2297–2304. https://doi.org/10.1038/sj.bjc.6601887
Chang, V.T., Hwang, S.S., Feuerman, M., & Kasimis, B.S. (2000). Symptom and quality of life survey of medical oncology patients at a Veterans Affairs medical center: A role for symptom assessment. Cancer, 88, 1175–1183.
Cheung, W.Y., Le, L.W., Gagliese, L., & Zimmerman, C. (2011). Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Supportive Care in Cancer, 19, 417–423. https://doi.org/10.1007/s00520-010-0865-2
Chow, E., Fan, G., Hadi, S., Wong, J., Kirou-Mauro, A., & Filipczak, L. (2008). Symptom clusters in cancer patients with brain metastases. Clinical Oncology, 20, 76–82. https://doi.org/10.1016/j.clon.2007.09.007
Coleman, J. (2010). Diarrhea. In C.G. Brown (Ed.), A guide to oncology symptom management (pp. 173–195). Pittsburgh, PA: Oncology Nursing Society.
Crippa, S., Dominquez, I., Rodriquez, J.R., Razo, O., Thayer, S.P., Ryan, D.P., . . . Fernández-del Castillo, C. (2008). Quality of life in pancreatic cancer: Analysis by stage and treatment. Journal of Gastrointestinal Surgery, 12, 783–793. https://doi.org/10.1007/s11605-007-0391-9
Dirksen, S.R., Belyea, M.J., Wong, W., & Epstein, D.R. (2016). Transitions in symptom cluster subgroups among men undergoing prostate cancer radiation therapy. Cancer Nursing, 39, 3–11. https://doi.org/10.1097/NCC.0000000000000236
Dodd, M.J., Miaskowski, C., & Lee, K.A. (2004). Occurrence of symptom clusters. Journal of the National Cancer Institute. Monographs, 32, 76–78. https://doi.org/10.1093/jncimonographs/lgh008
Dodd, M.J., Miaskowski, C., & Paul, S.M. (2001). Symptom clusters and their effect on the functional status of patients with cancer. Oncology Nursing Forum, 28, 465–470.
Franceschini, J., Jardim, J.R., Fernandes, A.L., Jamnik, S., & Santoro, I.L. (2013). Relationship between the magnitude of symptoms and the quality of life: A cluster analysis of lung cancer patients in Brazil. Jornal Brasileiro de Pneumologia, 39, 23–31.
Fras, I.L., Litin, E.M., & Pearson, J.S. (1967). Comparison of psychiatric symptoms in carcinoma of the pancreas with those in some other intra-abdominal neoplasms. American Journal of Psychiatry, 123, 1553–1562. https://doi.org/10.1176/ajp.123.12.1553
Frick, E., Tyroller, M., & Panzer, M. (2007). Anxiety, depression and quality of life of cancer patients undergoing radiation therapy: A cross-sectional study in a community hospital outpatient centre. European Journal of Cancer Care, 16, 130–136. https://doi.org/10.1111/j.1365-2354.2006.00720.x
Gleason, J.F., Jr., Case, D., Rapp, S.R., Ip, E., Naughton, M., Butler, J.M., Jr., . . . Shaw, E.G. (2007). Symptom clusters in patients with newly-diagnosed brain tumors. Journal of Supportive Oncology, 5, 427–433.
Gorsuch, R.L. (1983). Factor analysis (2nd ed.). Manwah, NJ: Lawrence Erlbaum Associates.
Green, A.I., & Austin, C.P. (1993). Psychopathology of pancreatic cancer. A psychobiologic probe. Psychosomatics, 34, 208–221. https://doi.org/10.1016/S0033-3182(93)71882-4
Heffernan, N., Cella, D., Webster, K., Odom, L., Martone, M., Passik, S., . . . Blumgart, L. (2002). Measuring health-related quality of life in patients with hepatobiliary cancers: The functional assessment of cancer therapy-hepatobiliary questionnaire. Journal of Clinical Oncology, 20, 2229–2239. https://doi.org/10.1200/JCO.2002.07.093
Hodgins, M.B. (2011). Pancreatic cancer. In C.H. Yarbro, D. Wujcik, & B.H. Gobal (Eds.) Cancer nursing: Principles and practice (7th ed., pp. 1581–1609). Sudbury, MA: Jones and Bartlett.
Huang, J., Gu, L., Zhang, L., Lu, X., Zhuang, W., & Yang Y. (2016). Symptom clusters in ovarian cancer patients with chemotherapy after surgery: A longitudinal survey. Cancer Nursing, 39, 106–116. https://doi.org/10.1097/NCC.0000000000000252
Huang, J.J., Yeo, C.J., Sohn, T.A., Lillemoe, K.D., Sauter, P.K., Coleman, J., . . . Cameron, J.L. (2000). Quality of life and outcomes after pancreaticoduodenectomy. Annals of Surgery, 231, 890–898.
Hüttner, F.J., Fitzmaurice, C., Schwarzer, G., Seiler, C.M., Antes, G., Büchler, M.W., & Diener, M.K. (2016). Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database of Systematic Reviews, CD006053. https://doi.org/10.1002/14651858.CD006053.pub6
Jarden, M., Nelausen, K., Hovgaard, D., Boesen, E., & Adamsen, L. (2009). The effect of a multimodal intervention on treatment-related symptoms in patients undergoing hematopoietic stem cell transplantation: A randomized controlled trial. Journal of Pain and Symptom Management, 38, 174–190. https://doi.org/10.1016/j.jpainsymman.2008.09.005
Karabulut, N., Erci, B., Ozer, N., & Ozdemir, S. (2010). Symptom clusters and experiences of patients with cancer. Journal of Advanced Nursing, 66, 1011–1021. https://doi.org/10.1111/j.1365-2648.2009.05254.x
Kim, H.J., Barsevick, A.M., Beck, S.L., & Dudley, W. (2012). Clinical subgroups of a psychoneurologic symptom cluster in women receiving treatment for breast cancer: A secondary analysis [Online exclusive]. Oncology Nursing Forum, 39, E20–E30. https://doi.org/10.1188/12.ONF.E20-E30
Kim, H.J., Barsevick, A.M., Tulman, L., & McDermott, P.A. (2008). Treatment-related symptom clusters in breast cancer: A secondary analysis. Journal of Pain and Symptom Management, 36, 468–479. https://doi.org/10.1016/j.jpainsymman.2007.11.011
Kim, H.J., McGuire, D.B., Tulman, L., & Barsevick, A.M. (2005). Symptom clusters: Concept analysis and clinical implications for cancer nursing. Cancer Nursing, 28, 270–282.
Knobf, M.T., Cooley, M.E., Duffy, S., Doorenbos, A., Eaton, L., Given, B., . . . Mallory, G. (2015). The 2014–2018 Oncology Nursing Society research agenda. Oncology Nursing Forum, 42, 450–465. https://doi.org/10.1188/15.ONF.450-465
Laird, B.J., Scott, A.C., Colvin, L.A, McKeon, A.L., Murray, G.D., Fearon, K.C., & Fallon, M.T. (2011). Pain, depression, and fatigue as a symptom cluster in advanced cancer. Journal of Pain and Symptom Management, 42, 1–11. https://doi.org/10.1016/j.jpainsymman.2010.10.261
Lavu, H., Lengel, H.B., Sell, N.M., Baiocco, J.A., Kennedy, E.P., Yeo, T.P., . . . Yeo, C.J. (2015). A prospective, randomized, double-blind, placebo controlled trial on the efficacy of ethanol celiac plexus neurolysis in patients with operable pancreatic and periampullary adenocarcinoma. Journal of the American College of Surgeons, 220, 497–508. https://doi.org/10.1016/j.jamcollsurg.2014.12.013
Lenz, E.R., & Pugh, L.C. (2008). Theory of unpleasant symptoms. In M.J. Smith and P.R. Liehr (Eds.), Middle range theory for nursing (2th ed., pp. 159–182). New York, NY: Springer.
Lenz, E.R., Pugh, L.C., Milligan, R.A., Gift, A.G., & Suppe, F. (1997). The middle-range theory of unpleasant symptoms: An update. Advances in Nursing Science, 19(3), 14–27.
Mackay, S., Hayes, T., & Yeo, A. (2006). Management of gastric cancer. Australian Family Physician, 35, 208–211.
Musselman, D.L., Miller, A.H., Porter, M.R., Manatunga, A., Gao, F., Penna, S., . . . Nemeroff, C.B. (2001). Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: Preliminary findings. American Journal of Psychiatry, 158, 1252–1257. https://doi.org/10.1176/appi.ajp.158.8.1252
Muthén, B., & Asparouhov, T. (2012). Bayesian structural equation modeling: A more flexible representation of substantive theory. Psychological Methods, 17, 313–335. https://doi.org/10.1037/a0026802
Nho, J.H., Reul Kim, S., & Nam, J.H. (2017). Symptom clustering and quality of life in patients with ovarian cancer undergoing chemotherapy. European Journal of Oncology Nursing, 30, 8–14. https://doi.org/10.1016/j.ejon.2017.07.007
Noquez, A.E. (2008). Cancer-related symptom clusters (Doctoral dissertation). Retrieved from ProQuest Dissertations and Theses Global. (Publication No. AAT 3314979).
Okada, K.I., Shimokawa, T., Hirono, S., Kawai, M., Sho, M., Satoi, S., . . . Yamaue, H. (2017). Effect of neoadjuvant nab-paclitaxel plus gemcitabine therapy on overall survival in patients with borderline resectable pancreatic cancer: A prospective multicenter phase II trial (NAC-GA trial). Oncology, 93, 343–346. https://doi.org/10.1159/000478660
Reyes-Gibby, C.C., Chan, W., Abbruzzese, J.L., Xiong, H.Q., Ho, L., Evans, D.B., . . . Crane, C. (2007). Patterns of self-reported symptoms in pancreatic cancer patients receiving chemoradiation. Journal of Pain and Symptom Management, 34, 244–252. https://doi.org/10.1016/j.jpainsymman.2006.11.007
Roiland, R.A., & Heidrich, S.M. (2011). Symptom clusters and quality of life in older adult breast cancer survivors. Oncology Nursing Forum, 38, 672–680. https://doi.org/10.1188/11.ONF.672-680
Sass, D.A., & Schmitt, T.A. (2010). A comparative investigation of rotation criteria within exploratory factor analysis. Multivariate Behavioral Research, 45, 73–103. https://doi.org/10.1080/00273170903504810
Scheingraber, S., Scheingraber, T., Brauckhoff, M., & Dralle, H. (2005). Comparison between a general and a disease-specific health-related quality-of-life questionnaire in patients after pancreatic surgery. Journal of Hepato-Biliary-Pancreatic Surgery, 12, 290–297. https://doi.org/10.1007/s00534-005-0973-4
Serrano, P.E., Herman, J.M., Griffith, K.A., Zalupski, M.M., Kim, E.J., Bekaii-Saab, T.S., . . . Wei, A.C. (2014). Quality of life in a prospective, multicenter phase 2 trial of neoadjuvant full-dose gemcitabine, oxaliplatin, and radiation in patients with resectable or borderline resectable pancreatic adenocarcinoma. International Journal of Radiation Oncology, Biology, Physics, 90, 270–277. https://doi.org/10.1016/j.ijrobp.2014.05.053
Siegel, R., Naishadham, D., & Jemal, A. (2013). Cancer statistics, 2013. CA: A Cancer Journal for Clinicians, 63, 11–30. https://doi.org/10.3322/caac.21166
Siegel, R.L., Miller, K.D., & Jemal, A. (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 68, 7–30. https://doi.org/10.3322/caac.21442
Skerman, H.M., Yates, P.M., & Battistutta, D. (2012). Cancer-related symptom clusters for symptom management in outpatients commencing adjuvant chemotherapy, at 6 months, and 12 months. Supportive Care in Cancer, 20, 95–105. https://doi.org/10.1007/s00520-010-1070-z
Sohn, T., & Yeo, C.J. (2002). Pancreatic and periampullary carcinoma (nonendocrine). In G.D. Zuidema and C.J. Yeo (Eds.), Shackelford’s surgery of the alimentary tract. (5th ed., pp. 63–87). Philadelphia, PA: W.B. Saunders Company.
Sommerville, C.A., Limongelli, P., Pai, M., Ahmad, R., Stamp, G., Habib, N.A, . . . Jiao, L.R. (2009). Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: An analysis of clinicopathological factors. Journal of Surgical Oncology, 100, 651–656. https://doi.org/10.1002/jso.21390
Starkweather, A.R., Lyon, D.E., Elswick, R.K., Jr., Montpetit, A., Conley, Y., & McCain, N.L. (2013). Symptom cluster research in women with breast cancer: A comparison of three subgrouping techniques. Advances in Breast Cancer Research, 2(4), 107–114. https://doi.org/10.4236/abcr.2013.24018
Sun, V., Ferrell, B., Juarez, G., Wagman, L.D., Yen, Y., & Chung, V. (2008). Symptom concerns and quality of life in hepatobiliary cancers [Online exclusive]. Oncology Nursing Forum, 35, E45–E52. https://doi.org/10.1188/08.ONF.E45-E52
Sun, V., Ruel, N., Chung, V., Singh, G., Leong, L., Fakih, M., . . . Ferrell, B. (2016). Pilot study of an interdisciplinary supportive care planning intervention in pancreatic cancer. Supportive Care in Cancer, 24, 3417–3424. https://doi.org/10.1007/s00520-016-3155-9
Vodermaier, A., Lucas, S., Linden, W., & Olson, R. (2017). Anxiety after diagnosis predicts lung cancer-specific and overall survival in patients with stage III non-small cell lung cancer: A population-based cohort study. Journal of Pain and Symptom Management, 53, 1057–1065. https://doi.org/10.1016/j.jpainsymman.2016.12.338
Wang, Y., O’Conner, M., Xu, Y., & Liu, X. (2012). Symptom clusters in Chinese patients with primary liver cancer [Online exclusive]. Oncology Nursing Forum, 39, E468–E479. https://doi.org/10.1188/12.ONF.E468-E479
Wikman, A., Johar, A., & Lagergren, P. (2014). Presence of symptom clusters in surgically treated patients with esophageal cancer: Implications for survival. Cancer, 120, 286–293
Xiao, C., Hanlon, A., Zhang, Q., Ang, K., Rosenthal, D.I., Nguyen-Tan, P.F., . . . Bruner, D.W. (2012). Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiation. Oral Oncology, 49, 360–366.
Yeo, T.P., Burrell, S.A., Sauter, P.K., Kennedy, E.P., Lavu, H., Leiby, B.E., & Yeo, C.J. (2012). A progressive postresection walking program significantly improves fatigue and health-related quality of life in pancreas and periampullary cancer patients. Journal of the American College of Surgeons, 214, 463–475. https://doi.org/10.1016/j.jamcollsurg.2011.12.017
Zabora, J., BrintzenhofeSzoc, K., Curbow, B., Hooker, C., & Piantadosi, S. (2001). The prevalence of psychosocial distress by cancer site. Psycho-Oncology, 10, 19–28.




