Nurse-Delivered Symptom Assessment for Individuals With Advanced Lung Cancer
Objectives: To assess an intervention derived from self-regulation theory (SRT) to promote well-being for individuals with advanced lung cancer.
Sample & Setting: 45 adults with advanced lung cancer who were receiving chemotherapy at an ambulatory cancer center.
Methods & Variables: Participants were randomized to the intervention group or usual care control group. Feasibility assessment focused on recruitment, retention, design, methods, and fidelity. Outcome measures of quality of life, symptoms, and distress were collected at four time points. The main research variables were symptoms, quality of life, and distress.
Results: The participation rate was 79%, and the retention rate was 62%. Participant loss was most often because of progressive disease and occurred early in the study. High fidelity was noted for delivery of the intervention as planned and outcome data collection by telephone. The mean number of interventions delivered was 5.5 of a planned 8. A high level of acceptability was reported for participants completing the intervention.
Implications for Nursing: Although delivering the SRT-derived intervention with fidelity was possible, feasibility findings do not warrant intervention replication in this population.
Jump to a section
One of the most challenging clinical problems in oncology for patients, families, and clinicians is the occurrence of multiple symptoms. Unrelieved symptoms result in decreased functional status and quality of life and increased distress and mortality (Cleeland et al., 2013; Flannery, Phillips, & Lyons, 2009; Reilly et al., 2013). Although the experience of multiple co-occurring symptoms is well established as a frequently occurring clinical issue, research establishing effective interventions for multiple symptoms has been minimal. Efforts have begun to identify interventions that are effective for more than one symptom, but research in the field is in its infancy, with limited studies in selected oncology populations examining specific clusters of symptoms (Berger, Yennu, & Million, 2013). Therefore, the continued finding of multiple co-occurring unrelieved symptoms warrants ongoing development and examination of effective nursing interventions.
One intriguing strategy that has been related to decreased symptom burden and improved patient outcomes is ongoing structured symptom assessment (Basch, Deal, et al., 2017; Cooley et al., 2015; Lobach et al., 2016). In these studies, standardized symptom assessment was followed with trigger alerts to clinicians and/or symptom management interventions. To capitalize on this finding, the current authors asked the question: “What if we could standardize and enhance the symptom assessment process so that it functions as an effective intervention for multiple symptoms?” Based on empirical findings that repeated symptom assessment is related to improved outcomes and on principles of self-regulation theory (SRT), the intervention standardized the symptom assessment process by asking questions that would guide an individual to develop a more detailed understanding of the symptom and promote the individual’s self-monitoring and focus on problem-solving strategies for symptom management. The primary purpose of this article is to report feasibility results of a pilot randomized clinical trial of a structured symptom assessment to promote functional well-being for individuals with advanced lung cancer.
Background
Assessment of symptoms to guide self-care and clinician recommendations has long been recognized as a primary component of oncology nursing practice. Traditionally, assessment is conceptualized as an information-gathering strategy; however, some limited evidence suggests that repeated assessment may reduce symptom distress and improve functional health (Hoekstra, de Vos, van Duijn, Schadé, & Bindels, 2006; Velikova et al., 2004). Researchers have examined the impact of an individual’s completion of a symptom checklist, which is shared with his or her clinician with the expectation that it will improve patient–provider communication and better direct symptom management recommendations. However, several authors reported unanticipated results; beneficial effects of the completed assessment for the participants were found even when assessment results were not shared with clinicians.
In a randomized, controlled trial (RCT), Hoekstra et al. (2006) examined the effects of a weekly administered symptom-monitoring instrument that was intended to be shared with the participants’ care providers. However, in carrying out the study, the completed symptom instrument was actually shared with the care provider only 18% of the time. Nevertheless, improvements in symptom occurrence and symptom severity were found for the intervention group. Although this study was underpowered for statistical significance and different findings were reported for different symptoms, findings suggest that symptom occurrence and severity may be altered by repeated assessment even when results are not communicated with clinicians.
Velikova et al. (2004) reported another RCT in which individuals with cancer who were receiving chemotherapy completed repeated quality-of-life assessments (including nine symptom items). Statistically significant improved functional well-being was reported in individuals completing assessments when compared to those receiving usual care. The study used a three-group design, with the attention control group completing weekly questionnaires that were not shared with clinicians. An unexpected finding was that the attention control group also had better outcomes than the usual care group, lending support to the hypothesis that the mechanism is not related to patient–provider interaction.
Despite beginning evidence of the relationship, little work has been directed at understanding the mechanisms responsible for the link between the assessment process and an individual’s redirection in self-care and coping activities to improve well-being. The framework for studying this area traditionally has been focused on patients as passive participants. The current authors propose using a framework in which patients are active participants who, when prompted to complete a structured symptom assessment, become involved in a very active information process. The premise of an active process is supported by findings from qualitative research interviews with individuals with lung cancer who described a complex cognitive evaluation and interpretation of their symptom experiences, which include symptom anticipation, impact of symptoms on daily life, familiarity of the symptom with experience, and attribution of the symptom to manageable causal factors (Lowe & Molassiotis, 2011).
Theoretical Framework
In the current study, the authors used SRT to build on preliminary findings that symptom assessment results in improved outcomes. SRT asserts that knowledge is stored in memory as mental representations, sometimes referred to as schemas (Johnson, 1999). These representations are described as “explanatory working models of reality” (Severtson, Baumann, & Brown, 2008, p. 540). For example, a person feels sore and achy and thinks of his or her representation of pain; depending on the content in his or her individual pain representation, he or she may think of distress, causative factors, or management strategies. If the information that is being processed from the representation has concrete objective features, “attention is focused on the concrete, objective aspect of the experience and coping is focused on problem-solving and direct actions” (Johnson, Fieler, Jones, Wlasowicz, & Mitchell, 1997, p. 1,042). Examples of concrete objective information are sensory-based aspects—what an experience feels like, the timing of the symptom experience, or things that make the symptom better or worse. In SRT, this sensory-based content activates the functional pathway rather than the emotional pathway; information that is emotion-focused would activate the emotional pathway. SRT-based interventions promoting cognitive processing, with preparatory information formatted as concrete objective sensory information, tested in individuals receiving radiation therapy and chemotherapy have consistently demonstrated improved functional outcomes (Burnish, Snyder, & Jenkins, 1991; Johnson et al., 1997; Johnson, Lauver, & Nail, 1989; Reuille, 2002).
Although research based on SRT has primarily focused on a nurse’s provision of concrete objective information as content for teaching about the treatment experience, SRT was extended to examine how a nurse’s structured symptom assessment questioning can direct an individual to the somatic aspects (rather than the emotional aspects) of the symptom experience in the current study. SRT suggests that a focus on these aspects activates a functional cognitive pathway that integrates problem solving into the representation and subsequently activates self-management skills. Rather than a vague, undeveloped mental representation of an overwhelming global symptom experience, the current authors posit that the intervention will promote a more detailed, precise, differentiated mental representation specific to each co-occurring symptom. Requesting for an individual to conduct repeated assessments promotes self-monitoring. More specifically, if individuals determine that a discrepancy exists between the symptom they have and their desired goal (e.g., no pain), this will serve as a motivational factor to engage in activities to achieve their desired goals, with ongoing opportunities to focus attention on the symptom experienced, recognize details surrounding the experience, and increase recognition of patterns that can lead to symptom self-management activities.
Although the intervention theoretically is applicable to any individual with multiple symptoms, the authors tested the intervention in individuals with advanced lung cancer who were receiving systemic therapy. This population provides a rigorous test of the intervention because, compared to people with other cancer diagnoses, individuals with lung cancer report the highest number of symptoms, worst symptom severity, and highest levels of symptom distress (Degner & Sloan, 1995; Iyer, Roughley, Rider, & Taylor-Stokes, 2014; McCorkle & Quint-Benoliel, 1983). In addition, lung cancer is the second most commonly occurring cancer in men and women and is the leading cause of cancer mortality (Siegel, Miller, & Jemal, 2017). Although much research has been focused on improving symptoms in this population, many interventions are complex and multicomponent and have not been widely adapted in practice (Cooley et al., 2015; Given et al., 2004).
The initial step in testing a new intervention is to conduct an examination of its feasibility (Bowen et al., 2009). The authors conducted a pilot RCT to examine the feasibility of a structured symptom assessment derived from SRT for individuals with advanced lung cancer. Of note, in this design choice, a control arm was included. The primary study purpose was to establish feasibility data, and an exploratory aim was to provide preliminary evaluation of efficacy data.
Methods
A pilot study with a randomized, controlled design was conducted. Participants were randomized to one of two conditions: the intervention group, which received an eight-week, telephone-delivered structured symptom assessment, or the usual care control group. The feasibility assessment focused on recruitment, retention, design, methods, and ability to deliver the intervention as planned (Thabane et al., 2010). The human subject review board at the University of Rochester Medical Center in New York approved the study.
Recruitment occurred at the Wilmot Cancer Institute in Rochester, New York, from December 2012 to May 2014. Participants were adult, nonhospitalized individuals who were diagnosed with lung cancer. The following were the inclusion criteria: being aged 18 years or older, having been diagnosed with advanced lung cancer (stage IIIB or greater non-small cell or extensive stage small cell), receiving oncology treatment currently (chemotherapy, radiation therapy, targeted treatment, or combined therapy), having reported pain since cancer diagnosis, being able to speak English, and having access to a telephone.
Report of pain was an inclusion criterion to target enrollment by individuals who were symptomatic at enrollment. Individuals were screened with the General Practitioner Assessment of Cognition (Brodaty, Kemp, & Low, 2004). Those with a score greater than 5 were ineligible to participate; however, no screened individuals had ineligible scores. The total sample size was 45. Sample size justification in pilot studies is based on the ability to provide useful information for determining feasibility (Thabane et al., 2010). Therefore, the sample size of 45 for the pilot RCT was selected to permit adequate feasibility assessment. A 2:1 group assignment was used with 30 experimental and 15 usual care control group members to facilitate adequate observations in the experimental arm. Randomization was programmed and generated by a computer program, with group assignment predetermined by study ID number.
Procedures and Intervention
The principal investigator (PI) met with potential participants in the clinic after a visit. The study was explained and, if the individual was interested, screening was conducted and written informed consent was obtained. Table 1 outlines the study procedures. All participants (usual care and intervention) received outcome telephone calls from one team of study personnel (blinded to treatment assignment) every three weeks times four. These telephone calls included questions related to symptoms, distress, and quality of life. In addition, participants in the intervention group received weekly telephone calls from a different study team of interventionists; this telephone call included structured symptom assessment. Participants’ health records were reviewed, and demographic and cancer data were extracted. All telephone calls were recorded (both outcome and intervention as a source for fidelity evaluation and as the raw data). Manuals for instruments with directions for use by telephone were used as the training manual. A research team member telephoned the participant and read the questions, and the researcher entered participant responses directly into a computer via REDCap. The program converted the responses into a database file, eliminating the need to record on paper and then enter data. 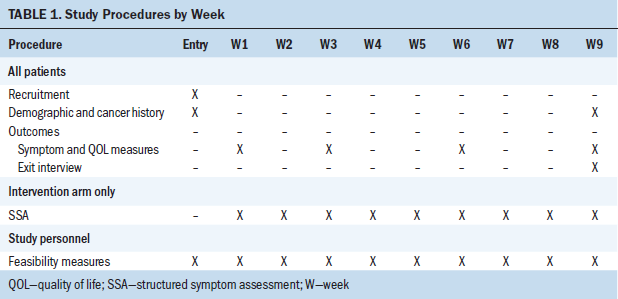
Individuals who were randomized to the intervention received weekly telephone calls for eight weeks. The intervention was a structured assessment of 16 common symptoms experienced by individuals with lung cancer (Mendoza et al., 2011). For any symptom endorsed as present, the interventionists asked a series of six structured questions that were based on SRT and focused on the somatic aspects of the symptom experience, consistent with SRT’s tenants (see Figure 1). This process was repeated for 16 specific symptoms, as identified on the MD Anderson Symptom Inventory–Lung Cancer (Mendoza et al., 2011). The symptom assessment was the intervention. The questions are basic and familiar and are readily transferable to practicing oncology nurses. The authors did not ask about symptom distress because that would involve the emotional pathway, which is not consistent with SRT. 
The PI conducted the intervention initially and trained two additional interventionists. Two independent team members conducted fidelity assessment by listening to the recorded call and reviewing data entered on REDCap. Thirty calls were reviewed, and two issues (skipped questions) were noted; both occurred early in the study and were addressed by the PI.
Measures
Demographic and cancer variables were obtained from a health record review. In addition, the Karnofsky Performance Status scale (KPS), a measure of functional status, was used; a single item was scored on a scale of 0–100 based on self-care ability, symptoms, and activity. The scale takes less than five minutes to complete, has been administered by telephone, and has been used in individuals with lung cancer. Construct validity has been reported as an indicator of overall physical functioning (Yates, Chalmer, & McKegney, 1980). Feasibility variables included recruitment rate, reasons for study refusal, percentage of symptom assessment administered as planned, percentage of completion of outcome calls, and attrition rate by group assignment. Feasibility questions for the research staff to answer were integrated through all phases of the design. For example, after completing each telephone call, research team members completed a series of feasibility questions that included the following:
• Time required to make the call
• If the participant was reached on the initial attempt
• If all questions were answered
• Assessment of the procedures and electronic database
A structured interview was completed at study exit to assess participant acceptability. Five semistructured questions were asked about study participation, as has been done in prior research; however, the psychometric properties were not established (Wells, Hepworth, Murphy, Wujcik, & Johnson, 2003).
For preliminary assessment of efficacy, the outcome variables of quality of life and symptoms were collected. Quality of life was measured with the Functional Assessment of Cancer Therapy–Lung (FACT-L), an instrument specific to lung cancer with 44 items in four domains of well-being (physical, social/family, emotional, and functional) (Cella et al., 2002). The scale takes 5–10 minutes to complete and has been administered by telephone. Reliability is reported as acceptable (Cronbach alpha = 0.86–0.9), and criterion validity has been established with SF-12® scores (p = 0.001) (Mendoza et al., 2011). A single item captured symptom-related distress on a numeric rating scale ranging from 0 (no distress) to 10 (as bad as imaginable). Single-item distress scales previously have been used with cancer samples, take less than a minute to complete, and have demonstrated construct validity (Wells, Murphy, Wujcik, & Johnson, 2003). A single item was used to capture global quality of life, scored 0 (none at all) to 100 (the best imaginable). Single-item quality-of-life data correlate well with multi-item instrument scores and are responsive to change over time (Bernhard, Sullivan, Hürny, Coates, & Rudenstam, 2001; Cunny & Perri, 1991). The MD Anderson Symptom Inventory–Lung Cancer was used to assess symptoms, including severity for 16 commonly occurring symptoms in individuals with lung cancer. Scoring ranges from 0 (none at all) to 10 (as bad as imaginable). The scale takes five minutes to complete and has been administered by telephone (Cleeland, 2016). The test, retest, and internal consistency (Cronbach alpha = 0.83 or higher) are adequate, and criterion validity has been established with the SF–12 and sensitivity to disease progression (Mendoza et al., 2011).
Statistical Analysis
Data were examined by checking frequencies and were cleaned as needed. IBM SPSS Statistics, version 22.0, was used for all analyses. Scoring guidelines were used for the FACT-L, and MD Anderson Symptom Inventory user guide instructions were used for administration and scoring. Summary scale scores were computed. For aim 1 of establishing feasibility, analysis with descriptive and nonparametric statistics was performed. For aim 2 of examination of preliminary efficacy data, nonparametric statistics were used to compare between-group differences because of the small sample size. Because this was a pilot study and findings were exploratory only, p values were not adjusted for multiple testing.
Results
Recruitment and Retention
A CONSORT (Consolidated Standards of Reporting Trials) diagram (see Figure 2) provides an overview of recruitment and retention. Recruitment was from the Wilmot Cancer Institute thoracic oncology clinic and was conducted by the PI at 65 half-day clinic sessions and completed within 15 months. At preclinic meetings, schedules were reviewed for potential eligible participants. A total of 145 were identified; of those, 95 were asked by their oncology provider if they would be willing to talk to the research team. Data were not formally collected on prescreened patients who were not asked to participate, but sometimes visits were canceled or the patient was too ill. Of the 95 patients who were asked by their oncologist, 76 agreed to talk to the researcher. When approached by the researcher, 19 of the 76 patients did not want to talk about a study. Fifty-seven individuals agreed to talk about the study and were asked to consent; 12 declined participation. Forty-five individuals consented for a 79% (45 of 57) participation rate. 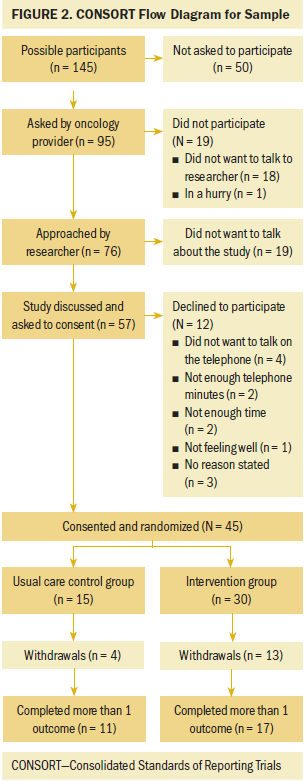
The authors defined completers as participants who completed more than one outcome assessment. Overall retention rate was 62% (28 of 45). Fifteen participants were randomized to the usual care control arm, and four did not complete the study (73% retention); one was a screen failure, one had disease progression, one died, and one moved out of state. For the 30 participants in the intervention group, 13 did not complete the study (57% retention). Eight participants were randomized but withdrew prior to receiving any symptom assessment calls, and five withdrew during the study. The reasons they withdrew included hospitalization, disease progression, not enough time, death in the family, or reason not stated. Most participant loss occurred in the first three weeks of the study. There was decreased retention in the intervention group compared to the usual care control group, but the difference was not statistically significant (chi-square = 0.34).
Sample Characteristics
Twenty-five participants were men, with a mean age of 62.62 years. Thirty-three participants were living with others, and 23 had less than a high school education. Forty participants had non-small cell lung cancer (33 were stage IV), and five had extensive stage small cell lung cancer. All were receiving systemic chemotherapy or targeted therapy, and 10 also were receiving concurrent radiation therapy. All participants had comorbid conditions, and 16 had a palliative care consultation. During the nine-week study, 14 participants had disease progression, 13 had a change in treatment, 6 had partial response, and 2 entered hospice care.
Feasibility Findings
Because of the differences noted in retention between the two study arms, the authors examined baseline differences in participants who were retained in the study and those who withdrew. As shown in Table 2, a consistent pattern showed that participants who completed two or more outcomes had a higher performance status, were living with a partner, had greater than a high school education, had a higher cognitive screening score, had a lower number and severity of symptoms, and had lower distress scores. Tested with chi-square (nominal variables) or t test (numeric variables), significant differences were found in the number and severity of symptoms and the level of distress (p < 0.05). Participants who remained in the study longer had lower distress and less severe symptoms than those who withdrew from the study. 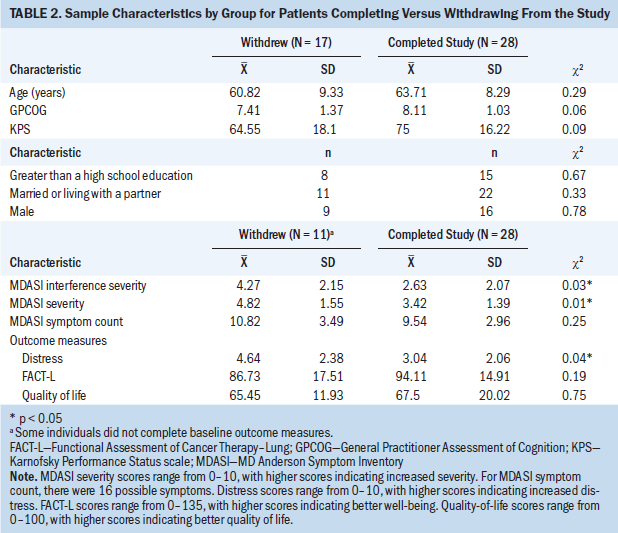
The authors completed 112 of 180 (62%) planned outcome calls. Sixty-eight calls were successfully completed on the first attempt, and 44 required additional attempts. Sixty-two calls were completed in less than 15 minutes; 50 required 15–30 minutes. For the 28 participants who were retained, 20 completed all four outcome calls as planned. Blinding of outcome data collectors was not maintained because many participants disclosed that they were also receiving intervention telephone calls.
The authors completed 121 of 240 (50%) planned symptom assessment calls; 70 required rescheduling or repeated attempts. Ninety-eight calls were completed in less than 15 minutes. The number of symptoms discussed in each telephone call ranged from 3–13, with the mean decreasing from 6.42 to 4.08 during the eight weeks. Of the 17 participants who were retained in the intervention group, the number of intervention calls received was a mean of 5.5 (SD = 2.48); 8 of 17 participants received all eight interventions.
Individuals completing the exit interview at week nine reported that participating in the study was easy or very easy. Three individuals (all in the intervention group) mentioned that calls were repetitive; one mentioned that answers were difficult because symptoms varied and the answers depended on the time of day when questions were asked. Seven individuals indicated that they appreciated the ability to learn things about themselves or that they were glad to participate in doing something to help others with cancer. All but one participant reported no problems with the telephone calls; one individual mentioned difficulty talking because of laryngectomy. All but one participant stated that they would recommend participating in the study to other individuals. When asked in what ways participating in the study changed the way they thought about their symptoms, about half of the participants (13 of 24) commented that they did not think differently. However, many individuals commented that study participation had changed their thinking (i.e., participation had made them “deal with what I got,” “am more aware,” “ accepted,” “think I’m lucky not to be worse,” “realize other people also deal with this,” and “opened my eyes”). Acceptability data were not collected for individuals who withdrew early.
Treatment Effect
The outcome variables included the FACT-L total and subscale scores, global quality of life (possible range = 0–100, with higher scores indicating better quality of life), and distress (possible range = 0–10, with higher scores indicating increased distress). Outcomes were assessed with a change score and, therefore, were examined for patients who completed more than one outcome measure. Because of the small sample size, the authors used nonparametric analysis and did not control for any covariates. See Table 3 for baseline scores by group assignment. The authors computed change scores from baseline to week nine for all outcome variables and examined group differences with the Kruskal–Wallis test (see Table 4). 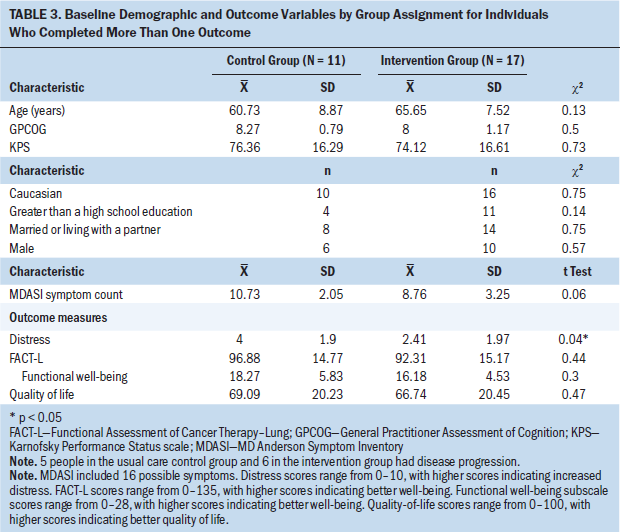
No statistically significant differences were found between the usual care control and intervention groups on outcomes. Examining mean scores for groups, from baseline to week nine, total FACT-L scores increased (improved) for the intervention group and decreased for the usual care control group. Functional well-being improved for both groups, with a larger mean increase for the intervention arm. Global single-item quality-of-life scores decreased six points for the control group and increased three points for the intervention group. Distress scores increased for both groups. 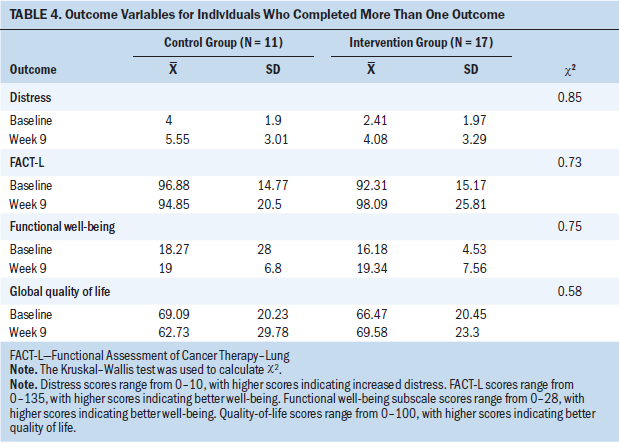
Discussion
The primary rationale for conducting this pilot study was to address an important clinical problem (multiple symptoms), following up on limited but intriguing empirical findings that symptom assessment can have a beneficial effect on an individual’s well-being. SRT provided a possible theoretical explanation for this effect and guided the development of a nurse-delivered structured symptom assessment as the intervention. The primary aim of the current study was to establish the feasibility of this intervention. Feasibility findings were mixed. Feasibility was established for the method of telephone collection of outcome measures, intervention delivery, and fidelity of intervention delivery. Feasibility assessment also provided specific data on time requirements for study personnel and participants. Feasibility concerns included issues related to recruitment, retention, and inability to deliver the planned dose of the intervention. Acceptability of the study was rated as high by participants who remained in the study for nine weeks.
Recruitment of this population was feasible but time-intensive. The recruitment of 45 participants took more than a year and a half; this rate of accrual would not be scalable to a larger clinical trial. The recruitment occurred on 65 clinic days (about one day a week) and would need to be expanded to additional clinics and providers for timelier enrollment. Considering reasons for study refusal, six individuals’ reasons for refusal were related to not wanting to talk on the telephone or use telephone minutes for study-related calls. This is particularly relevant, because telephones are increasingly being used for delivery of research measures and clinical care; this may be a concern for a subset of patients who do not make the telephone a preferred patient-centered approach.
Examining the challenges to retention, the participants who did not complete the study were the high-risk patients who the authors most wanted to reach with their intervention (i.e., those who were sicker, had more symptoms, and were more distressed). When working with individuals with advanced lung cancer during a nine-week interval, some attrition was expected, and the primary reason for participant withdrawal was worsening disease. The randomized, controlled design allowed the authors to identify that withdrawal was higher in the intervention group than in the usual care control group (although not statistically significant). The planned dose of the intervention was not able to be delivered. On average, participants in the intervention group received 5.5 of the planned eight weekly telephone calls. Although receiving every-three-week outcome calls appeared to be an acceptable burden, the planned weekly telephone calls for eight weeks were only able to be delivered to slightly more than one-third of participants.
The exploratory aim was to obtain preliminary data on the efficacy of the intervention. Examination of the effect of the intervention was hampered by small sample size, variable retention to study arms, and decreased dose of the intervention. No statistically significant differences were found. Findings tended to be in the direction favoring the intervention arm for participants who remained in the study. These findings should be interpreted with extreme caution because of the small sample size. The study was not designed to establish efficacy, and these findings do not provide support to recommend the intervention to practicing oncology nurses.
Although the overall feasibility findings do not support replication, much was learned during the conduct of this pilot study. The choice of a randomized, controlled design was a major strength because it not only allowed examination of the intervention, but also provided a comparison group. The choice of a 2:1 randomization scheme made it more difficult to detect the pattern of differential loss in the two groups, and, in future pilots, a 1:1 randomization model will be selected. There are many alternative explanations for the findings. The assessment may not have been done at the correct dosing interval, the two trained interventionists were not experienced oncology nurses, the selected population of individuals with advanced lung cancer receiving systemic treatment may have been too ill or may have had too great a symptom burden, or the underlying rationale may have been incorrect.
Although it was possible to develop and deliver an SRT-based intervention, the current study did not provide support for the extension of SRT to examine this clinical problem in this population. SRT-based interventions were originally conducted with less ill and symptomatic individuals. The intervention involved a series of questions that prompted individuals to conduct a focused self-assessment of any symptom they were experiencing. Participants were not provided any symptom-management recommendations by study personnel; they received usual care from their clinicians. Participants in the intervention arm identified experiencing multiple symptoms, so they had to access multiple representations; it is possible that this diluted the ability to change content in any specific symptom representations. Other authors have advocated for using an SRT/representational approach for education around a solitary symptom (Donovan et al., 2007; Reuille, 2002) or specific subset of symptoms and as a nurse-delivered educational intervention.
Limitations
This was a pilot feasibility study and was not designed to establish efficacy or effectiveness of the intervention. Feasibility results raise concern about the ability to retain and deliver this intervention to high-risk patients. Although the authors attempted to have data collectors blinded, this was not maintained because participants often disclosed that they were receiving additional symptom-assessment calls.
Implications for Nursing
The clinical problem of multiple unrelieved symptoms continues for many individuals with advanced cancer. Assessment of symptoms has long been recognized as a primary component of oncology nursing practice. Although some differences exist in content, virtually all national guidelines for symptom management (e.g., pain, fatigue), including those developed by the National Comprehensive Cancer Network (NCCN), the Oncology Nursing Society, and the American College of Chest Physicians, provide recommendations for repeated symptom assessment (Beck, Erickson, & Shun, 2004; Griffin, Koch, Nelson, & Cooley, 2007; NCCN, 2018a, 2018b). Although use of standardized symptom assessment tools is increasing, this practice remains highly variable across oncology settings (Cooley & Siefert, 2016). It is critical that oncology nurses continue to conduct systematic and repeated symptom assessments as the initial step in the process of minimizing symptom burden. The structured symptom assessment questions are familiar assessment questions to practicing oncology nurses (e.g., timing of symptom, aggravating factors) and remain appropriate for oncology nurses in assessment of symptoms. 
Conclusion
In designing an intervention to address the clinical problem of multiple symptoms, the literature review revealed intriguing and potentially promising findings on the benefit to patients of participating in research studies that focused on repeated symptom assessment. Since the initiation of this study, researchers have continued to examine the effects of routine collection of symptom assessments from patients (often referred to as patient-reported outcomes). Designs often integrate data capture into the electronic health record, with real-time availability to clinicians, and occasionally integrate electronic delivery of symptom management interventions to patients (Basch, Pugh, et al., 2017; Berry et al., 2014; Cooley & Siefert, 2016). Basch, Deal, et al. (2017) reported a survival benefit for individuals with advanced cancer who were randomized to electronic patient symptom reporting. Despite this growing body of work, a knowledge gap remains; the underlying mechanism that explains the efficacy of repeated symptom assessment is not established. The intervention delivered in the current study was based on one theory (SRT) that provided a plausible argument on why repeated symptom assessment would be associated with improved outcomes. However, feasibility results did not provide support for continuing to use the intervention with this high-risk, very ill population. Continued research is needed to build the science and establish evidence-based interventions that are easily transferable to practicing oncology clinicians.
The authors gratefully acknowledge the patients, nurses, physicians, and research staff.
About the Author(s)
Marie Flannery, PhD, RN, AOCN®, is an assistant professor and Karen F. Stein, PhD, RN, is a Ruth Miller Brody and Bernard Brody endowed professor, both in the School of Nursing at the University of Rochester in New York; David W. Dougherty, MD, MBA, is the director of regional operations at the Wilmot Cancer Institute, Supriya Mohile, MD, is an associate professor in the Department of Medicine, and Joseph Guido, MS, is a statistician in the Department of Surgery, all at the University of Rochester Medical Center in New York; and Nancy Wells, DNSc, RN, FAAN, is the director of nursing research at the Vanderbilt University Medical Center in Nashville, TN. This research was funded by a Purdue Pharma Trish Greene Pain Assessment and Management Research Grant from the ONS Foundation and by a grant from the National Cancer Institute (NCI R25-CA10618 and UG1CA189961). Mohile has previously consulted for Seattle Genetics. Flannery, Stein, Dougherty, and Wells contributed to the conceptualization and design. Flannery and Dougherty completed the data collection. Flannery and Guido provided statistical support. Flannery, Dougherty, and Mohile provided the analysis. Flannery, Stein, Dougherty, Mohile, and Wells contributed to the manuscript preparation. Flannery can be reached at marie_flannery@urmc.rochester.edu, with copy to ONFEditor@ons.org. (Submitted August 2017. Accepted March 21, 2018.)
References
Basch, E., Deal, A.M., Dueck, A.C., Scher, H.I., Kris, M.G., Hudis, C., & Schrag, D. (2017). Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA, 318, 197–198. https://doi.org/10.1001/jama.2017.7156
Basch, E., Pugh, S.L., Dueck, A.C., Mitchell, S.A., Berk, L., Fogh, S., . . . Bruner, D.W. (2017). Feasibility of patient reporting of symptomatic adverse events via the patient-reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) in a chemoradiotherapy cooperative group multicenter clinical trial. International Journal of Radiation Oncology, Biology, Physics, 98, 409–418. https://doi.org/10.1016/j.ijrobp.2017.02.002
Beck, S.L., Erickson, J., & Shun, S.C. (2004). Measuring oncology nursing-sensitive patient outcomes: Measurement summary.
Berger, A.M., Yennu, S., & Million, R. (2013). Update on interventions focused on symptom clusters: What has been tried and what have we learned? Current Opinion in Supportive and Palliative Care, 7, 60–66. https://doi.org/10.1097/SPC.0b013e32835c7d88
Bernhard, J., Sullivan, M., Hürny, C., Coates, A.S., & Rudenstam, C.M. (2001). Clinical relevance of single item quality of life indicators in cancer clinical trials. British Journal of Cancer, 84, 1156–1165. https://doi.org/10.1054/bjoc.2001.1785
Berry, D.L., Hong, F., Halpenny, B., Partridge, A., Fox, E., Fann, J.R., . . . Ford, R. (2014). The electronic self report assessment and intervention for cancer: Promoting patient verbal reporting of symptom and quality of life issues in a randomized controlled trial. BMC Cancer, 14, 513. https://doi.org/10.1186/1471-2407-14-513
Bowen, D.J., Kreuter, M., Spring, B., Cofta-Woerpel, L., Linnan, L., Weiner, D., . . . Fernandez, M. (2009). How we design feasibility studies. American Journal of Preventive Medicine, 36, 452–457.
Brodaty, H., Kemp, N.M., & Low, L.F. (2004). Characteristics of the GPCOG, a screening tool for cognitive impairment. International Journal of Geriatric Psychiatry, 19, 870–874.
Burnish, T.G., Snyder, S.L., & Jenkins, R.A. (1991). Preparing patients for cancer chemotherapy: Effect of coping preparation and relaxation interventions. Journal of Consulting and Clinical Psychology, 59, 518–525.
Cella, D., Eton, D.T., Fairclough, D.L., Bonomi, P., Heyes, A.E., Silberman, C., . . . Johnson, D.H. (2002). What is a clinically meaningful change on the Functional Assessment of Cancer Therapy–Lung (FACT-L) questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. Journal of Clinical Epidemiology, 55, 285–295.
Cleeland, C.S. (2016). The MD Anderson Symptom Inventory: User guide [v.1]. Houston, TX: University of Texas MD Anderson Cancer Center.
Cleeland, C.S., Sloan, J.A., Cella, D., Chen, C., Dueck, A.C., Janjan, N.A., . . . Woodruff, J.F. (2013). Recommendations for including multiple symptoms as endpoints in cancer clinical trials: A report from the ASCPRO (Assessing the Symptoms of Cancer Using Patient-Reported Outcomes) Multisymptom Task Force. Cancer, 119, 411–420. https://doi.org/10.1002/cncr.27744
Cooley, M.E., Blonquist, T.M., Catalano, P.J., Lobach, D.F., Halpenny, B., McCorkle, R., . . . Abrahm, J.L. (2015). Feasibility of using algorithm-based clinical decision support for symptom assessment and management in lung cancer. Journal of Pain and Symptom Management, 49, 13–26.
Cooley, M.E., & Siefert, M.L. (2016). Assessment of multiple co-occuring cancer symptoms in the clinical setting. Seminars in Oncology Nursing, 32, 361–372.
Cunny, K.A., & Perri, M., III. (1991). Single-item vs multiple-item measures of health-related quality of life. Psychological Reports, 69, 127–130. https://doi.org/10.2466/pr0.1991.69.1.127
Degner, L.F., & Sloan, J.A. (1995). Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. Journal of Pain and Symptom Management, 10, 423–431.
Donovan, H.S., Ward, S.E., Song, M.K., Heidrich, S.M., Gunnarsdottir, S., & Pillips, C.M. (2007). An update on the representational approach to patient education. Journal of Nursing Scholarship, 39, 259–265. https://doi.org/10.1111/j.1547-5069.2007.00178.x
Flannery, M., Phillips, S.M., & Lyons, C.A. (2009). Examining telephone calls in ambulatory oncology. Journal of Oncology Practice, 5, 57–60. https://doi.org/10.1200/JOP.0922002
Given, C., Given, B., Rahbar, M., Jeon, S., McCorkle, R., Cimprich, B., . . . Bowie, E. (2004). Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. Journal of Clinical Oncology, 22, 507–516.
Griffin, J.P., Koch, K.A., Nelson, J.E., & Cooley, M.E. (2007). Palliative care consultation, quality-of-life measurements, and bereavement for end-of-life care in patients with lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest, 132(Suppl. 3), 404S–422S.
Hoekstra, J., de Vos, R., van Duijn, N.P., Schadé, E., & Bindels, P.J. (2006). Using the symptom monitor in a randomized controlled trial: The effect on symptom prevalence and severity. Journal of Pain and Symptom Management, 31, 22–30. https://doi.org/10.1016/j.jpainsymman.2005.06.014
Iyer, S., Roughley, A., Rider, A., & Taylor-Stokes, G. (2014). The symptom burden of non-small cell lung cancer in the USA: A real-world cross-sectional study. Supportive Care in Cancer, 22, 181–187. https://doi.org/10.1007/s00520-013-1959-4
Johnson, J.E. (1999). Self-regulation theory and coping with physical illness. Research in Nursing and Health, 22, 435–448.
Johnson, J.E., Fieler, V.K., Jones, L.S., Wlasowicz, G.S., & Mitchell, M.L. (1997). Self-regulation theory: Applying theory to your practice. Pittsburgh, PA: Oncology Nursing Society.
Johnson, J.E., Lauver, D.R., & Nail, L.M. (1989). Process of coping with radiation therapy. Journal of Consulting and Clinical Psychology, 57, 358–364. https://doi.org/10.1037/0022-006X.57.3.358
Lobach, D.F., Johns, E.B., Halpenny, B., Saunders, T.A., Brzozowski, J., Del Fiol, G., . . . Cooley, M.E. (2016). Increasing complexity in rule-based clinical decision support: The symptom assessment and management intervention. JMIR Medical Informatics, 4(4), e36.
Lowe, M., & Molassiotis, A. (2011). A longitudinal qualitative analysis of the factors that influence patient distress within the lung cancer population. Lung Cancer, 74, 344–348.
McCorkle, R., & Quint-Benoliel, J. (1983). Symptom distress, current concerns and mood disturbance after diagnosis of life threatening disease. Social Science and Medicine, 17, 431–438.
Mendoza, T.R., Wang, X.S., Lu, C., Palos, G.R., Liao, Z., Mobley, G.M., . . . Cleeland, C.S. (2011). Measuring the symptom burden of lung cancer: The validity and utility of the lung cancer module of the MD Anderson symptom inventory. Oncologist, 16, 217–227.
National Comprehensive Cancer Network. (2018a). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Adult cancer pain [v.1.2018]. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf
National Comprehensive Cancer Network. (2018b). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Distress management [v.2.2018]. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
Reilly, C.M., Bruner, D.W., Mitchell, S.A., Minasian, L.M., Basch, E., Dueck, A.C., . . . Reeve, B.B. (2013). A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Supportive Care in Cancer, 21, 1525–1550. https://doi.org/10.1007/s00520-012-1688-0
Reuille, K.M. (2002). Using self-regulation theory to develop an intervention for cancer-related fatigue. Clinical Nurse Specialist, 16, 312–319. https://doi.org/10.1097/00002800-200211000-00015
Severtson, D.J., Baumann, L.C., & Brown, R.L. (2008). Applying the common sense model to measure representations of arsenic contaminated well water. Journal of Health Communication, 13, 538–554. https://doi.org/10.1080/10810730802281627
Siegel, R.L., Miller, K.D., & Jemal, A. (2017). Cancer statistics, 2017. CA: A Cancer Journal for Clinicians, 67, 7–30. https://doi.org/10.3322/caac.21387
Thabane, L., Ma, J., Chu, R., Cheng, J., Ismaila, A., Rios, L.P., . . . Goldsmith, C.H. (2010). A tutorial on pilot studies: The what, why and how. BMC Medical Research Methodology, 10, 1.
Velikova, G., Booth, L., Smith, A.B., Brown, P.M., Lynch, P., Brown, J.M., & Selby, P.J. (2004). Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. Journal of Clinical Oncology, 22, 714–724. https://doi.org/10.1200/JCO.2004.06.078
Wells, N., Hepworth, J.T., Murphy, B.A., Wujcik, D., & Johnson, R. (2003). Improving cancer pain management through patient and family education. Journal of Pain and Symptom Management, 25, 344–356. https://doi.org/10.1016/S0885-3924(02)00685-1
Wells, N., Murphy, B., Wujcik, D., & Johnson, R. (2003). Pain-related distress and interference with daily life of ambulatory patients with cancer with pain. Oncology Nursing Forum, 30, 977–986. https://doi.org/10.1188/03.ONF.977-986
Yates, J.W., Chalmer, B., & McKegney, F.P. (1980). Evaluation of patients with advanced cancer using the Karnofsky Performance Status. Cancer, 45, 2220–2224.




