Mental Health and Substance Use Disorders in Patients Diagnosed With Cancer: An Integrative Review of Healthcare Utilization
Problem Identification: The impact of mental health disorders (MHDs) and substance use disorders (SUDs) on healthcare utilization (HCU) in patients with cancer is an understudied phenomenon.
Literature Search: A literature search of studies published prior to January 2018 that examined HCU in patients with preexisting MHDs or SUDs diagnosed with cancer was conducted.
Data Evaluation: The research team evaluated 22 studies for scientific rigor and examined significant trends in HCU, as well as types of the MHD, SUD, and cancer studied.
Synthesis: The heterogeneity of HCU outcome measures, MHD, SUD, sample sizes, and study settings contributed to inconsistent study findings. However, study trends indicated higher rates of HCU by patients with depression and lower rates of HCU by patients with schizophrenia. In addition, the concept of HCU measures is evolving, addressing not only volume of health services, but also quality and efficacy.
Implications for Research: Oncology nurses are essential to improving HCU in patients with MHDs and SUDs because of their close connections with patients throughout the stages of cancer care. Additional prospective studies are needed to examine specific MHDs and different types of SUDs beyond alcohol use, improving cancer care and the effectiveness of HCU in this vulnerable population.
Jump to a section
Approximately one in five adults in the United States are diagnosed with a mental health disorder (MHD) or a substance use disorder (SUD) annually (Substance Abuse and Mental Health Services Administration, 2017). More than 43 million adults in the United States live with MHDs. Among the 20 million adults in the United States with an SUD, 50% have a concurrent MHD (Substance Abuse and Mental Health Services Administration, 2015). MHDs and SUDs can have a significant effect on morbidity and mortality. MHDs and SUDs decrease life expectancy by 10 years and double the relative risk for mortality compared to those without MHDs or SUDs (Walker, McGee, & Druss, 2015). A meta-analysis by Singer, Das-Munshi, and Brähler (2010) found that, at the beginning of cancer treatment, 32% of patients with cancer also had a concurrent MHD or SUD. This is concerning because adults in the United States living with serious mental illness die, on average, 25 years earlier than adults without a serious mental illness, largely related to treatable physical comorbidities, including cancer (National Association of State Mental Health Program Directors Council, 2006). In addition, a systematic review of published studies from 1996 to 2006 found that, in patients with MHDs, the incidence of cancer remained consistent with the general population; however, once patients with MHDs were diagnosed with cancer, they had significantly higher mortality than people without MHDs or SUDs (De Hert et al., 2011; Leucht, Burkard, Henderson, Maj, & Sartorius, 2007).
In the general population, MHDs and SUDs are associated with increased healthcare utilization (HCU) (Fogarty, Sharma, Chetty, & Culpepper, 2008). MHDs and SUDs accounted for about 6% of all inpatient stays in the United States in 2014, an increase of 20% from 2005 (Mcdermott, Elixhauser, Sun, & Cost, 2017). In addition, in patients with SUDs, significant increases in HCU are noted for cardiovascular, respiratory, infectious diseases, injuries, and accidents with associated costs that are almost twice that of matched controls (De Weert-Van Oene, Termorshuizen, Buwalda, & Heerdink, 2017).
• Aim 1: Identify the conceptualization and operationalization of MHDs and SUDs in the context of cancer.
• Aim 2: Identify the conceptualization and operationalization of HCU of the outcome variables used to define HCU in the context of MHDs, SUDs, and cancer.
• Aim 3: Determine the association between preexisting MHDs or SUDs on HCU in patients with cancer.
Methods
Data Extraction
After consulting a health sciences librarian, the research team searched the MEDLINE®, CINAHL®, and PsycINFO® electronic databases. The literature search included healthcare utilization, healthcare accessibility, mental disorders, and neoplasms as Medical Subject Heading (MeSH) terms, as well as substance use, mental, cancer, comorbidities, use of health services, admission, emergency, length of stay, and substance-related disorder as additional search terms. Because reviewers found that MHDs and SUDs in patients with cancer is an understudied phenomenon, all studies published longitudinally prior to January 2018 were included in the review.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009) directed the methodology of this integrative review. Prior to the initial literature search, investigators jointly adopted a plan for sampling the literature, article collection, and critical analysis of studies. The initial search produced 1,426 citations that were downloaded into a Microsoft Excel® spreadsheet for independent, manual review by two investigators. Investigators narrowed the search by candidate titles, then abstracts, and finally a full-text review of articles based on the inclusion and exclusion criteria. Investigators retained articles for abstract and full-text review if they met the inclusion and exclusion criteria. Throughout the process, two investigators (J.W. and J.V.C.) worked independently to evaluate each article and then met to discuss decisions and resolve differences. To ensure reliability and validity of the finding from the review, the two investigators independently documented rationale for decisions on article inclusion and exclusion in the Excel spreadsheet.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows:
• Prospective and retrospective cohort, case-control, and cross-sectional studies published in peer-reviewed journals prior to January 2018
• Studies provided a definition or diagnostic criteria for the MHD studied that were present before the measure of HCU. MHDs were operationalized broadly as any neurocognitive, neurodevelopmental, and psychiatric disorders that fall within the 20 disorder chapters of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) (American Psychiatric Association, 2013) present in patients prior to measurement of HCU.
• Studies described patients diagnosed with cancer that used health services as a part of managing their cancer care and physical health.
• Studies provided a definition or diagnostic criteria for SUDs studied. SUDs were operationalized using any preexisting alcohol misuse, alcohol abuse, and illicit or prescription drug use disorders that fall within substance and addictive disorders codes of the DSM-5 (American Psychiatric Association, 2013).
• English language articles with adults aged 18 years or older
• Investigators operationalized HCU as all study outcome variables that are measures of the volume of health services in the context of physical health and cancer care (e.g., hospital stays lengthened because of complications from cancer treatment) based on Andersen’s (1968, 2008) behavioral model of health services use.
The exclusion criteria were as follows:
• Articles in which HCU consisted solely of mental health or SUD treatment outside of the context of cancer care
• Articles that did not describe some form of diagnostic criterion or definition for a MHD or SUD
• Studies involving acute, organic delirium not associated with co-occurring chronic dementia that developed during the same time as the measurement of HCU
Investigators identified 1,426 relevant candidate titles and 87 abstracts for review, of which 37 studies underwent full-text review. Ultimately, 22 studies met inclusion and exclusion criteria (see Figure 1). 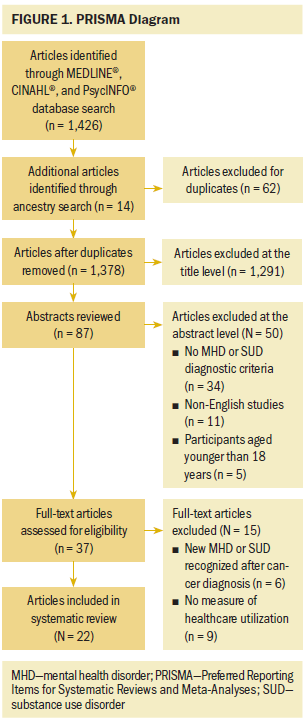
Quality Appraisal
Each study was examined for bias and other aspects of study quality using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria (Benchimol et al., 2015). A team of epidemiologists, methodologists, statisticians, researchers, and journal editors developed the STROBE checklist to improve the scientific rigor, scientific reporting, and internal and external validity of observational studies including cohort, case control, and cross-sectional study designs (Benchimol et al., 2015). The STROBE checklist includes 22 criteria for observational studies. A number of biomedical and cancer journals endorse the STROBE guidelines to improve the quality of scientific reporting for observational studies (Papathanasiou & Zintzaras, 2010). All 22 studies were observational, quantitative studies with moderate to high quality scores (see Table 1). 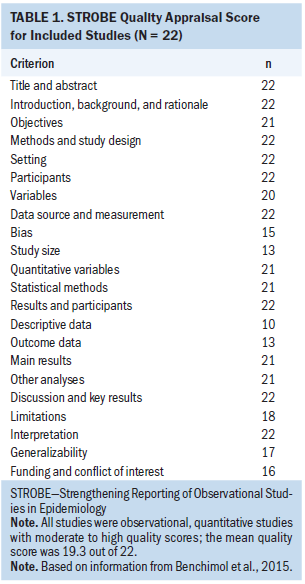
Results
Characteristics of Studies
This review identified 22 relevant studies. All studies used an observational study design with a convenience sample. Nineteen of 22 studies were retrospective with cohort, case-control, or cross-sectional study designs. Three studies were prospective cohort studies. Sample sizes ranged from 41 to 1,238,895 participants. The majority of studies used large federal, state, or health system databases or electronic health records (n = 16 studies) as their source of data, with more than half of the studies examining 10,000 or more participants.
In reviewing demographic traits of study participants, mean ages ranged from 38 to 89 years (Morin, Beaussant, Aubry, Fastbom, & Johnell, 2016; Spies et al., 1996); however, the majority of studies focused on participants who were middle aged or older (n = 16 studies). Both genders were equally represented, with some homogenous male or female samples related to the types of cancer studied (e.g., breast, prostate, gynecologic). Participants were predominantly Caucasian (n = 12 studies). Four studies were conducted in Japan and China (Aoyanagi, Iizuka, & Watanabe, 2007; Chang, Hou, et al., 2013; Chang, Kao, et al., 2013; Ishikawa, Yasunaga, Matsui, Fushimi, & Kawakami, 2016), and five studies did not report on race or ethnicity (Bhattarai, Charlton, Rudisill, & Gulliford, 2013; Ganzini, Socherman, Duckart, & Shores, 2010; Morin et al., 2016; Spies et al., 1996; Wancata, Benda, Windhaber, & Nowotny, 2001).
Types of cancer studied varied considerably. Four studies looked at neoplasms as a broad category, three of which were focused on hospice care (Ganzini et al., 2010; Legler, Bradley, & Carlson, 2011; Morin et al., 2016; Wancata et al., 2001). The most prevalent cancers studied were colon (n = 5 studies) (Aoyanagi et al., 2007; Bhattarai et al., 2013; Himelhoch, Weller, Wu, Anderson, & Cooper, 2004; Ishikawa et al., 2016; Wieghard, Hart, Herzig, Lu, & Tsikitis, 2015), head and neck (n = 4 studies) (Chang, Hou, et al., 2013; Chang, Kao, et al., 2013; Genther & Gourin, 2012; Spies et al., 1996), and breast cancer (n = 4 studies) (Farasatpour et al., 2013; Himelhoch et al., 2004; Mahabaleshwarkar et al., 2015; Zhang, Ivy, Payton, & Diehl, 2010). The only specific relationship between certain types of MHDs or SUDs and cancer was alcohol abuse in patients with head and neck cancer (n = 3 studies) (Chang, Kao, et al., 2013; Genther & Gourin, 2012; Spies et al., 1996).
Aim 1
To operationalize MHDs and SUDs, the majority of studies (n = 20) used organizational diagnostic codes from the International Classification of Diseases developed by the World Health Organization and the DSM-5 from the American Psychiatric Association (Deyo, Cherkin, & Ciol, 1992; Regier, Kuhl, & Kupfer, 2013). Studies identified an MHD or SUD either by direct collection of diagnostic codes or through designation by study investigators based on written patient history extracted from medical records. Two studies also used physician diagnoses from patients’ records to classify MHDs (Nakayama, Ou, Friedman, Smolkin, & Duska, 2015; Spies et al., 1996).
Aim 2
The most common outcome measures used to operationalize HCU were length of stay, hospital admissions, emergency department visits, and outpatient visits. Table 2 contains a review of all outcome measures used to operationalize HCU. Two studies provided a definition for HCU. They used the terms health services use (Chhatre, Metzger, Malkowicz, Woody, & Jayadevappa, 2014) or utilization of acute medical service (Himelhoch et al., 2004). Of the two studies, Himelhoch et al. (2004) was the only study to create a conceptual model when defining HCU. The conceptual model demonstrated the bidirectional relationship between symptom burden of MHDs and other medical illness that is also influenced by the illness behaviors in patients with MHDs (Himelhoch et al., 2004).
Aim 3
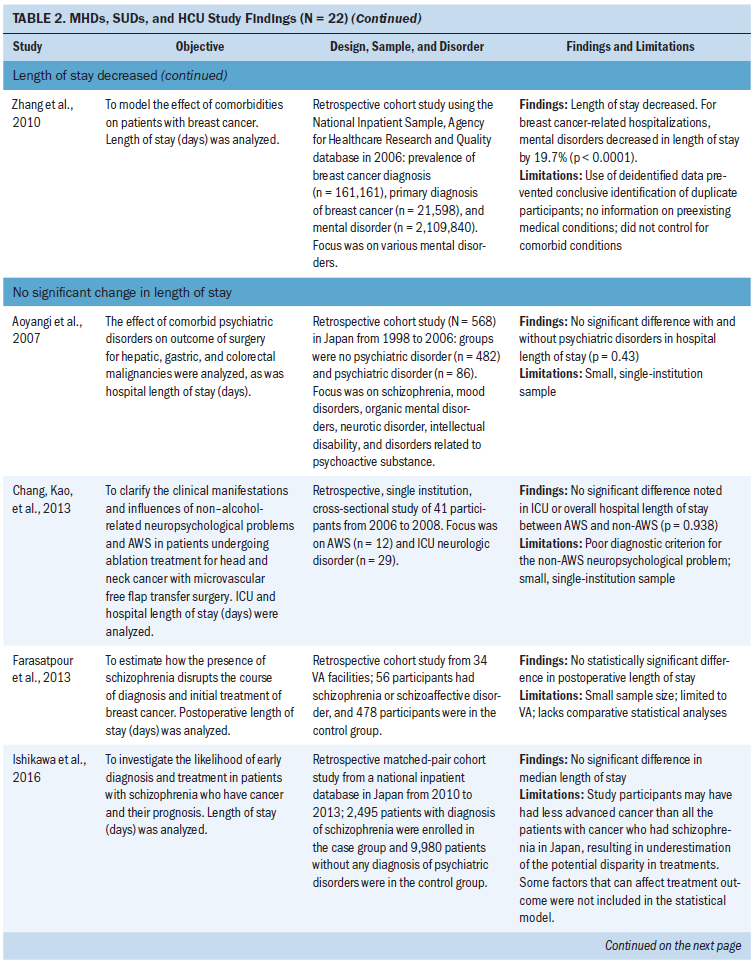
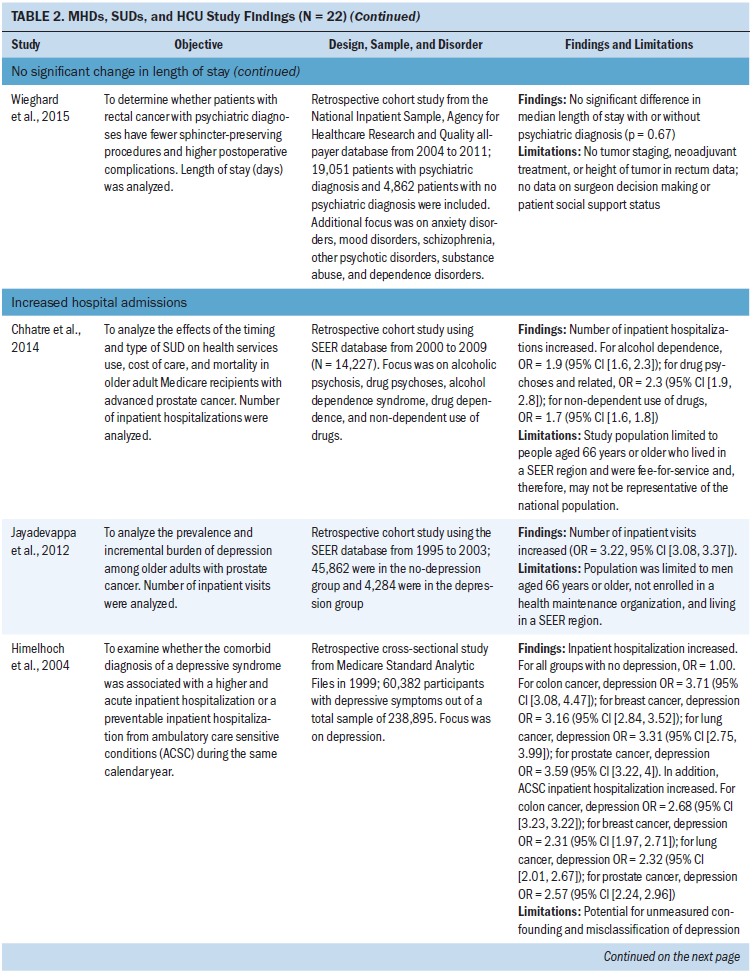
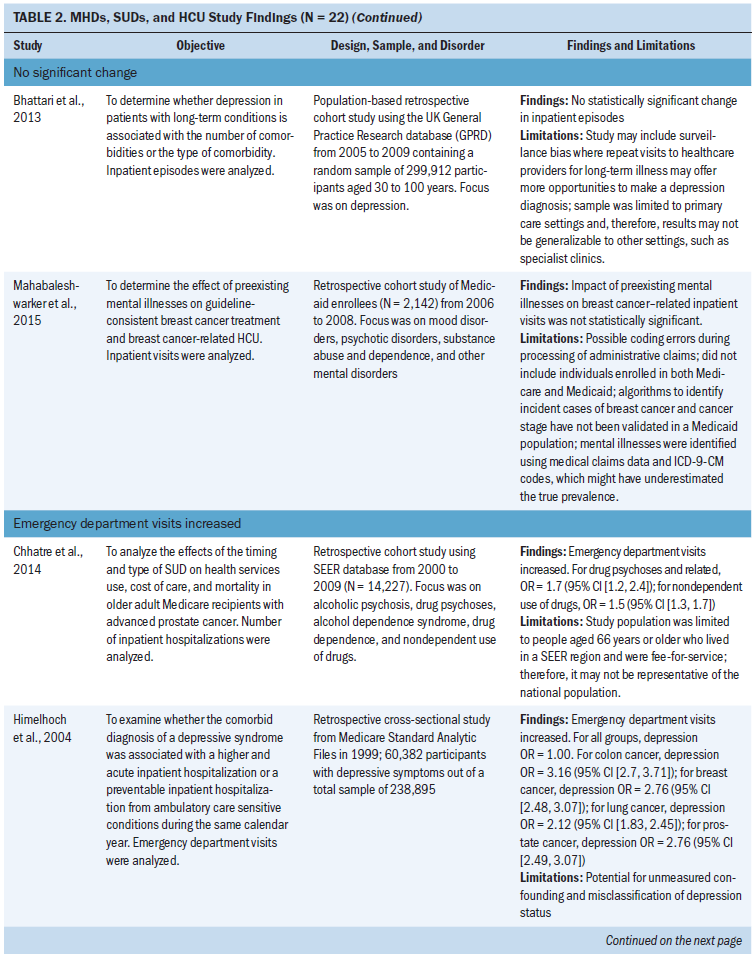
Length of stay: Eleven studies examined length of stay. Five studies did not find a statistically significant change in length of stay in patients with MHDs, SUDs, and cancer (Aoyanagi et al., 2007; Chang, Kao, et al., 2013; Farasatpour et al., 2013; Ishikawa et al., 2016; Wieghard et al., 2015). In addition, four studies (Ganzini et al., 2010; Genther & Gourin, 2012; Prieto et al., 2002; Spies et al., 1996) found an increase and two studies (Wancata et al., 2001; Zhang et al., 2010) found a decrease in length of stay.
The most common types of cancer studied included head and neck (n = 3 studies) (Chang, Kao, et al., 2013; Genther & Gourin, 2012; Spies et al., 1996) and gastric or colorectal (n = 3 studies) (Aoyanagi et al., 2007; Ishikawa et al., 2016; Wieghard et al., 2015). The most common type of MHD studied was all MHDs as a broad category (n = 4 studies) (Aoyanagi et al., 2007; Prieto et al., 2002; Wieghard et al., 2015) and schizophrenia (n = 3 studies) (Farasatpour et al., 2013; Ganzini et al., 2010; Ishikawa et al., 2016). The most common type of SUD studied was all SUDs as a broad category (n = 4 studies) (Aoyanagi et al., 2007; Wancata et al., 2001; Wieghard et al., 2015; Zhang et al., 2010) and alcohol use disorders (n = 3 studies) (Chang, Kao, et al., 2013; Genther & Gourin, 2012; Spies et al., 1996).
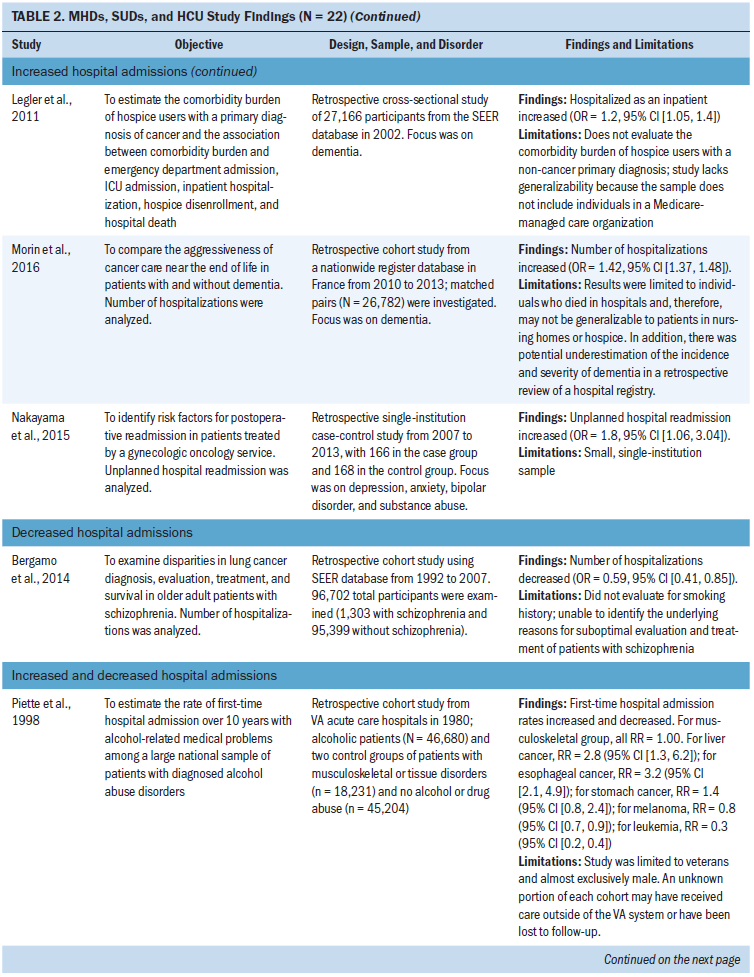
Hospital admissions: Ten studies focused on hospital admissions. Six of those studies found that hospital admissions increased in patients with MHDs and SUDs, with odds ratios ranging from 1.1 to 3.7 compared to patients without MHDs and SUDs (Chhatre et al., 2014; Himelhoch et al., 2004; Jayadevappa, Malkowicz, Chhatre, Johnson, & Gallo, 2012; Legler et al., 2011; Morin et al., 2016; Nakayama et al., 2015). In addition, two studies (Bhattarai et al., 2013; Mahabaleshwarkar et al., 2015) did not find a statistically significant change in hospital admissions. Bergamo, Sigel, Mhango, Kale, and Wisnivesky (2014) found a decrease in hospitalizations in patients with schizophrenia, and Piette, Barnett, and Moos (1998) found increases and decreases in hospital admissions depending on the type of cancer studied.
The most common type of cancer studied in relation to hospital admissions was prostate cancer (n = 3 studies) (Chhatre et al., 2014; Himelhoch et al., 2004; Jayadevappa et al., 2012). Depression (n = 3 studies) was the most common type of MHD studied and was primarily examined in studies that found an increase in hospital admissions in patients with cancer (Bhattarai et al., 2013; Himelhoch et al., 2004; Jayadevappa et al., 2012). SUDs focused on all SUDs as a broad category (n = 2 studies) (Mahabaleshwarkar et al., 2015; Nakayama et al., 2015) and alcohol use disorders (n = 1 study) (Piette et al., 1998). Chhatre et al. (2014) found an increase in hospital admissions and was the only study in the integrative review that specifically examined illicit drug use or prescriptive drug use disorders.
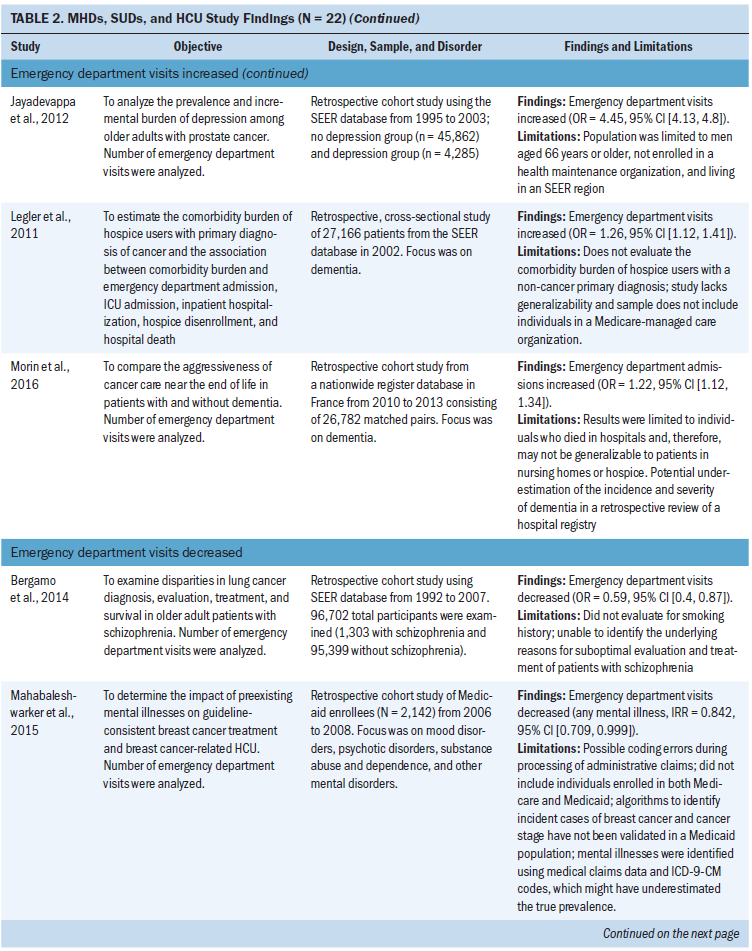
Emergency department visits: Seven studies examined emergency department visits. Five of those studies found that emergency department visits increased in patients with MHDs and SUDs, with odds ratios ranging from 1.2 to 4.5 compared to patients without MHDs and SUDs (Chhatre et al., 2014; Himelhoch et al., 2004; Jayadevappa et al., 2012; Legler et al., 2011; Morin et al., 2016). In addition, two studies examining lung and breast cancer found a decrease in emergency department visits in patients with schizophrenia and MHD studied as a broad category (Bergamo et al., 2014; Mahabaleshwarkar et al., 2015).
The most common type of cancer studied in relation to emergency department visits was prostate cancer (n = 3 studies) (Chhatre et al., 2014; Himelhoch et al., 2004; Jayadevappa et al., 2012). The most common type of MHDs studied were depression (n = 2 studies) (Himelhoch et al., 2004; Jayadevappa et al., 2012) and dementia (n = 2 studies) (Legler et al., 2011; Morin et al., 2016). Depression and dementia were examined in studies with an increase in emergency department visits. Chhatre et al. (2014) examined illicit drug use or prescriptive drug use disorders, and Mahabaleshwarkar et al. (2015) combined all SUDs with MHDs into a broad category for analysis.
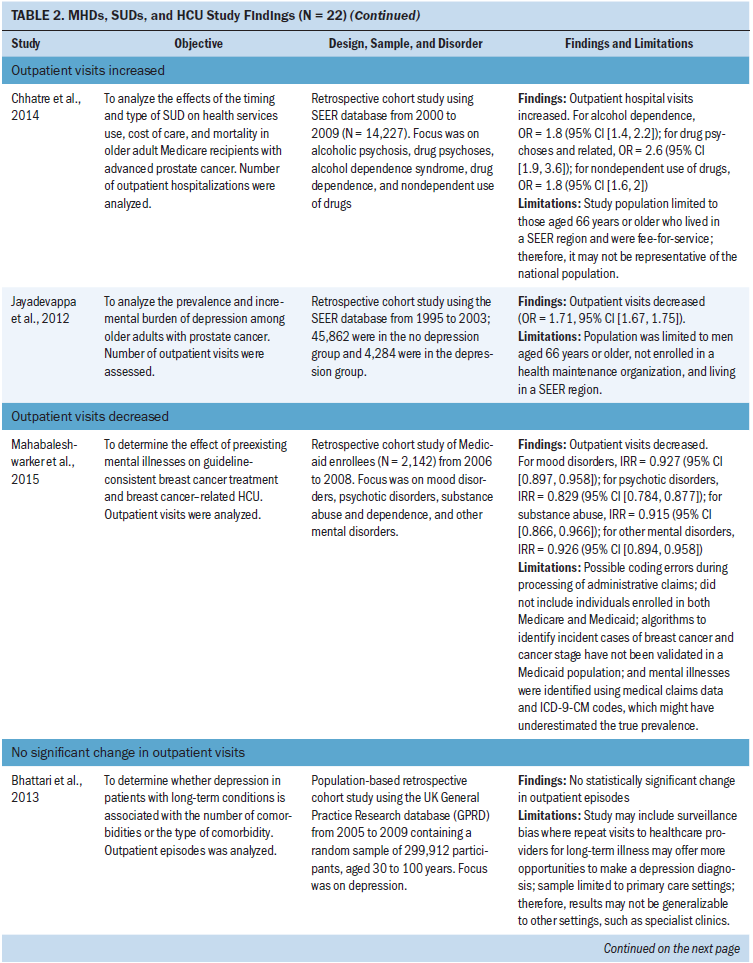
Outpatient visits: Of the four studies that focused on outpatient visits, two found increases in outpatient visits in patients with MHDs, SUDs, and prostate cancer (Chhatre et al., 2014; Jayadevappa et al., 2012). The most common type of MHD studied was depression (n = 2 studies) (Bhattarai et al., 2013; Jayadevappa et al., 2012). All SUDs were studied as a broad category (Chhatre et al., 2014; Mahabaleshwarkar et al., 2015).
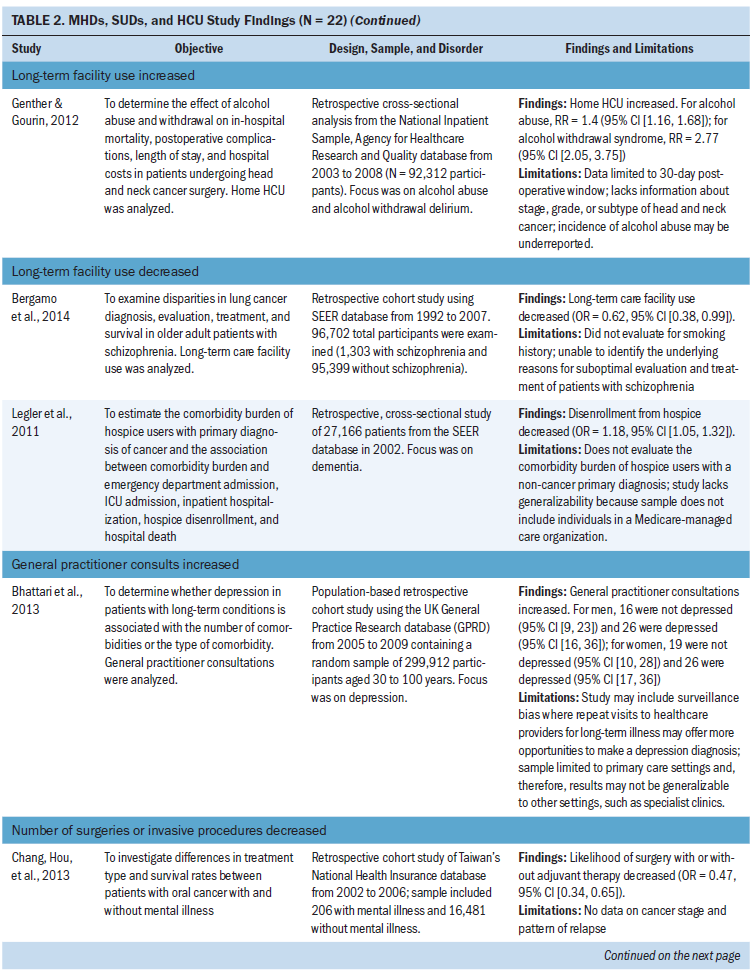
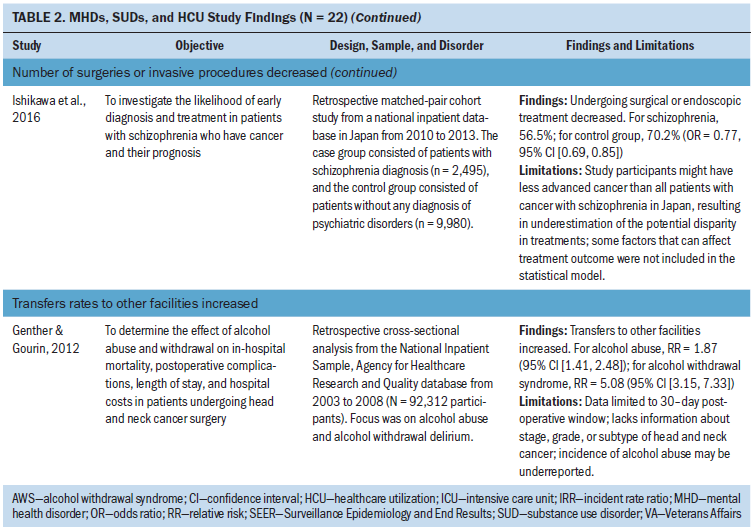
Other outcome measures: Other outcome measures identified included long-term facility use (n = 3 studies), general practitioner consultations (n = 1 study), transfer rates to other facilities (n = 1 study), and number of surgeries or invasive treatments (n = 2 studies) (Bergamo et al., 2014; Bhattarai et al., 2013; Chang, Hou, et al., 2013; Genther & Gourin, 2012; Ishikawa et al., 2016; Legler et al., 2011). In two studies of long-term care, facility use decreased in patients with schizophrenia and dementia during treatment for lung cancer and in hospice care (Bergamo et al., 2014; Legler et al., 2011). In two studies, the number of surgeries or invasive procedures for head and neck, gastric, and colorectal cancer decreased in patients with schizophrenia (Chang, Hou, et al., 2013; Ishikawa et al., 2016). Bhattari et al. (2013) found an increase in general practitioner consultations in patients with depression and colorectal cancer. Genther and Gourin (2012) examined patients with head and neck cancer and found an increase in home HCU and transfer rates to other facilities in patients with alcohol use disorders. 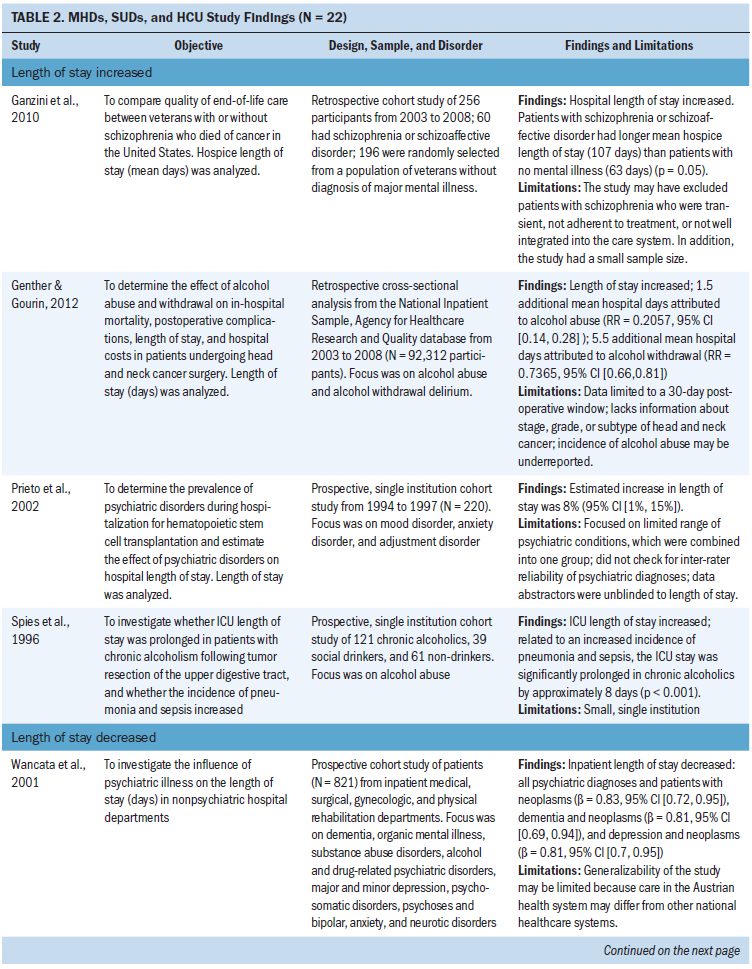
Discussion
This review examined 22 studies and found that, across all cancers, outcomes for HCU in patients with MHDs and SUDs were inconsistent, with both increases and decreases of HCU. The heterogeneity of HCU outcome measures, MHDs, SUDs, sample sizes, and study settings can all contribute to inconsistent study findings. Although hospital admission rates and length of stay were common measures of HCU, the authors counted eight different measures of HCU. Therefore, standardized measures of HCU are needed to determine the nature of the association between preexisting MHDs or SUDs and HCU in patients with cancer.
Policy initiatives regarding insurance reimbursement may create barriers to standardized HCU measures with the use of new outcome measures focused on value in health care. Health payers will increasingly use value-based programs to determine insurance reimbursement policies across the entire healthcare delivery system, evaluating multiple providers and settings as a single episode of patient care (Agency for Healthcare Research and Quality, 2016; National Quality Forum, 2009; Van Cleave, Smith-Howell, & Naylor, 2016). The Centers for Medicare and Medicaid Services (2018) has implemented seven value-based programs, each using new HCU measures as opposed to traditional fee-for-service payments (Agency for Healthcare Research and Quality, 2016; Fried & Sherer, 2016). In addition, in an effort to address quality in health care, large data projects, such as the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project and the National Quality Forum’s Cost and Resource Use Measures project, are also changing the way HCU is operationalized. HCU is increasingly measured using outcome measures for quality, including healthcare safety, timeliness, effectiveness, equity, and patient-centeredness when examining HCU rather than volume of health services alone (Farquhar, 2008; National Quality Forum, 2010). Based on this evidence, future researchers may develop standardized outcome measures for HCU that also reflect quality in healthcare delivery and cost effectiveness in cancer care.
In this review, nine studies combined MHDs into a single category for statistical analysis. This combination of MHDs has important research implications in that prevalence rates of specific MHDs and SUDs may confound study findings. For example, this integrative review suggests that depression was associated with increased hospitalization, whereas schizophrenia was associated with decreased hospitalization. Prevalence rates for depression in patients with cancer are higher than other forms of MHDs, ranging from 8% to 24% (Krebber et al., 2014). The global prevalence rate of schizophrenia ranges from 0.3% to 0.7%, and the incidence rate of cancer in patients with schizophrenia ranges from 0.79% to 0.9% (Catts, Catts, O’Toole, & Frost, 2008; Lie et al., 2015). Therefore, study findings regarding HCU could change depending on the prevalence rates of specific MHDs and SUDs included in study samples.
In addition, the unique symptomatology of specific types of MHDs and SUDs may influence the overall relationship with HCU. For example, higher rates of HCU in patients with depression may be attributed to some aspects of symptom amplification present in patients with depression, including greater awareness of somatic, physical symptoms reported to healthcare providers (Kapfhammer, 2006). In addition, the relationship between depression and certain types of cancer has been investigated as a reciprocal interaction where several studies suggest depression is an early indicator of poor cancer survival and increased HCU (Mayr & Schmid, 2010). In contrast, the symptomatology of schizophrenia involves acute psychotic exacerbations and cognitive deficits involving verbal memory, attention, and executive function (Irwin, Henderson, Knight, & Pirl, 2014). Cognitive deficits in patients with schizophrenia have been shown to impair patients’ ability to live independently and make navigating complex treatment regimens for cancer difficult (Green, Kern, & Heaton, 2004; Irwin et al., 2014). Therefore, cognitive deficits associated with schizophrenia may negatively affect HCU, and specific symptomatology unique to each MHD may affect study results.
Another finding regarding MHDs from the current review was the lack of studies focused on neurocognitive disorders, with only three studies focused on dementia (Legler et al., 2011; Morin et al., 2016; Wancata et al., 2001) and one study focused on neurodevelopmental delays (Aoyanagi et al., 2007). The prevalence of dementia is estimated to be around 6% in people aged older than 65 years and 30% in people aged older than 90 years (Butler, 2014). With an aging global population, the number of people living with dementia is expected to almost double from 35.7 to 65.7 million in 2030 (Prince et al., 2013). Age is a risk factor for a number of cancers, and estimates indicate that, by 2030, 67% of all cancer diagnoses will occur in patients aged 65 years or older (Smith, Smith, Hurria, Hortobagyi, & Buchholz, 2009). Dementia can lead to altered decision-making capacity that can have significant consequences regarding the ability to process information and manage the intricacies of cancer treatment, altering HCU and health outcomes (Karuturi et al., 2016). Therefore, additional research is needed to focus on neurocognitive and neurodevelopmental disorders, including dementia, and their unique relationships with HCU.
When examining SUDs, researchers focused primarily on alcohol use disorders, with only one study specifically examining illicit drug or prescription drug use disorders (Chhatre et al., 2014). A number of prominent research studies illustrate the strong association between alcohol consumption and several types of cancer, including oral cavity, pharynx, larynx, esophageal, liver, breast, and colorectal cancers (Baan et al., 2007; Secretan et al., 2009). In contrast, the relationship between illicit drug and prescription drug use disorders and cancer is an understudied area of research. Patients with cancer have unique risk factors for SUDs and are a part of an aging population in which the number of patients with SUDs undergoing cancer treatment is rising (Paice, 2018). In light of the growing opioid crisis, oncology providers should expect that HCU in patients with cancer increasingly includes management of illicit drug and prescription drug use disorders (Paice, 2018). Consequently, additional research is needed to understand the relationship between prescriptive drug use and other drug use disorders as an emerging health risk for patients with cancer. Oncology nurses need to incorporate new policies in their practice for assessing risk factors for SUDs and managing cancer pain for patients with current and past SUDs (Paice, 2018).
Finally, this review contributes to the literature regarding increased morbidity and mortality in patients with MHDs and SUDs. In addition to HCU outcomes, the majority of studies also identified negative health outcomes as a part of their study findings. Patients with MHDs, SUDs, and cancer were more likely to undergo major surgery, require more invasive surgery, have more medical and surgical complications during hospital admissions, accrue higher healthcare costs, and have greater mortality than controls (Chhatre et al., 2014; Genther & Gourin, 2012; Ishikawa et al., 2016; Spies et al., 1996). Therefore, additional HCU research is needed to identify evidence-based clinical initiatives and treatments tailored to improving cancer care for patients with MHDs and SUDs.
Limitations
All 22 studies were observational, quantitative studies with moderate to high STROBE quality scores. However, the research team identified limitations within the literature. The majority of studies in this review were retrospective with nonrandomized samples where there is a potential for bias. Five studies collected data from single hospitals, which increases the risk of selection bias in recruitment of participants and decreases the external validity (generalizability) of study findings. Another source of selection bias is that some studies identified statistically significant differences in baseline demographic characteristics, socioeconomic status, and physical health characteristics compared to a control group, which could affect HCU. There also was significant potential for source bias and information bias because the majority of studies were retrospective, using secondary data analysis when data collection occurred prior to formulating the study aims and design.
There were also limitations to the findings of this integrative review. Although investigators established operational definitions to guide the identification and conceptualization of HCU, HCU outcome measures included in the review are subject to interpretation. Reviewers used a two-person consensus model for inclusion of all MHDs, SUDs, and HCU outcome measures to try to mitigate subjective, individual interpretation. Although study findings suggested that depression was associated with increased HCU and schizophrenia was associated with decreased HCU, the heterogeneity of MHDs and types of cancer limits the ability of reviewers to draw strong conclusions about the relationship between specific MHDs, types of cancer, and HCU until more data are available for review. Additional limitations are the exclusion of non-English studies and the exclusive use of large, online literature search engines.
Implications for Nursing
These findings have significant implications for clinical nursing, as well as future research. Cancer treatment is a multifaceted combination of surgery, radiation, and chemotherapy. Patients with MHDs and SUDs may require additional mental health and cancer screening to ensure they receive adequate assistance navigating the complexities of cancer care. The unique clinical symptomatology of mental health and SUDs can influence HCU. For example, underreporting, denial, symptom minimization, and poor insight are all common phenomena in patients with schizophrenia that may influence mental health and cancer screening (Carney & Jones, 2006).
Oncology nurses are essential to addressing HCU because of their direct patient contact throughout the multiple stages of care, including screening for postoperative complications and adverse drug reactions, patient and family education, discharge planning, and outpatient care transitions (Naylor, Aiken, Kurtzman, Olds, & Hirschman, 2011). Some clinical areas of concern include more intensive monitoring for postoperative complications and adverse drug reactions in patients with MHDs and SUDs. Farasatpour et al. (2013) found that patients who presented with psychotic symptoms were more likely to not only delay or refuse treatment, but also to exhibit disruptive behavior in the hospital setting. These patients with psychotic symptoms were also at greater risk of postoperative surgical complications like deep and superficial wound infection, pneumonia, and mastectomy flap necrosis (Farasatpour et al., 2013). In addition, oncology medications can have significant pharmacodynamic and pharmacokinetic drug interactions with psychotropic drugs, resulting in negative side effects and noncompliance (Zhou et al., 2010). Finally, oncology nurses are essential to assessing and advocating for patients in chronic pain, as well as monitoring for prescription drug use disorders and other SUDs as the health system in the United States manages a growing opioid crisis (Manchikanti et al., 2012). Greater education regarding the specific needs of patients with MHDs and SUDs throughout oncology nursing is necessary to optimize health outcomes and HCU. 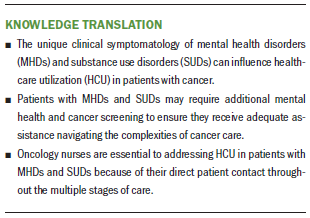
Conclusion
This is one of the first integrative reviews focused on examining the conceptualization and operationalization of HCU in the context of MHDs, SUDs, and cancer. As HCU evolves from volume of health services to a marker of healthcare value and efficiency, standardized HCU measures are needed in research that reflect how HCU is evolving into a measure of healthcare quality. Additional research and clinical initiatives are needed to improve not only effective HCU, but also cancer health outcomes in this unique, high-risk population.
About the Author(s)
Joanna Woersching, MSN, RN, is a doctoral student, Janet H. Van Cleave, PhD, RN, is an assistant professor, and Judith Haber, PhD, FAAN, APRN-BC, is the Ursula Springer Leadership Professor in Nursing, all in the Rory Meyers College of Nursing at New York University in New York; and Deborah Chyun, PhD, RN, FAHA, FAAN, is the dean of the School of Nursing at the University of Connecticut in Mansfield. No financial relationships to disclose. Woersching, Van Cleave, Haber, and Chyun completed the data collection and provided the analysis. All authors contributed to the conceptualization and design and the manuscript preparation. Woersching can be reached at jwoersch@gmail.com, with copy to ONFEditor@ons.org. (Submitted September 2018. Accepted December 11, 2018.)
References
Agency for Healthcare Research and Quality. (2016). Prevention quality indicators overview. Retrieved from http://www.qualityindicators.ahrq.gov/Modules/pqi_resources.aspx
American Psychatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing.
Andersen, R.M. (1968). A behavioral model of families’ use of health services. Chicago, IL: University of Chicago.
Andersen, R.M. (2008). National health surveys and the behavioral model of health services use. Medical Care, 46, 647–653.
Aoyanagi, N., Iizuka, I., & Watanabe, M. (2007). Surgery for digestive malignancies in patients with psychiatric disorders. World Journal of Surgery, 31, 2323–2328.
Baan, R., Straif, K., Grosse, Y., Secretan, B., El Ghissassi, F., Bouvard, V., . . . Cogliano, V. (2007). Carcinogenicity of alcoholic beverages. Lancet Oncology, 8, 292–293. https://doi.org/10.1016/S1470-2045(07)70099-2
Benchimol, E.I., Smeeth, L., Guttmann, A., Harron, K., Moher, D., Petersen, I., . . . Committee, R.W. (2015). The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLOS Medicine, 12(10), e1001885. http://doi.org/10.1371/journal.pmed.1001885
Bergamo, C., Sigel, K., Mhango, G., Kale, M., & Wisnivesky, J.P. (2014). Inequalities in lung cancer care of elderly patients with schizophrenia. Psychosomatic Medicine, 76, 215–220. https://doi.org/10.1097/PSY.0000000000000050
Bhattarai, N., Charlton, J., Rudisill, C., & Gulliford, M.C. (2013). Prevalence of depression and utilization of health care in single and multiple morbidity: A population-based cohort study. Psychological Medicine, 43, 1423–1431. https://doi.org/10.1017/S0033291712002498
Butler, R. (2014). Clinical evience handbook: Dementia. American Family Physician, 89, 117–118. Retrieved from http://www.aafp.org/afp/2014/0115/p117.html
Carney, C.P., & Jones, L.E. (2006). The influence of type and severity of mental illness on receipt of screening mammography. Journal of General Internal Medicine, 21, 1097–1104. https://doi.org/10.1111/j.1525-1497.2006.00565.x
Catts, V.S., Catts, S.V, O’Toole, B.I., & Frost, A.D. (2008). Cancer incidence in patients with schizophrenia and their first-degree relatives—A meta-analysis. Acta Psychiatrica Scandinavica, 117, 323–336. https://doi.org/10.1111/j.1600-0447.2008.01163.x
Centers for Medicare and Medicare Services. (2018). CMS value-based programs. Retrieved from https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Ins…
Chang, C.C., Kao, H.K., Huang, J.J., Tsao, C.K., Cheng, M.H., & Wei, F.C. (2013). Postoperative alcohol withdrawal syndrome and neuropsychological disorder in patients after head and neck cancer ablation followed by microsurgical free tissue transfer. Journal of Reconstructive Microsurgery, 29, 131–136.
Chang, T.S., Hou, S.J., Su, Y.C., Chen, L.F., Ho, H.C., Lee, M.S., . . . Lee, C.C. (2013). Disparities in oral cancer survival among mentally ill patients. PLOS ONE, 8(8), e70883. https://doi.org/10.1371/journal.pone.0070883
Chhatre, S., Metzger, D.S., Malkowicz, S.B., Woody, G., & Jayadevappa, R. (2014). Substance use disorder and its effects on outcomes in men with advanced-stage prostate cancer. Cancer, 120, 3338–3345. https://doi.org/10.1002/cncr.28861
Damjanovic, A., Ivkovic, M., Jasović-Gasic, M., & Paunović, V. (2006). Comorbidity of schizophrenia and cancer: Clinical recommendations for treatment. Psychiatria Danubina, 18, 55–60.
De Hert, M., Cohen, D., Bobes, J., Cetkovich-Bakmas, M., Leucht, S., Ndetei, D.M., . . . Correll, C.U. (2011). Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry, 10, 138–151.
De Weert-Van Oene, G.H., Termorshuizen, F., Buwalda, V.J.A., & Heerdink, E.R. (2017). Somatic health care utilization by patients treated for substance use disorders. Drug and Alcohol Dependence, 178, 277–284.
Deyo, R.A., Cherkin, D.C., & Ciol, M.A. (1992). Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology, 45, 613–619.
Farasatpour, M., Janardhan, R., Williams, C.D., Margenthaler, J.A., Virgo, K.S., & Johnson, F.E. (2013). Breast cancer in patients with schizophrenia. American Journal of Surgery, 206, 798–804. https://doi.org/10.1016/j.amjsurg.2012.06.013
Farquhar, M. (2008). AHRQ quality indicators. In R.G. Hughes, Patient safety and quality: An evidence-based handbook for nurses. Rockville, MD: Agency for Healthcare Research and Quality.
Fogarty, C.T., Sharma, S., Chetty, V.K., & Culpepper, L. (2008). Mental health conditions are associated with increased health care utilization among urban family medicine patients. Journal of the American Board of Family Medicine, 21, 398–407.
Fried, B.M., & Sherer, J.D. (2016). Value based reimbursement: The rock thrown into the health care pond. Health Affairs. Retrieved from https://www.healthaffairs.org/do/10.1377/hblog20160708.055764/full
Ganzini, L., Socherman, R., Duckart, J., & Shores, M. (2010). End-of-life care for veterans with schizophrenia and cancer. Psychiatric Services, 61, 725–728.
Genther, D.J., & Gourin, C.G. (2012). The effect of alcohol abuse and alcohol withdrawal on short-term outcomes and cost of care after head and neck cancer surgery. Laryngoscope, 122, 1739–1747. https://doi.org/10.1002/lary.23348
Green, M.F., Kern, R.S., & Heaton, R.K. (2004). Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophrenia Research, 72, 41–51.
Heslin, K.C., & Weiss, A.J. (2015). Hospital readmissions involving psychiatric disorders, 2012 [HCUP Statistical Brief #189]. Agency for Healthcare Research and Quality. Retrieved from https://www.hcup-us.ahrq.gov/reports/statbriefs/sb189-Hospital-Readmiss…
Himelhoch, S., Weller, W.E., Wu, A.W., Anderson, G.F., & Cooper, L.A. (2004). Chronic medical illness, depression, and use of acute medical services among Medicare beneficiaries. Medical Care, 42, 512–521.
Irwin, K.E., Henderson, D.C., Knight, H.P., & Pirl, W.F. (2014). Cancer care for individuals with schizophrenia. Cancer, 20, 232–334. https://doi.org/10.1002/cncr.28431
Ishikawa, H., Yasunaga, H., Matsui, H., Fushimi, K., & Kawakami, N. (2016). Differences in cancer stage, treatment and in-hospital mortality between patients with and without schizophrenia: Retrospective matched-pair cohort study. British Journal of Psychiatry , 208, 239–244. https://doi.org/10.1192/bjp.bp.114.156265
Jayadevappa, R., Malkowicz, S.B., Chhatre, S., Johnson, J.C., & Gallo, J.J. (2012). The burden of depression in prostate cancer. Psycho-Oncology, 21, 1338–1345. https://doi.org/10.1002/pon.2032
Kapfhammer, H.P. (2006). Somatic symptoms in depression. Dialogues in Clinical Neuroscience, 8, 227–239.
Karuturi, M., Wong, M.L., Hsu, T., Kimmick, G.G., Lichtman, S.M., Holmes, H.M., . . . Mohile, S. (2016). Understanding cognition in older patients with cancer. Journal of Geriatric Oncology, 7, 258–269. https://doi.org/10.1016/j.jgo.2016.04.004
Krebber, A.M., Buffart, L.M., Kleijn, G., Riepma, I.C., de Bree, R., Leemans, C.R., . . . Verdonck-de Leeuw, I.M. (2014). Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psycho-Oncology, 23, 121–130. https://doi.org/10.1002/pon.3409
Legler, A., Bradley, E.H., & Carlson, M.D. (2011). The effect of comorbidity burden on health care utilization for patients with cancer using hospice. Journal of Palliative Medicine, 14, 751–756.
Leucht, S., Burkard, T., Henderson, J., Maj, M., & Sartorius, N. (2007). Physical illness and schizophrenia: A review of the literature. Acta Psychiatrica Scandinavica, 116, 317–333.
Lie, H.C., Hjermstad, M.J., Fayers, P., Finset, A., Kaasa, S., & Loge, J.H. (2015). Depression in advanced cancer—Assessment challenges and associations with disease load. Journal of Affective Disorders, 173, 176–184. https://doi.org/10.1016/j.jad.2014.11.006
Mahabaleshwarkar, R., Khanna, R., Banahan, B., West-Strum, D., Yang, Y., & Hallam, J.S. (2015). Impact of preexisting mental illnesses on receipt of guideline-consistent breast cancer treatment and health care utilization. Population Health Management, 18, 449–458. https://doi.org/10.1089/pop.2014.0146
Manchikanti, L., Helm, S., Fellows, B., Janata, J.W., Pampati, V., Grider, J.S., & Boswell, M.V. (2012). Opioid epidemic in the United States. Pain Physician, 15(3, Suppl.), ES9–ES38.
Mariotto, A.B., Yabroff, K.R., Shao, Y., Feuer, E.J., & Brown, M.L. (2011). Projections of the cost of cancer care in the United States: 2010–2020. Journal of the National Cancer Institute, 103, 117–128. https://doi.org/10.1093/jnci/djq495
Mayr, M., & Schmid, R.M. (2010). Pancreatic cancer and depression: Myth and truth. BMC Cancer, 10, 569.
Mcdermott, K.W., Elixhauser, A., Sun, R., & Cost, T.H. (2017). Statistical brief # 225: Trends in hospital inpatient stays in the United States, 2005–2014. Retrieved from www.hcup-us.ahrq.gov/faststats/landing.jsp
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D.G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology, 62, 1006–1012. https://doi.org/10.1016/j.jclinepi.2009.06.005
Morin, L., Beaussant, Y., Aubry, R., Fastbom, J., & Johnell, K. (2016). Aggressiveness of end-of-life care for hospitalized individuals with cancer with and without dementia: A nationwide matched-cohort study in France. Journal of the American Geriatrics Society, 64, 1851–1857. https://doi.org/10.1111/jgs.14363
Nakayama, J.M., Ou, J.P., Friedman, C., Smolkin, M.E., & Duska, L.R. (2015). The risk factors of readmission in postoperative gynecologic oncology patients at a single institution. International Journal of Gynecological Cancer , 25, 1697–1703.
National Association of State Mental Health Program Directors Council. (2006). Morbidity and mortality in people with serious mental illness. Retrieved from https://bit.ly/2kjSnsf
National Quality Forum. (2009). Cost and resource use measures. Retrieved from https://bit.ly/2TRFKBD
National Quality Forum. (2010). Measurement framework: Evaluating efficiency across patient-focused episodes of care. Retrieved from http://www.qualityforum.org/Publications/2010/01/Measurement_Framework_…
Naylor, M.D., Aiken, L.H., Kurtzman, E.T., Olds, D.M., & Hirschman, K.B. (2011). The care span: The importance of transitional care in achieving health reform. Health Affairs, 30, 746–754. https://doi.org/10.1377/hlthaff.2011.0041
Paice, J.A. (2018). Cancer pain management and the opioid crisis in America: How to preserve hard-earned gains in improving the quality of cancer pain management. Cancer, 124, 2491–2497.
Papathanasiou, A.A., & Zintzaras, E. (2010). Assessing the quality of reporting of observational studies in cancer. Annals of Epidemiology, 20, 67–73.
Piette, J.D., Barnett, P.G., & Moos, R.H. (1998). First-time admissions with alcohol-related medical problems: A 10-year follow-up of a national sample of alcoholic patients. Journal of Studies on Alcohol, 59, 89–96.
Prieto, J.M., Blanch, J., Atala, J., Carreras, E., Rovira, M., Cirera, E., & Gasto, C. (2002). Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. Journal of Clinical Oncology, 20, 1907–1917. https://doi.org/10.1200/JCO.2002.07.101
Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., & Ferri, C.P. (2013). The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s and Dementia, 9, 63–75. https://doi.org/10.1016/J.JALZ.2012.11.007
Regier, D.A., Kuhl, E.A., & Kupfer, D.J. (2013). The DSM-5: Classification and criteria changes. World Psychiatry, 12, 92–98.
Sambamoorthi, U., Tan, X., & Deb, A. (2015). Multiple chronic conditions and healthcare costs among adults. Expert Review of Pharmacoeconomics and Outcomes Research, 15, 823–832.
Sarfati, D., Koczwara, B., & Jackson, C. (2016). The impact of comorbidity on cancer and its treatment. CA: A Cancer Journal for Clinicians, 66, 337–350.
Secretan, B., Straif, K., Baan, R., Grosse, Y., El Ghissassi, F., Bouvard, V., . . . Cogliano, V. (2009). A review of human carcinogens—Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncology, 10, 1033–1034.
Singer, S., Das-Munshi, J., & Brähler, E. (2010). Prevalence of mental health conditions in cancer patients in acute care—A meta-analysis. Annals of Oncology, 21, 925–930.
Smith, B.D., Smith, G.L., Hurria, A., Hortobagyi, G.N., & Buchholz, T.A. (2009). Future of cancer incidence in the United States: Burdens upon an aging, changing nation. Journal of Clinical Oncology, 27, 2758–2765.
Spies, C.D., Nordmann, A., Brummer, G., Marks, C., Conrad, C., Berger, G., . . . Schaffartzik, W. (1996). Intensive care unit stay is prolonged in chronic alcoholic men following tumor resection of the upper digestive tract. Acta Anaesthesiologica Scandinavica, 40, 649–656.
Substance Abuse and Mental Health Services Administration. (2015). Results from the 2015 national survey on drug use and health: Summary of the effects of the 2015 NSDUH questionnaire redesign: Implications for data users. Retrieved from https://bit.ly/2X0PWtd
Substance Abuse and Mental Health Services Administration. (2017). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Retrieved from https://bit.ly/2X0PWtd
Van Cleave, J.H., Smith-Howell, E., & Naylor, M.D. (2016). Achieving a high-quality cancer care delivery system for older adults: Innovative models of care. Seminars in Oncology Nursing, 32, 122–133. https://doi.org/10.1016/j.soncn.2016.02.006
Walker, E.R., McGee, R.E., & Druss, B.G. (2015). Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA Psychiatry, 72, 334–341. https://doi.org/10.1001/jamapsychiatry.2014.2502
Wancata, J., Benda, N., Windhaber, J., & Nowotny, M. (2001). Does psychiatric comorbidity increase the length of stay in general hospitals? General Hospital Psychiatry, 23, 8–14.
Wieghard, N.E., Hart, K.D., Herzig, D.O., Lu, K.C., & Tsikitis, V.L. (2015). Psychiatric illness is a disparity in the surgical management of rectal cancer. Annals of Surgical Oncology, 22(Suppl. 3), S573–S579. https://doi.org/10.1245/s10434-015-4791-x
Zhang, S., Ivy, J.S., Payton, F.C., & Diehl, K.M. (2010). Modeling the impact of comorbidity on breast cancer patient outcomes. Health Care Management Science, 13, 137–154.
Zhou, T., Duan, J.J., Zhou, G.P., Cai, J.Y., Huang, Z.H., Zeng, Y.T., & Xu, F. (2010). Impact of depression mood disorder on the adverse drug reaction incidence rate of anticancer drugs in cancer patients. Journal of International Medical Research, 38, 2153–2159.

