Anthracycline Chemotherapy–Induced Cardiotoxicity in Breast Cancer Survivors: A Systematic Review
Problem Identification: This review identifies specific cardiotoxicity related to anthracycline chemotherapy, specific risk factors related to increased anthracycline chemotherapy–induced cardiotoxicity, and underlying mechanisms of action of anthracycline chemotherapy–induced cardiotoxicity.
Literature Search: PubMed®, CINAHL®, Embase®, and Web of Science were searched in May 2018 using keywords related to heart diseases, anthracycline chemotherapy, and breast cancer.
Data Evaluation: Data were extracted, and study quality was assessed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Synthesis: 1,117 articles were identified through the literature search. After a review of the abstracts and articles, 15 clinical studies were identified for the final analysis by using exclusion and inclusion criteria.
Implications for Practice: Nurses should recognize the critical elements for prevention and early detection of anthracycline chemotherapy–induced cardiotoxicity.
Jump to a section
Anthracycline chemotherapy is commonly indicated for the treatment of breast cancer. These agents include doxorubicin, epirubicin, daunorubicin, and idarubicin (Smith et al., 2010). They are used singularly or in combination with other chemotherapy agents to increase the overall survival rate of breast cancer survivors (BCSs) (Gianni et al., 2009; Menna, Salvatorelli, Gianni, & Minotti, 2008). Anthracyclines are the most frequently used chemotherapy regimen in breast cancer and may reduce mortality from breast cancer by about 33% (Early Breast Cancer Trialists’ Collaborative Group, 2012). Research shows that the adoption of anthracycline-based chemotherapy regimens not only increases long-term breast cancer survival rates, but also reduces the risk of relapse and death for people with early-stage breast cancer after surgery (Smith et al., 2010). Because all chemotherapy agents may cause some adverse reactions among BCSs, cardiotoxicity is a concern. The cytotoxic effects of anthracycline agents are from the generation of oxygen-derived free radicals, which can cause myocyte apoptosis. The loss of cardiac myocytes results in cardiomyopathy and cardiac failure (Ewer & Lippman, 2005). The use of anthracyclines among BCSs is restricted because of the severe cardiac side effects.
Although anthracycline chemotherapy–induced cardiotoxicity in people with breast cancer has been identified as a major health concern (Brower, 2013; Smith et al., 2010), a need remains for information about what is known and what is not. Studies have shown that anthracycline chemotherapy may cause a decrease in left and right ventricular systolic and diastolic functioning (Abdar Esfahani, Mokarian, & Karimipanah, 2017; Appel, Jensen, Nielsen, Ryberg, & Zerahn, 2010; Boyd et al., 2017; Narayan et al., 2017; Sawaya et al., 2012; Schneeweiss et al., 2018; Serrano et al., 2015), impaired myocardial contraction measured by strain imaging (Cheng et al., 2017; Khouri et al., 2014; Stoodley et al., 2013), impaired left atrial electrical conduction (Yaylali, Saricopur, Yurtdas, Senol, & Gokoz-Dogu, 2016), and cardiac myopathy and cardiac failure (Bowles et al., 2012). The relationships between anthracycline chemotherapy–induced cardiotoxicity and type or stage of breast cancer, patient age, specific drug dosage, previous cardiovascular status, and risk factors prior to chemotherapy still need to be examined.
The major aim of this systematic review was to identify specific cardiac problems after treatment related to use of anthracycline chemotherapy among early- and late-stage BCSs. The review also identified specific risk factors that increase the risk and the underlying mechanism of anthracycline chemotherapy–induced cardiotoxicity, as well as the clinical implications of anthracycline chemotherapy–induced cardiotoxicity for BCSs.
Methods
Search Strategies
The search included studies published from January 2008 to June 2018 using PubMed®, CINAHL®, Embase®, and Web of Science. The authors used specified MeSH (Medical Subject Headings) terms and keywords related to anthracycline chemotherapy–induced cardiotoxicity in patients with breast cancer. Combination terms associated with heart disease or heart diseases, anthracycline or drug therapy or chemotherapy, and breast cancer or breast neoplasm or breast neoplasms were used. All publications were cross-checked to retrieve the articles using the selection criteria. The final search strategy used for PubMed was also used for each electronic health journal database. Additional publications were also identified by reviewing the reference lists from eligible articles.
Inclusion and Exclusion Criteria
Citations from all search results were downloaded and merged by using the reference management software package EndNote X7. This was followed by screening study titles and abstracts for potential inclusion and then reviewing full-text articles to determine the eligibility for inclusion. Reported studies met the following inclusion criteria:
• Clinical trial research focused on anthracycline chemotherapy–induced cardiac issues among BCSs aged 18 years or older
• Original article publications
• Published in peer-reviewed journals
• Published in English
• Published from January 2008 to June 2018
Studies were excluded if they were not clinical trial research, research on chemotherapy drugs used for a cancer other than breast cancer, not focused on anthracycline chemotherapy–induced cardiotoxicity, focused on chemotherapy drugs other than anthracycline among BCSs, or published before January 1, 2008. For the purpose of this review, cardiotoxicity is defined as left ventricular ejection fraction (LVEF) decreased by 10% or greater, acute or chronic cardiac failure, and other cardiac problems caused by anthracycline chemotherapy (Boyd et al., 2017; Finkelman et al., 2017; Grover et al., 2015; Narayan et al., 2017; Sawaya et al., 2012; Serrano et al., 2015).
Study designs included randomized controlled trials (RCTs), cohort studies (prospective observational studies), cross-sectional studies, and retrospective analytical studies. Study participants were restricted to patients with breast cancer who were prescribed, were undergoing, or had completed anthracycline chemotherapy. 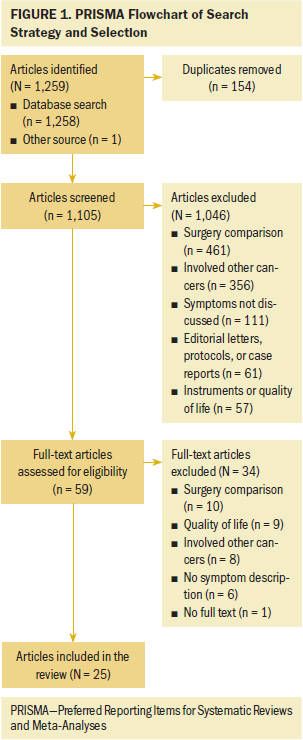
Search Outcome
After applying inclusion and exclusion criteria to the titles and abstracts of the initial search, 27 publications met the criteria. Upon review of each of the 27 articles, 15 fulfilled all inclusion criteria and were included in the systematic review. A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of the selection process is presented in Figure 1. A standard spreadsheet was used to extract data related to authors, setting, design, intervention, sample characteristics, outcome measures, and results (see Table 1). 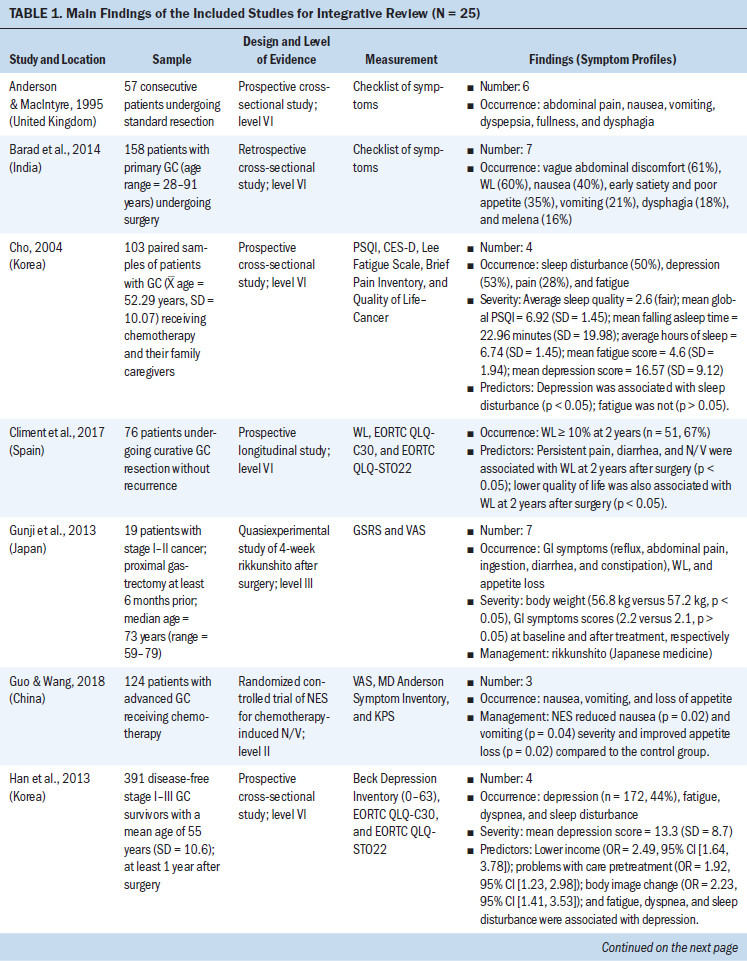
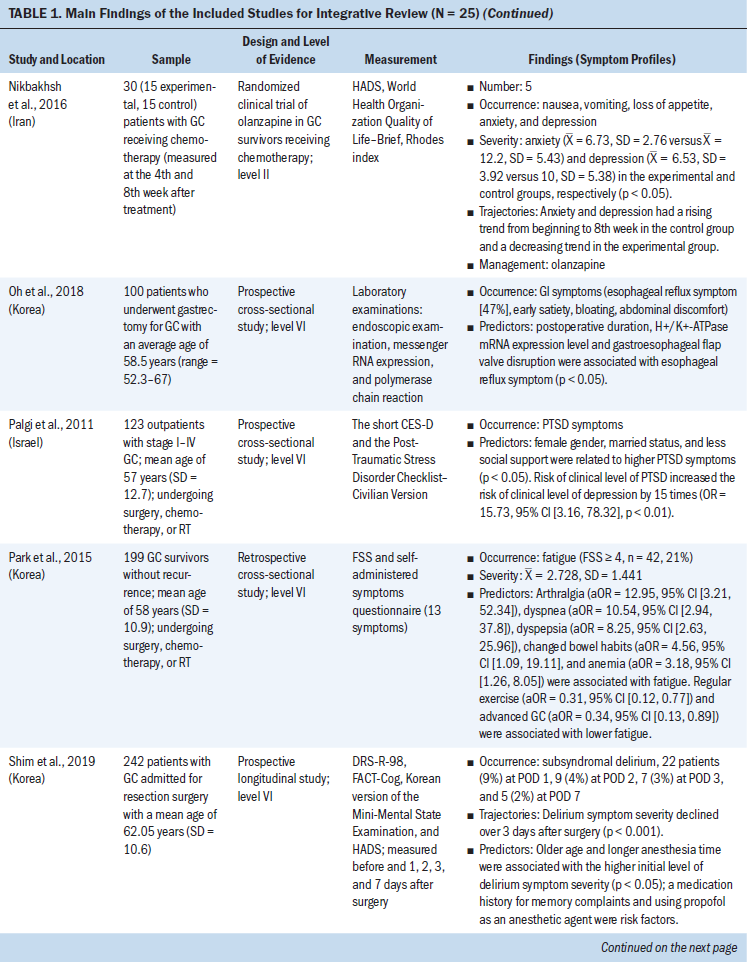
Data Evaluation
Table 2 summarizes the methodologic quality assessment of the 15 articles included in this systematic review. The Quality Assessment Tool for Quantitative Studies developed by the Effective Public Health Practice Project was used as the assessment tool (National Collaborating Centre for Methods and Tools, 2017). This instrument provides a standardized means to assess study quality for review of articles related to public health. It also provides an overall methodologic quality rating of evidence using a scale of strong, moderate, or weak rating in eight areas (i.e., selection bias, study design, confounders, blinding, data collection methods, withdrawals and dropouts, intervention integrity, and analysis) (National Collaborating Centre for Methods and Tools, 2017). Thirteen of the 15 studies received a moderate global quality rating (Abdar Esfahani et al., 2017; Appel et al., 2010; Cheng et al., 2017; Finkelman et al., 2017; Grover et al., 2015; Ho et al., 2010; Khouri et al., 2014; Kotwinski et al., 2016; Narayan et al., 2017; Sawaya et al., 2012; Serrano et al., 2015; Stoodley et al., 2013; Yaylali et al., 2016). Two studies received a strong global rating (Bowles et al., 2012; Boyd et al., 2017). None of the studies received a weak global quality rating. Overall, the quality rating for the 15 reviewed studies was moderate (Zeng et al., 2015). 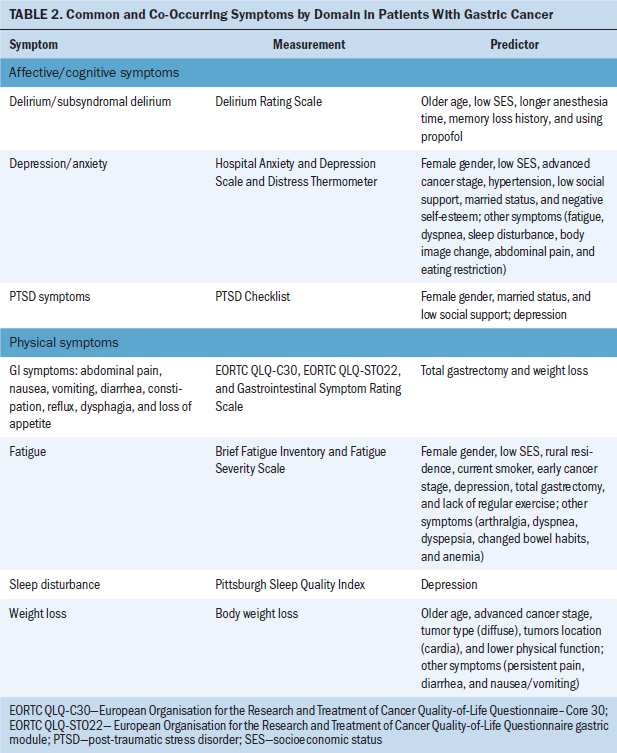
Results
Study Characteristics
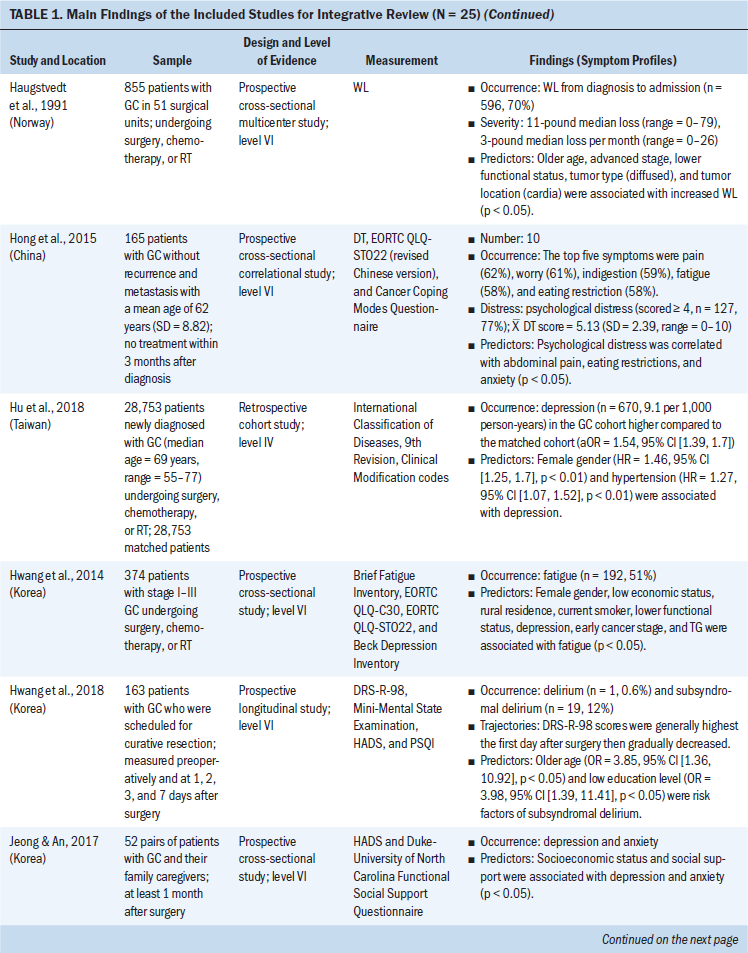
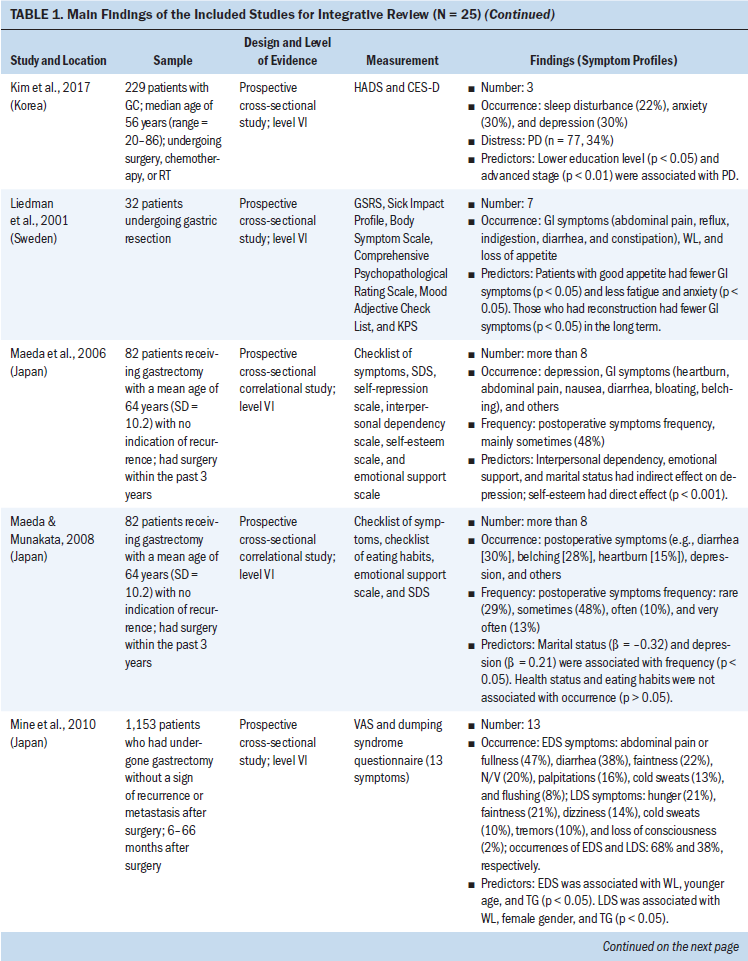
The 15 clinical studies consisted of three retrospective studies, four cross-sectional studies, and eight prospective studies. For the eight prospective studies, six studies assessed echocardiograms to measure left ventricular longitudinal systolic and diastolic myocardial strain, early and subsequent changes in left ventricular ejection, and symptoms of cardiac failure (Boyd et al., 2017; Cheng et al., 2017; Narayan et al., 2017; Sawaya et al., 2012; Serrano et al., 2015; Stoodley et al., 2013). One study used cardiovascular magnetic resonance to measure the changes of LVEF before and after anthracycline-based chemotherapy (Kotwinski et al., 2016). One study used a blood sample to measure early changes in arginine-nitric oxide metabolite levels and the association with cancer therapeutics–related cardiac dysfunction (Finkelman et al., 2017).
Participant Characteristics
The number of participants ranged from 27 to 12,500 BCSs. The mean age of participants was 60 years (range = 18–99). The total population was 13,781 BCSs, with the majority having advanced-stage breast cancer. All were prescribed anthracycline or anthracycline plus other chemotherapy. Studies were made up of BCSs who completed their anthracycline chemotherapy within seven days to eight years after anthracycline treatment. Most of the BCSs (n = 12,956) were from the United States; the remaining participants were from Denmark, Iran, Australia, Canada, Ireland, the United Kingdom, Spain, China, and Turkey. BCSs with preexisting cardiovascular diseases were excluded from the clinical studies (Bowles et al., 2012; Serrano et al., 2015; Yaylali et al., 2016).
Post-Treatment Cardiac Issues After Anthracycline Chemotherapy
This review identified specific post-treatment cardiac issues related to anthracycline chemotherapy among BCSs. The type of acute and chronic cardiotoxicity caused by anthracycline chemotherapy (ranging from months to years after treatment) in patients with breast cancer included the following:
• Risk of developing chronic or acute cardiac failure and decreased LVEF (Appel et al., 2010; Boyd et al., 2017; Kotwinski et al., 2016; Narayan et al., 2017; Sawaya et al., 2012; Stoodley et al., 2013)
• Decreased myocardial function and impaired global longitudinal strain, a parameter assessed by 2D speckle-tracking echocardiography (2DSTE) to evaluate left ventricular function (Bowles et al., 2012; Khouri et al., 2014)
• Moderately elevated baseline blood pressure (Kotwinski et al., 2016)
• Atrial electromechanical delay and impaired atrial electronic conduction and aortic function (Grover et al., 2015; Yaylali et al., 2016)
• Risk of decreased right ventricular systolic and diastolic function (Abdar Esfahani et al., 2017; Serrano et al., 2015)
• Increase in the duration of contraction mainly by increasing the isovolumic contraction time (Cheng et al., 2017)
• Early alterations in arginine-nitric oxide metabolite levels (Finkelman et al., 2017).
When monitoring anthracycline chemotherapy–induced cardiotoxicity, evaluation was typically focused on measuring the left ventricular systolic function by using 2DSTE (Stoodley et al., 2013). The most common cardiotoxicity after anthracycline treatment is left ventricular systolic dysfunction and possible arrhythmias (Yaylali et al., 2016). Chronic and acute cardiac failure is the major concern of anthracycline chemotherapy–induced cardiotoxicity, and the risk of cardiac failure is high if patient’s LVEF is less than 50% (Appel et al., 2007).
Significant Risk Factors for Cardiotoxicity
Results of this review identified significant risk factors related to anthracycline chemotherapy–induced cardiotoxicity. Cumulative anthracycline dose, prior trastuzumab therapy, elevated blood pressure, increased height, high body mass index (BMI), and high body surface area (BSA) are all associated with increased chronic progressive anthracycline cardiotoxicity (Guenancia et al., 2016; Kotwinski et al., 2016). The risk of cardiac failure and/or cardiomyopathy was significantly increased among BCSs treated with trastuzumab alone or anthracycline plus trastuzumab as compared to BCSs treated with anthracycline alone (Bowles et al., 2012; Finkelman et al., 2017; Guglin, Cutro, & Mishkin, 2008). A five-year cumulative incidence rate for cardiac failure and/or cardiomyopathy was associated with anthracycline use in late-stage breast cancer and with older age (Bowles et al., 2012). Risk factors for anthracycline chemotherapy–induced diastolic dysfunction include blood pressure, BMI, and age (Yaylali et al., 2016). Jordan et al. (2016) found that risk factors for anthracycline chemotherapy–induced cardiovascular events and myocardial fibrosis included increased body weight, high heart rate, and high systolic blood pressure, as well as history of coronary artery disease, diabetes mellitus, hyperlipidemia, or hypertension. In addition, myocardial fibrosis increased with age and was higher among women (Jordan et al., 2016).
Underlying Mechanisms of Cardiotoxicity
Results of this review identified the proposed underlying mechanisms of anthracycline chemotherapy–induced cardiotoxicity. Although the exact mechanism for anthracycline chemotherapy–induced myocardial dysfunction is unclear, there are several hypotheses. Anthracycline chemotherapy–induced cardiotoxicity is identified as a type I cardiotoxicity and reported to be irreversible because of myocardial damage (Kotwinski et al., 2016). Because of the multiple toxic effects of anthracycline, mitochondrial dysfunction (DNA damage and impaired bioenergetics), possible cellular senescence (shortening of telomere length and loss of cardiac progenitor cells) may play a role in cardiac dysfunction and cardiac failure (Kotwinski et al., 2016). Intracellular oxidative stress resulting in mitochondrial dysfunction, cell apoptosis, and myocyte necrosis is thought to be associated with myocardial damage (Stoodley et al., 2013). The majority of research on anthracycline chemotherapy–induced cardiotoxicity focused on myocellular injury. Jordan et al. (2016) found that anthracycline treatment can cause chronic and persistent elevated extracellular volume, which is associated with subclinical development of diffuse myocardial fibrosis with the fibrotic tissue lowering left ventricular myocellular mass. The elevated extracellular volume reflects the interstitial process that may influence changes in myocellular composition (intramyocellular edema or deposition of protein, lipids, or iron); it also may change myocardial oxygenation or perfusion. Increased subclinical interstitial myocardial fibrosis coincides with decreased LVEF and myocardial mass among BCSs treated with anthracycline chemotherapy (Jordan et al., 2016). To assess the atrial mechanical alteration and prolonged left intra- and interatrial electromechanical features by anthracycline therapy, Yaylali et al. (2016) suggested that the change of cardiac function after anthracycline treatment is the result of the ventricular proarrhythmogenic mechanism. The left ventricular diastolic dysfunction and delayed electrical conduction creates an arrhythmogenic substrate, which leads to a decrease in intracardiac conduction and a heterogeneous dispersion of repolarization. Cardiac arrhythmias are caused by these two effects. Prolonged electromechanical intervals in the left intra- and interatrial are because of anthracycline chemotherapy–induced oxidative stress, which may cause autonomic dysfunction (Yaylali et al., 2016).
Discussion
Among all chemotherapy drugs, anthracyclines are the most common and effective antineoplastic agents (Patel, Balakrishnan, Kumar, Naswa, & Malhotra, 2010). Anthracyclines are recommended in the majority of triple-negative, HER2-positive and high-risk luminal HER2-negative tumors (Early Breast Cancer Trialists’ Collaborative Group, 2012). Anthracyclines are not routinely recommended for use concomitant with endocrine therapy for estrogen receptor–positive breast cancer (Albain et al., 2009). The benefit of anthracyclines is undermined by potential cardiotoxicities that could be life-threatening (Stoodley et al., 2013). Therefore, healthcare providers should be vigilant to diagnose anthracycline chemotherapy–induced cardiotoxicity early so that actions or interventions can be implemented to prevent irreversible damage to the patient’s heart (Abdar Esfahani et al., 2017). If anthracyclines remain as one of the cornerstones of chemotherapy for breast cancer, a need exists for cardio-oncology expertise to balance the use of anthracycline chemotherapy with the risk for cardiotoxicity (Groarke & Nohria, 2015).
Traditionally, hypertension, diabetes mellitus, hyperlipidemia, family history of premature coronary artery disease, and history of smoking are included as risk factors for cardiovascular disease (Negishi et al., 2013). Research also indicates that age is an established risk factor for anthracycline chemotherapy–induced cardiotoxicity among BCSs (Jordan et al., 2016). Evidence shows that a BMI of more than 27 kg/m2 was significantly associated with left ventricular dysfunction after anthracycline therapy in BCSs (Fumoleau et al., 2006). Body weight of more than 70 kg is identified as another risk factor of anthracycline chemotherapy–induced cardiotoxicity in patients with breast cancer (Dranitsaris et al., 2008). Among anthracycline agents, doxorubicin and epirubicin are most frequently used for BCSs (Khasraw, Bell, & Dang, 2012). Doxorubicin-containing chemotherapy causes dose-dependent myocardial injury for people with early-stage breast cancer (McGowan et al., 2017).
The mechanisms of anthracycline chemotherapy–induced cardiotoxicity in breast cancer are complex (Mele et al., 2016). According to a study by Cardinale et al. (2015), anthracycline chemotherapy–induced cardiotoxicity represents a continuum that begins with subclinical myocardial cell injury, followed by an early asymptomatic LVEF reduction, and then progresses to symptomatic cardiac failure if untreated. Oxidative stress has been identified as a central mechanism in anthracycline-related myocardial cell injury (Xu, Tang, Qian, & Ashraf, 2001). Because the heart is susceptible to oxidative stress, anthracycline therapy can cause toxic free radicals that increase oxidative stress and lead to cardiovascular injury (Grover et al., 2015). Nitric oxide synthase–dependent endothelial dysfunction is identified as the primary mechanism for doxorubicin-induced cardiotoxicity (Deng et al., 2009). The arginine-nitric oxide metabolism pathway in anthracycline chemotherapy–induced cardiotoxicity causes endothelial dysfunction and edema secondary to increased oxidative stress in the vascular wall (Wolf & Baynes, 2006). Doxorubicin is reported to suppress endothelin production, which makes the myocytes susceptible to death. Structural changes of the vascular matrix can be caused by interference of the vascular tone regulated by endothelin, increasing aortic stiffness and the risk of cardiovascular disease (Keltai et al., 2010).
Implications for Practice and Research
No generally accepted method exists to provide protection for cardiac damage induced by anthracyclines, often leading to symptomatic cardiac failure and death during or after chemotherapy treatment. Clinical guidelines are needed for better surveillance and management of significant complications of anthracycline chemotherapy–induced cardiotoxicity among BCSs (Groarke & Nohria, 2015).
Research shows that the timing of detection rather than the timing of occurrence is reflected in late-onset anthracycline chemotherapy–induced cardiotoxicity in patients with cancer (Groarke & Nohria, 2015). Once LVEF reduction is detected or clinical symptoms are noted in the patient, anthracycline chemotherapy–induced cardiotoxicity is not reversible, and the prognosis is very poor (Felker et al., 2000; Jensen, Skovsgaard, & Nielsen, 2002). Doppler-based myocardial deformation imaging, such as 2DSTE, should be used to monitor patients’ cardiac function during anthracycline chemotherapy and in long-term follow-up to prevent potential cardiac failure (Jurcut et al., 2008; Stoodley et al., 2013). Because early changes in global longitudinal strain are associated with subsequent declination in LVEF, this finding supports using global longitudinal strain detected by 2DSTE as an early indicator for anthracycline chemotherapy–induced cardiotoxicity in clinical practice (Narayan et al., 2017). Cardinale et al. (2015) found that anthracycline chemotherapy–induced cardiotoxicity in patients with cancer occurs almost exclusively within the first year after treatment; therefore, cardiac surveillance during this high-risk period is critical (Groarke & Nohria, 2015). Early initiation of cardioprotective agents in patients with breast cancer following anthracycline chemotherapy has been found to be beneficial (Boyd et al., 2017). Dexrazoxane can reduce the incidence of contractile dysfunction in patients with breast cancer treated with anthracycline (Sun, Shi, & Geng, 2016). The American Society of Clinical Oncology Chemotherapy and Radiotherapy Expert Panel offers a guideline to support the use of dexrazoxane in patients with breast cancer who received high-dose doxorubicin (Sawyer, Peng, Chen, Pentassuglia, & Lim, 2010). The American Heart Association/American College of Cardiology guidelines recommend the use of cardioprotective drugs, such as angiotensin-converting enzyme (ACE) inhibitors or beta blockers, in asymptomatic patients with abnormal LVEF after chemotherapy (Sawaya et al., 2012). Initiation of cardioprotective drugs after the early detection of anthracycline chemotherapy–induced cardiotoxicity has been found to be associated with an 82% recovery rate in a mean period of eight months (SD = 5) (Cardinale et al., 2015). The concept of type I cardiotoxicity was challenged by these data, indicating a significant potential for reversibility of anthracycline chemotherapy–induced cardiotoxicity if early detection and treatment occurs (Groarke & Nohria, 2015).
Assessment of resting LVEF for patients before administration of anthracyclines as a standard of care for people with breast cancer is recommended by the European Society of Cardiology and the 2013 American College of Cardiology Foundation/American Heart Association guidelines for management of heart failure (Eschenhagen et al., 2011; Yancy et al., 2013). Late development of chronic cardiac failure is reported to be related to epirubicin dose and patient entry-level blood pressure and inversely related to post-epirubicin LVEF. Therefore, maximum cumulated doses of doxorubicin (550 mg/m2) and epirubicin (900 mg/m2) are recommended for use (Appel et al., 2010; Ryberg et al., 1998). Recognition of hypertension and initiation of hypertension therapy before patients start anthracycline therapy is suggested. BSA indexing of anthracycline dose may be causal because of the association between increasing BSA and subclinical chronic progressive anthracycline chemotherapy–induced cardiotoxicity (Kotwinski et al., 2016). The 2012 American Society of Clinical Oncology practice guideline recommends adjusting anthracycline dosing based on patient’s BSA (Griggs, Mangu, Temin, & Lyman, 2012). Because of post-treatment cardiotoxicity, non–anthracycline-based regimens may be used as an alternative treatment for patients with breast cancer who are at risk of cardiac complications (Jones et al., 2009). Trastuzumab is not routinely recommended for concomitant administration with anthracyclines because of its cardiotoxicity (Perez et al., 2014). Anthracyclines and taxanes are recommended to be sequentially administered rather than used concomitantly (Shao et al., 2012).
Evidence from this review identifies the importance for nurses to recognize the critical elements for prevention and early detection of anthracycline chemotherapy–induced cardiotoxicity. Early detection and assessment may affect long-term patient outcomes related to cardiac symptoms. BCSs who are at high risk, particularly those receiving anthracycline and trastuzumab, will need close monitoring through a cardio-oncology program during and after treatment. In addition, careful cardiology management and use of preventive medications may be vital for prevention of adverse outcomes. Larger, long-term, multicenter prospective trials are needed to validate the relationships of specific risk factors and determine if they may be causal in nature. 
Conclusion
Anthracycline chemotherapy may cause acute and chronic cardiotoxicity among BCSs. The major concerns of anthracycline chemotherapy–induced cardiotoxicity include myocardial dysfunction and cardiac failure. Risk factors associated with anthracycline chemotherapy–induced cardiotoxicity include age, gender, obesity, BSA, cumulative anthracycline dose, and preexisting patient cardiovascular conditions. Monitoring anthracycline chemotherapy–induced cardiotoxicity and early identification of risk factors are essential for treatment management. Developing and testing new programs to enhance the quality of life of BCSs is critical.
About the Author(s)
Katherine Jinghua Lin, RN, MSN, is an assistant professor at Pasco-Hernando State College in Wesley Chapel Florida, and Cecile A. Lengacher, RN, PhD, FAAN, FAPOS, is a professor in the College of Nursing at the University of South Florida in Tampa. No financial relationships to disclose. Both authors contributed to the conceptualization and design, provided the analysis, and contributed to the manuscript preparation. Lin completed the data collection and provided statistical support. Lin can be reached at jlin2@health.usf.edu, with copy to ONFEditor@ons.org. (Submitted September 2018. Accepted February 12, 2019.)
References
Abdar Esfahani, M., Mokarian, F., & Karimipanah, M. (2017). Alterations in the echocardiographic variables of the right ventricle in asymptomatic patients with breast cancer during anthracycline chemotherapy. Postgraduate Medical Journal, 93, 271–274. https://doi.org/10.1136/postgradmedj-2016-134286
Albain, K.S., Barlow, W.E., Ravdin, P.M., Farrar, W.B., Burton, G.V., Ketchel, S.J., . . . Osborne, C.K. (2009). Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: A phase 3, open-label, randomised controlled trial. Lancet, 374, 2055–2063. https://doi.org/10.1016/S0140-6736(09)61523-3
Appel, J.M., Jensen, B.V., Nielsen, D.L., Ryberg, M., & Zerahn, B. (2010). Systolic versus diastolic cardiac function variables during epirubicin treatment for breast cancer. International Journal of Cardiovascular Imaging, 26, 217–223. https://doi.org/10.1007/s10554-009-9518-2
Appel, J.M., Nielsen, D., Zerahn, B., Jensen, B.V., & Skagen, K. (2007). Anthracycline-induced chronic cardiotoxicity and heart failure. Acta Oncologica, 46, 576–580. https://doi.org/10.1080/02841860601156165
Bowles, E.J., Wellman, R., Feigelson, H.S., Onitilo, A.A., Freedman, A.N., Delate, T., . . . Wagner, E.H. (2012). Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. Journal of the National Cancer Institute, 104, 1293–1305. https://doi.org/10.1093/jnci/djs317
Boyd, A., Stoodley, P., Richards, D., Hui, R., Harnett, P., Vo, K., . . . Thomas, L. (2017). Anthracyclines induce early changes in left ventricular systolic and diastolic function: A single centre study. PLOS ONE, 12, e0175544. https://doi.org/10.1371/journal.pone.0175544
Brower, V. (2013). Cardiotoxicity debated for anthracyclines and trastuzumab in breast cancer. Journal of the National Cancer Institute, 105, 835–836. https://doi.org/10.1093/jnci/djt161
Cardinale, D., Colombo, A., Bacchiani, G., Tedeschi, I., Meroni, C.A., Veglia, F., . . . Cipolla, C.M. (2015). Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation, 131, 1981–1988. https://doi.org/10.1161/CIRCULATIONAHA.114.013777
Cheng, K.H., Handschumacher, M.D., Assuncao, B.M.B.L., Sebag, I.A., Halpern, E.F., & Scherrer-Crosbie, M. (2017). Contraction timing patterns in patients treated for breast cancer before and after anthracyclines therapy. Journal of the American Society of Echicardiography, 30, 454–460. https://doi.org/10.1016/j.echo.2016.12.013
Deng, S., Kruger, A., Schmidt, A., Metzger, A., Yan, T., Gödtel-Armbrust, U., . . . Wojnowski, L. (2009). Differential roles of nitric oxide synthase isozymes in cardiotoxicity and mortality following chronic doxorubicin treatment in mice. Naunyn-Schmiedeberg’s Archives of Pharmacology, 380, 25–34. https://doi.org/10.1007/s00210-009-0407-y
Dranitsaris, G., Rayson, D., Vincent, M., Chang, J., Gelmon, K., Sandor, D., & Reardon, G. (2008). The development of a predictive model to estimate cardiotoxic risk for patients with metastatic breast cancer receiving anthracyclines. Breast Cancer Research and Treatment, 107, 443–450. https://doi.org/10.1007/s10549-007-9803-5
Early Breast Cancer Trialists’ Collaborative Group. (2012). Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet, 379, 432–444. https://doi.org/10.1016/S0140-6736(11)61625-5
Eschenhagen, T., Force, T., Ewer, M.S., de Keulenaer, G.W., Suter, T.M., Anker, S.D., . . . Shah, A.M. (2011). Cardiovascular side effects of cancer therapies: A position statement from the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure, 13, 1–10. https://doi.org/10.1093/eurjhf/hfq213
Ewer, M.S., & Lippman, S.M. (2005). Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. Journal of Clinical Oncology, 23, 2900–2902. https://doi.org/10.1200/JCO.2005.05.827
Felker, G.M., Thompson, R.E., Hare, J.M., Hruban, R.H., Clemetson, D.E., Howard, D.L., . . . Kasper, E.K. (2000). Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. New England Journal of Medicine, 342, 1077–1084. https://doi.org/10.1056/NEJM200004133421502
Finkelman, B.S., Putt, M., Wang, T., Wang, L., Narayan, H., Domchek, S., . . . Ky, B. (2017). Arginine-nitric oxide metabolites and cardiac dysfunction in patients with breast cancer. Journal of the American College of Cardiology, 70, 152–162. https://doi.org/10.1016/j.jacc.2017.05.019
Fumoleau, P., Roché, H., Kerbrat, P., Bonneterre, J., Romestaing, P., Fargeot, P., . . . Luporsi, E. (2006). Long-term cardiac toxicity after adjuvant epirubicin-based chemotherapy in early breast cancer: French Adjuvant Study Group results. Annals of Oncology, 17, 85–92. https://doi.org/10.1093/annonc/mdj034
Gianni, L., Norton, L., Wolmark, N., Suter, T.M., Bonadonna, G., & Hortobagyi, G.N. (2009). Role of anthracyclines in the treatment of early breast cancer. Journal of Clinical Oncology, 27, 4798–4808. https://doi.org/10.1200/JCO.2008.21.4791
Griggs, J.J., Mangu, P.B., Temin, S., & Lyman, G.H. (2012). Appropriate chemotherapy dosing for obese adult patients with cancer: American society of clinical oncology clinical practice guideline. Journal of Oncology Practice, 8(4), e59–e61. https://doi.org/10.1200/JOP.2012.000623
Groarke, J.D., & Nohria, A. (2015). Anthracycline cardiotoxicity: A new paradigm for an old classic. Circulation, 131, 1946–1949. https://doi.org/10.1161/CIRCULATIONAHA.115.016704
Grover, S., Lou, P.W., Bradbrook, C., Cheong, K., Kotasek, D., Leong, D.P., . . . Selvanayagam, J.B. (2015). Early and late changes in markers of aortic stiffness with breast cancer therapy. Internal Medicine Journal, 45, 140–147. https://doi.org/10.1111/imj.12645
Guenancia, C., Lefebvre, A., Cardinale, D., Yu, A.F., Ladoire, S., Ghiringhelli, F., . . . Vergely, C. (2016). Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: A systematic review and meta-analysis. Journal of Clinical Oncology, 34, 3157–3165. https://doi.org/10.1200/JCO.2016.67.4846
Guglin, M., Cutro, R., & Mishkin, J.D. (2008). Trastuzumab-induced cardiomyopathy. Journal of Cardiac Failure, 14, 437–444. https://doi.org/10.1016/j.cardfail.2008.02.002
Ho, E., Brown, A., Barrett, P., Morgan, R.B., King, G., Kennedy, M.J., & Murphy, R.T. (2010). Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: A speckle tracking echocardiographic study. Heart, 9, 701–707. https://doi.org/10.1136/hrt.2009.173997
Jensen, B.V., Skovsgaard, T., & Nielsen, S.L. (2002). Functional monitoring of anthracycline cardiotoxicity: A prospective, blinded, long-term observational study of outcome in 120 patients. Annals of Oncology, 13, 699–709.
Jones, S., Holmes, F.A., O’Shaughnessy, J., Blum, J.L., Vukelja, S.J., McIntyre, K.J., . . . Savin, M.A. (2009). Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. Journal of Clinical Oncology, 27, 1177–1183. https://doi.org/10.1200/JCO.2008.18.4028
Jordan, J.H., Vasu, S., Morgan, T.M., D’Agostino, R.B., Jr., Meléndez, G.C., Hamilton, C.A., . . . Hundley, W.G. (2016). Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circulation: Cardiovascular Imaging, 9(8), 1–9. https://doi.org/10.1161/CIRCIMAGING.115.004325
Jurcut, R., Wildiers, H., Ganame, J., D’hooge, J., De Backer, J., Denys, H., . . . Voigt, J.U. (2008). Strain rate imaging detects early cardiac effects of pegylated liposomal Doxorubicin as adjuvant therapy in elderly patients with breast cancer. Journal of the American Society of Echocardiograpy, 21, 1283–1289. https://doi.org/10.1016/j.echo.2008.10.005
Keltai, K., Cervenak, L., Makó, V., Doleschall, Z., Zsáry, A., & Karádi, I. (2010). Doxorubicin selectively suppresses mRNA expression and production of endothelin-1 in endothelial cells. Vascular Pharmacology, 53, 209–214. https://doi.org/10.1016/j.vph.2010.08.001
Khasraw, M., Bell, R., & Dang, C. (2012). Epirubicin: Is it like doxorubicin in breast cancer? A clinical review. Breast, 21, 142–149. https://doi.org/10.1016/j.breast.2011.12.012
Khouri, M.G., Hornsby, W.E., Risum, N., Velazquez, E.J., Thomas, S., Lane, A., . . . Jones, L.W. (2014). Utility of 3-dimensional echocardiography, global longitudinal strain, and exercise stress echocardiography to detect cardiac dysfunction in breast cancer patients treated with doxorubicin-containing adjuvant therapy. Breast Cancer Research and Treatment, 143, 531–539. https://doi.org/10.1007/s10549-013-2818-1
Kotwinski, P., Smith, G., Cooper, J., Sanders, J., Ma, L., Teis, A., . . . Montgomery, H. (2016). Body surface area and baseline blood pressure predict subclinical anthracycline cardiotoxicity in women treated for early breast cancer. PLOS ONE, 11, e0165262. https://doi.org/10.1371/journal.pone.0165262
McGowan, J.V., Chung, R., Maulik, A., Piotrowska, I., Walker, J.M., & Yellon, D.M. (2017). Anthracycline chemotherapy and cardiotoxicity. Cardiovascular Drugs and Therapy, 31, 63–75. https://doi.org/10.1007/s10557-016-6711-0
Mele, D., Nardozza, M., Spallarossa, P., Frassoldati, A., Tocchetti, C.G., Cadeddu, C., . . . Mercuro, G. (2016). Current views on anthracycline cardiotoxicity. Heart Failure Reviews, 21, 621–634. https://doi.org/10.1007/s10741-016-9564-5
Menna, P., Salvatorelli, E., Gianni, L., & Minotti, G. (2008). Anthracycline cardiotoxicity. Topics in Current Chemistry, 283, 21–44. https://doi.org/10.1007/128_2007_11
Narayan, H.K., Finkelman, B., French, B., Plappert, T., Hyman, D., Smith, A.M., . . . Ky, B. (2017). Detailed echocardiographic phenotyping in breast cancer patients: Associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation, 135, 1397–1412. https://doi.org/10.1161/CIRCULATIONAHA.116.023463
National Collaborating Centre for Methods and Tools. (2017). Quality assessment tool for quantitative sutdies. Retrieved from http://www.nccmt.ca/resources/search/14
Negishi, K., Negishi, T., Hare, J.L., Haluska, B.A., Plana, J.C., & Marwick, T.H. (2013). Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. Journal of the American Society of Echocardiogry, 26, 493–498. https://doi.org/10.1016/j.echo.2013.02.008
Patel, C.D., Balakrishnan, V.B., Kumar, L., Naswa, N., & Malhotra, A. (2010). Does left ventricular diastolic function deteriorate earlier than left ventricular systolic function in anthracycline cardiotoxicity? Hellenic Journal of Nuclear Medicine, 13, 233–237.
Perez, E.A., Romond, E.H., Suman, V.J., Jeong, J.H., Sledge, G., Geyer, C.E., Jr., . . . Wolmark, N. (2014). Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. Journal of Clinical Oncology, 32, 3744–3752. https://doi.org/10.1200/JCO.2014.55.5730
Ryberg, M., Nielsen, D., Skovsgaard, T., Hansen, J., Jensen, B.V., & Dombernowsky, P. (1998). Epirubicin cardiotoxicity: An analysis of 469 patients with metastatic breast cancer. Journal of Clinical Oncology, 16, 3502–3508. https://doi.org/10.1200/JCO.1998.16.11.3502
Sawaya, H., Sebag, I.A., Plana, J.C., Januzzi, J.L., Ky, B., Tan, T.C., . . . Scherrer-Crosbie, M. (2012). Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circulation: Cardiovascular Imaging, 5, 596–603. https://doi.org/10.1161/CIRCIMAGING.112.973321
Sawyer, D.B., Peng, X., Chen, B., Pentassuglia, L., & Lim, C.C. (2010). Mechanisms of anthracycline cardiac injury: Can we identify strategies for cardioprotection? Progress in Cardiovascular Disease, 53, 105–113. https://doi.org/10.1016/j.pcad.2010.06.007
Schneeweiss, A., Chia, S., Hickish, T., Harvey, V., Eniu, A., Waldron-Lynch, M., . . . Cortés, J. (2018). Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. European Journal of Cancer, 89, 27–35. https://doi.org/10.1016/j.ejca.2017.10.021
Serrano, J.M., González, I., Del Castillo, S., Muñiz, J., Morales, L.J., Moreno, F., . . . Alonso, J.J. (2015). Diastolic dysfunction following anthracycline-based chemotherapy in breast cancer patients: Incidence and predictors. Oncologist, 20, 864–872. https://doi.org/10.1634/theoncologist.2014-0500
Shao, N., Wang, S., Yao, C., Xu, X., Zhang, Y., Zhang, Y., & Lin, Y. (2012). Sequential versus concurrent anthracyclines and taxanes as adjuvant chemotherapy of early breast cancer: A meta-analysis of phase III randomized control trials. Breast, 21, 389–393. https://doi.org/10.1016/j.breast.2012.03.011
Smith, L.A., Cornelius, V.R., Plummer, C.J., Levitt, G., Verrill, M., Canney, P., & Jones, A. (2010). Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer, 10, 337. https://doi.org/10.1186/1471-2407-10-337
Stoodley, P.W., Richards, D.A., Boyd, A., Hui, R., Harnett, P.R., Meikle, S.R., . . . Thomas, L. (2013). Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. European Heart Journal: Cardiovascular Imaging, 14, 228–234. https://doi.org/10.1093/ehjci/jes139
Sun, F., Shi, J., & Geng, C. (2016). Dexrazoxane improves cardiac autonomic function in epirubicin-treated breast cancer patients with type 2 diabetes. Medicine, 95, e5228. https://doi.org/10.1097/MD.0000000000005228
Wolf, M.B., & Baynes, J.W. (2006). The anti-cancer drug, doxorubicin, causes oxidant stress-induced endothelial dysfunction. Biochimica et Biophysica Acta, 1760, 267–271. https://doi.org/10.1016/j.bbagen.2005.10.012
Xu, M.F., Tang, P.L., Qian, Z.M., & Ashraf, M. (2001). Effects by doxorubicin on the myocardium are mediated by oxygen free radicals. Life Sciences, 68, 889–901.
Yancy, C.W., Jessup, M., Bozkurt, B., Butler, J., Casey, D.E., Jr., Drazner, M.H., . . . Wilkoff, B.L. (2013). 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation, 128, 1810–1852. https://doi.org/10.1161/CIR.0b013e31829e8807
Yaylali, Y.T., Saricopur, A., Yurtdas, M., Senol, H., & Gokoz-Dogu, G. (2016). Atrial function in patients with breast cancer after treatment with anthracyclines. Arquivos Brasileiros de Cardiologica, 107, 411–419. https://doi.org/10.5935/abc.20160146
Zeng, X., Zhang, Y., Kwong, J.S., Zhang, C., Li, S., Sun, F., . . . Du, L. (2015). The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. Journal of Evidence-Based Medicine, 8, 2–10. https://doi.org/10.1111/jebm.12141




