Development and Psychometric Properties of the Self-Blame Attributions for Cancer Scale
Objectives: To adapt the Cardiac Self-Blame Attributions Scale into the Self-Blame Attributions for Cancer Scale (SBAC) for use in patients with cancer and analyze its psychometric properties.
Sample & Setting: 113 patients receiving radiation therapy at the University of Kansas Cancer Center.
Methods & Variables: The SBAC and other self-report measures were administered during outpatient oncology appointments for radiation therapy to establish the psychometric properties of the SBAC.
Results: A two-factor structure represented behavioral and characterological self-blame attributions. Reliability estimates for each factor were excellent and evidence of convergent and discriminant validity was found, indicating support for the SBAC as a valid and reliable measure of self-blame attributions in patients with cancer.
Implications for Nursing: The SBAC may help healthcare providers, including nursing staff, to identify the self-blame patterns exhibited by patients with cancer. Future research can assess the reliability and validity of SBAC across stages of treatment and establish the predictive validity of the scale in individuals with cancer.
Jump to a section
Threatening life events, such as receiving a cancer diagnosis, may trigger the need to identify an explanation for the threatening event or create causal attributions (Bennett, 2018). Creating attributions is a fundamental cognitive task, with wide-reaching implications for interpersonal functioning, psychological adjustment, and mental and physical health (Fiske & Taylor, 2017). Because of the aversive and unpredictable nature of the disease, patients with cancer may search for a cause or reason for their diagnosis, such as behavioral risk factors that are associated with the development of cancer (e.g., alcohol consumption, smoking) (Maasland, van den Brandt, Kremer, Goldbohm, & Schouten, 2014). A study by Taylor (1983), which analyzed the adjustment processes of 78 women with breast cancer, found that more than 95% of participants reported a causal attribution for their diagnosis. Therefore, the development of attributions is an important cognitive process that may allow patients with cancer to effectively adapt to their diagnoses (Taylor, 1983).
Abramson, Seligman, and Teasdale (1978) made distinctions between the various types of attributions developed by individuals. A theory proposed by Janoff-Bulman (1979) highlights the cognitive process for creating a self-blame attribution or attributing the occurrence of a stress-inducing event to oneself. Self-blame attributions are deconstructed into two types: behavioral self-blame (BSB) and characterological self-blame (CSB). BSB, an internal attribution, is defined as the tendency to blame one’s own behaviors for a threatening event. BSB is believed to be associated with better adjustment because of the malleability of behavior; although support and resources may be needed, individuals can theoretically change their own behaviors with relative ease. CSB is also considered an internal attribution, but because the blame is attributed to one’s own character or personality—which cannot be modified easily—it is considered maladaptive for adjustment. Although both attributions reflect an internal locus, their perceived malleability varies.
Previous studies of self-blame attributions in patients with chronic health conditions support Janoff-Bulman’s (1979) theory, particularly the suggested link between CSB and negative health outcomes. In a study by Plaufcan, Wamboldt, and Holm (2012), CSB was associated with more depressive symptoms, whereas BSB was associated with less depressive symptoms in patients with chronic obstructive pulmonary disease (COPD). According to Voth and Sirois (2009), patients with inflammatory bowel disease who created person-focused attributions (i.e., CSB) used more avoidant-focused coping strategies, were less likely to accept the limitations and difficulties of their illness, and were less effective at coping with illness-related stress than patients who did not create CSB attributions. Patients in Voth and Sirois’ (2009) study who created BSB attributions used fewer avoidant coping strategies, reported better acceptance of their limitations and difficulties, and coped with stress more effectively.
Bennett, Howarter, and Clark (2013), who examined the relationship between self-blame attributions and psychological distress in the context of cardiovascular disease (CVD), found that blaming one’s own character for a recent cardiac event at the beginning of cardiac rehabilitation was unrelated to distress concurrently or at 12 weeks following the end of rehabilitation. However, BSB was positively related to symptoms of anxiety and depression concurrently and at a 12-month follow-up. In addition, although CSB was not associated with depressive symptoms at three months following diagnosis, it did predict increased cardiac symptom experiences (e.g., chest pain) at 18 months following cardiac rehabilitation (Harry, Bennett, Clark, Howarter, & Eways, 2015). Conversely, BSB positively predicted depressive symptoms at a three-month follow-up but was unrelated to cardiac symptom experiences at 18 months following cardiac rehabilitation.
Literature on the effects of self-blame in patients with cancer is limited. In their study of women with newly diagnosed breast cancer, Bennett, Compas, Beckjord, and Glinder (2005) found that BSB and CSB attributions created at diagnosis were cross-sectionally and positively related to psychological distress at four months and predicted increased levels of distress at 7 and 12 months following diagnosis. In a study of patients with head and neck or lung cancer, BSB—defined in the study as the extent to which patients believed that their cancer was caused by tobacco use or alcohol consumption—was positively associated with psychological distress but unrelated to cancer-related stigma perceptions (Lebel et al., 2013). These results are supported by Friedman et al. (2007), who also reported cross-sectional associations between BSB attributions and distress in patients diagnosed with breast cancer.
However, many studies on self-blame attributions have been inconclusive. This may be a result of using single-item questions or measures adapted from other scales, which can present methodologic and statistical concerns (Bennett et al., 2005, 2013; Harry et al., 2015; Lebel et al., 2013; Plaufcan et al., 2012). Reliability coefficients cannot be calculated for single-item measures (Clark & Watson, 1995), and one item may not accurately capture the complexity of the construct of self-blame attributions (Hoeppner, Kelly, Urbanoski, & Slaymaker, 2011). Adapted items or measures may also introduce potential measurement errors if they are not subjected to sufficient psychometric scrutiny (Voth & Sirois, 2009). Therefore, a multi-item, psychometrically valid self-blame attribution measurement tool is needed to examine this construct in patients with cancer.
In response to the limitation of using a single-item measure, Harry et al. (2018) validated the Cardiac Self-Blame Attributions Scale for measuring self-blame attributions in patients with CVD, which represents significant improvements in the measurement of self-blame attributions in patients with medical conditions. However, because the use of the Cardiac Self-Blame Attributions Scale is limited to patients with CVD, the authors adapted the scale for use in patients with cancer. The purpose of this study was to explore the psychometric properties of the Self-Blame Attributions for Cancer Scale (SBAC). Using Harry et al.’s (2018) study as a framework, the following was hypothesized:
• Results of an exploratory factor analysis would yield a two-factor structure representing BSB and CSB.
• Each factor would demonstrate good internal consistency (indicated by a Cronbach alpha coefficient greater than 0.85).
• Each factor would not be significantly associated with sleep disturbance, indicating discriminant validity.
• Each factor would be positively and significantly related to a measure of cancer-specific internal locus, indicating convergent validity.
Methods
Sample, Setting, and Procedures
The outpatient clinic schedules of radiation oncology providers at the University of Kansas (KU) Cancer Center were screened to identify eligible patients. Patients were eligible if they were aged 18 years or older, English-speaking, literate, and receiving radiation therapy. Eligible patients could not have any cognitive or physical impairments that impeded their ability to provide voluntary consent or participate in the study procedures.
Eligible patients were approached by a member of the research team during their outpatient radiation oncology clinic visits. If the patient provided consent, the research team member administered a self-report questionnaire packet, which included the SBAC scale, demographic questions, and measures of convergent and discriminant validity. Patients completed the questionnaire packets in the clinic examination room and returned them to the research team member. The study was approved by the KU Cancer Center Institutional Review Board.
Measures
The KU Cancer Center’s standard sociodemographic form was used to collect patient demographic information, including age, cancer diagnosis, gender, race and ethnicity, sexual orientation, relationship status, religious affiliation, employment status, and education level.
Self-Blame Attributions for Cancer Scale: The Cardiac Self-Blame Attributions Scale was adapted to assesses self-blame attributions in patients with cancer. Developed and validated by Harry et al. (2018), the Cardiac Self-Blame Attributions Scale is an 11-item, Likert-type scale that consists of six items measuring BSB attributions and five items measuring CSB attributions. Total BSB scores range from 0–24, and total CSB scores range from 0–20. Items are scored using a five-point scale ranging from 0 (not at all) to 4 (completely), with higher scores reflecting higher levels of that specific type of reported self-blame (BSB or CSB). The reliability for each factor (BSB and CSB) was good to excellent (Cronbach alpha = 0.93 and 0.87, respectively). To expand the use of the Cardiac Self-Blame Attributions Scale in patients with cancer, the scale was adapted by replacing the phrase “cardiovascular disease” with “cancer” for each item. The same five-point, Likert-type scale for the Cardiac Self-Blame Attributions Scale was used with the SBAC. Reliability and validity statistics are reported in the results of this article.
Validity assessment: The six-item sleep disturbance subscale of the Patient-Reported Outcomes Measurement Information System®–43 (PROMIS-43), version 2.0, was used to assess the discriminant validity of the SBAC. The PROMIS-43 is a self-report measure that assesses patient-reported symptoms and health-related quality of life, and the sleep disturbance subscale assesses patient perceptions of sleep quality during the past seven days (Cella et al., 2010). Items are scored using a five-point, Likert-type scale ranging from 1 (very poor) to 5 (very good) for the first item and from 1 (not at all) to 5 (very much) for the subsequent items. Reverse scoring is used for the first two questions. Values are summed and raw scores are transformed into norms-based t scores, with a mean of 50 and a standard deviation of 10. Higher scores indicate greater sleep disturbance. The PROMIS-43 is a reliable and valid measure of multiple dimensions of health-related quality of life in patients with cancer, with Cronbach alpha coefficients ranging from 0.89–0.95 for the individual subscales (Jensen et al., 2015, 2017). In this study, the Cronbach alpha for the PROMIS-43 sleep disturbance subscale was 0.84.
The six-item Form C of the Multidimensional Health Locus of Control (MHLC) scales was used to assess the convergent validity of the SBAC. Form C of the MHLC scales evaluates the extent to which patients view their cancer diagnoses and their trajectory as something that they can self-manage (Wallston, Stein, & Smith, 1994). Although the MHLC scales assess perceived control, the internal locus of control subscale was selected because its items capture aspects of behavioral and dispositional causes for health conditions. Items are ranked on a Likert-type scale ranging from 1 (strongly disagree) to 6 (strongly agree). Higher total scores indicate a higher internal locus. Previous research has shown that the internal subscale has good internal consistency, with a Cronbach alpha ranging from 0.85–0.87 (Wallston, 2005; Wallston et al., 1994). In this study, the Cronbach alpha was 0.84.
Data Analysis
Descriptive and inferential statistics were performed using IBM SPSS Statistics, version 24.0. Because the number of missing values was less than two percent of the total data, expectation maximization was used to impute missing data (Cole, 2008). An exploratory factor analysis with principal axis factoring using an oblimin rotation was used to examine the factor structure of the 11-item SBAC. The number of factors to extract was determined using parallel analysis, and convergent and discriminant validity were determined using Pearson correlation analyses.
Results
Sample Characteristics
From July 2017 to May 2018, 146 patients were approached for the study, with 120 patients agreeing to participate. Patients declined because of a lack of interest (n = 20), time constraints (n = 4), and not feeling well (n = 2). In addition, five patients who initially provided consent did not complete the questionnaire packet. Two additional patients were excluded from the analyses because of a significant amount of missing data. The final convenience sample consisted of 113 patients with cancer who were actively receiving radiation therapy. According to scale development and validation recommendations (subject-to-item ratio of 10:1), the number of participants was deemed sufficient (Costello & Osborne, 2005).
Patient ages ranged from 20–88 years, with an approximate equal number of men (n = 54) and women (n = 59). Most patients were White, partnered, and straight or heterosexual. A majority of patients were retired and had completed at least some college. Head and neck, breast, and gynecologic cancer were the most common cancer diagnoses in this sample. Patient characteristics are presented in Table 1. 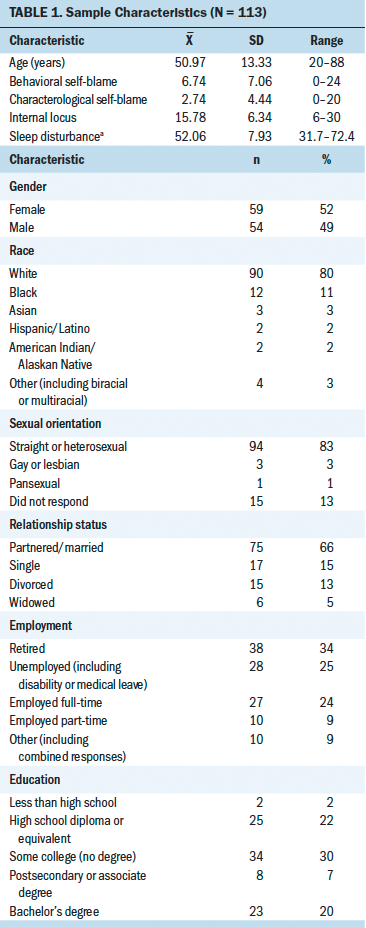
Exploratory Factor Analysis
Descriptive statistics, pattern and structure coefficients, and communalities of the SBAC are presented in Table 2. The Kaiser–Meyer–Olkin (KMO) measure confirmed that the sampling adequacy for this study was excellent (KMO = 0.9). Parallel analysis revealed that two factors should be extracted, and the exploratory factor analysis indicated that the two factors represented BSB (six items) and CSB (five items), explaining the 75.7% of the variance in the construct. Factor 1 (BSB) consisted of the first six items, and Factor 2 (CSB) consisted of the remaining five items. Primary pattern coefficients produced by the final factor solution ranged from 0.59–0.99, with no cross-loadings above 0.35. Internal consistency estimates for each subscale were excellent (Cronbach alpha = 0.95 and 0.93 for BSB and CSB, respectively). The correlation between Factors 1 and 2 was 0.65. Inter-item correlations are presented in Table 3. 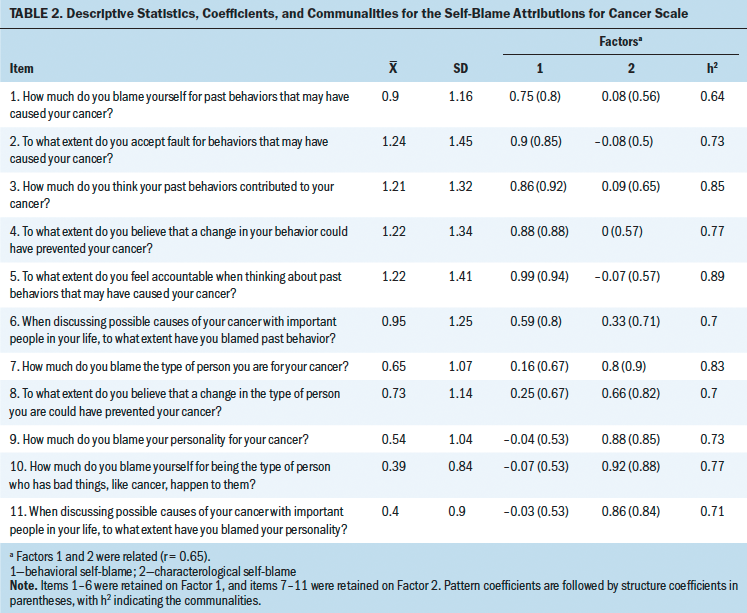
Convergent and Discriminant Validity
Significant and positive correlations were found between Form C of MHLC scales and the BSB (r = 0.33, p < 0.001) and CSB subscales (r = 0.29, p < 0.01), indicating convergent validity. Comparatively, weak and non-significant correlations were found between the sleep disturbance subscale of the PROMIS-43 and the BSB and CSB subscales (r = 0.11 and r = 0.09, respectively), indicating discriminant validity (p > 0.05).
Discussion
This study explored the psychometric properties of the SBAC, an adapted multi-item measure of self-blame attributions in patients with cancer. The SBAC revealed a two-factor structure representing BSB and CSB attributions, confirming that patients with cancer make distinctions between behavioral and characterological causes for their diagnoses. In addition, the two factors demonstrated excellent internal consistency, with each subscale showing good discriminant validity in the form of non-significant correlations with sleep disturbance. Convergent validity was also indicated via positive and significant correlations between the BSB and CSB subscales and Form C of the MHLC scales. Therefore, the results support the use of the SBAC in patients with cancer. [[{"fid":"54371","view_mode":"default","fields":{"format":"default","alignment":"","field_file_image_alt_text[und][0][value]":false,"field_file_image_title_text[und][0][value]":false},"link_text":null,"type":"media","field_deltas":{"1":{"format":"default","alignment":"","field_file_image_alt_text[und][0][value]":false,"field_file_image_title_text[und][0][value]":false}},"attributes":{"class":"media-element file-default","data-delta":"1"}}]]
Overall, this study’s results parallel the findings of Harry et al. (2018), but some differences were determined. In Harry et al.’s (2018) study, the mean scores of BSB (mean = 12.87) and CSB (mean = 5.47) were greater than those identified in the current study, suggesting that self-blame attributions vary by disease group. The discrepancy in BSB between the samples also suggests that patients with cancer are more likely to search for nonbehavioral causes for their illness. For example, genetic components are often emphasized over behavioral risk factors for patients with breast cancer (Shiovitz & Korde, 2015). Given the differing depictions of breast cancer and CVD in the media, this emphasis is unsurprising. The media often focuses on the search for a cure for breast cancer or new treatment options, whereas CVD is portrayed as a disease that is manageable through behavioral changes, such as dieting, exercising, and taking medication (Atkin, Smith, McFeters, & Ferguson, 2008; Wakefield, Loken, & Hornik, 2010). The varying degrees of self-blame attributions across patient populations is further illustrated by evaluating previous studies that used single-item measures for BSB and CSB. Bennett et al. (2005) and Harry et al. (2015) examined self-blame attributions in patients with newly diagnosed breast cancer and patients participating in cardiac rehabilitation using the same one-item measures for CSB and BSB. Consistent with the current study’s results, the mean scores of BSB (mean = 1.52) and CSB (mean = 1.3) reported by patients with breast cancer were found to be relatively lower than mean scores of BSB (mean = 2.55) and CSB (mean = 1.74) reported by patients with CVD.
The moderate cross-loading of item 6 (“When discussing possible causes of your cancer with important people in your life, to what extent have you blamed past behavior?”) on both factors in the current study was another difference found between the Cardiac Self-Blame Attributions Scale and the SBAC. Because patients with cancer may be less likely than patients with CVD to attribute their diagnoses to their own behaviors, the manner in which they discuss the potential causes for their disease with loved ones may also be affected. It is also possible that patients’ loved ones believe that cancer is less behaviorally mediated than CVD, and, therefore, when patients discuss their attributions for their cancer, friends and family may be less likely to focus on the patients’ behaviors. Patients who choose to discuss behavioral attributions may not receive supportive feedback, which may also cause them to question their own assumptions about the behavioral causes of their disease. The belief that having a strong support system eschews BSB attributions is best considered in the context of a specific cancer type. For example, Lobchuk, Murdoch, McClement, and McPherson (2008) found that caregivers of patients with lung cancer identified the patient as the primary locus of causality for their illness because of a history of smoking. These views may be less common in patients with cancers for which the causes are unknown or less obvious, such as head and neck, breast, and gynecologic. Future research should explore whether caregivers’ perceptions of the causes of a patient’s disease vary by disease type and influence patient–caregiver interactions.
Information on patients’ causal self-blame attributions can be integrated into cancer care by healthcare providers following a cancer diagnosis. Previous research has demonstrated the benefits of targeting patients’ perceptions of the causes of their illnesses in medical settings. Broadbent, Ellis, Thomas, Gamble, and Petrie (2009) tested an inpatient intervention aimed at shaping patients’ causal attributions for a heart attack. Following the intervention, patients reported significantly different causal explanations, accurately identifying high cholesterol and lack of exercise as the reasons for their disease. The intervention group also reported higher rates of returning to work, lower levels of anxiety about returning to work, a better understanding of medical information, and greater increases in exercise. Patients also described feeling better prepared to be discharged from the hospital and expressed a greater intention to attend cardiac rehabilitation following discharge. The results of Broadbent et al.’s (2009) study suggest that interventions can enhance outcomes through the modification of patients’ attributions for their medical conditions. The intervention techniques used in Broadbent et al.’s (2009) study can be implemented by clinic staff in other settings without advanced mental health training. Brief nurse-delivered psychosocial interventions, such as cognitive therapy, have also been shown to effectively reduce patients’ symptoms of depression and anxiety and improve overall quality of life (Chambers et al., 2014; Lee, Lim, Yoo, & Kim, 2011; Moorey et al., 2009).
Using a validated measure can also help to analyze the influence of BSB attributions on patient health outcomes. The protective effects of behavioral-based attributions have been explored in previous studies of patients with chronic conditions (Plaufcan et al., 2012; Voth & Sirois, 2009). Plaufcan et al. (2012) suggest that patients with behavior-based conditions (e.g., COPD) may experience perceptions of controllability over disease progression if they blame modifiable behaviors such as smoking. Patients with behavior-mediated cancers may, therefore, experience similar benefits from identifying modifiable behaviors as the cause of their diagnosis.
However, BSB attributions have been shown to adversely affect outcomes in patients receiving treatment for cancer (Bennett et al., 2005; Friedman et al., 2007; Lebel et al., 2013). According to Lebel et al. (2013), blaming specific behaviors (e.g., alcohol consumption, tobacco use) may elicit harmful effects in patients with behavior-mediated tumors. Administering the validated BSB subscale in oncology settings can help to clarify these mixed results and understand whether they are caused by poor measurement techniques, variations in attribution processes based on diagnosis, or another variable.
Limitations
In addition, the SBAC was administered to patients with heterogeneous cancer types. Although this enhances generalizability, the attribution processes experienced by patients from individual disease groups could not be examined. Because causal self-blame attributions may vary by disease type, future research should examine self-blame attributions in patients who have cancers, such as lung, head and neck, and skin, which are often associated with specific behaviors (e.g., smoking, alcohol use, sun exposure). The patients in this study were recruited from radiation therapy clinics, which does not effectively represent patients who are exclusively receiving other treatment types, such as surgery, chemotherapy, and immunotherapy. In this sample, race, ethnicity, and education level were also relatively limited; most patients were White and had completed some college. Future research should explore self-blame attributions in more diverse patient populations from a variety of outpatient oncology settings. Although it was consistent with scale development sampling recommendations (Costello & Osborne, 2005), the current study’s sample was relatively small. Future research should explore the psychometric properties of the SBAC with larger samples to reduce the risk of sampling error.
Implications for Nursing
The results from this study have important clinical and research implications. Because it has been consistently linked to poor outcomes across patient populations, nurses should discourage patients with cancer from engaging in CSB (Bennett et al., 2005, 2013; Harry et al., 2015; Janoff-Bulman, 1979; Plaufcan et al., 2012). Administering the CSB subscale in the clinical setting would allow for a better understanding of the extent to which patients create these attributions and their long-term effects. In addition, accurate measurement is necessary for understanding patients’ attribution processes so that appropriate interventions can be implemented to improve outcomes. Retraining patients’ attribution processes is feasible. Cognitive-based therapies for mental health concerns, which are effective at improving outcomes, can help to identify and restructure maladaptive cognitions in patients with cancer (Rubenstein, Freed, Shapero, Fauber, & Alloy, 2016).
Understanding the effects of BSB attributions can also provide clinicians with guidance about the appropriateness of interventions for patients engaging in such behaviors. If the BSB attributions of patients appear to be adaptive, healthcare providers can help to identify the specific behaviors the patients are blaming and facilitate discussions on how to modify those behaviors. However, if BSB is determined to be maladaptive and does not facilitate behavioral changes, healthcare providers may instead focus on using interventions similar to those described for CSB (i.e., discourage any type of self-blame by restructuring patients’ thought patterns and alleviating the symptoms of distress associated with them). Acceptance-based approaches, which have a goal of encouraging acceptance of the actual causes of cancer rather than modifying casual attributions, may be beneficial for patients reporting BSB. These approaches simultaneously help patients commit to behaviors more consistent with their values, such as physical health, family, and work (Hayes, Pistorello, & Levin, 2012). Future research evaluating the predictive validity of the SBAC is needed to determine the specific effects of BSB and CSB attributions on health outcomes in patients with cancer. Because the SBAC is a practical and feasible 11-item scale, with an administration and scoring time of about five minutes on average, it can be easily integrated into standard clinical workflows in outpatient oncology settings to help predict patient outcomes. 
Conclusion
This study provides preliminary evidence for the reliability and convergent and discriminant validity of the SBAC. As the first multi-item measure of self-blame attributions for patients with cancer, the SBAC fills an important gap in the literature and has many clinical implications, including better characterization of the self-blame attributions reported by patients with cancer and revealing potential targets for interventions for patients experiencing maladaptive types or levels of self-blame. Additional research evaluating the use of the SBAC in patients with cancer can help to further establish its psychometric properties and determine the effects of BSB and CSB attributions on psychological and physical health outcomes.
About the Author(s)
Kalon R. Eways, MA, is a clinical psychology doctoral candidate and Kymberley K. Bennett, PhD, is an associate professor, both in the Department of Psychology at the University of Missouri–Kansas City; Jessica L. Hamilton, PhD, is an assistant professor and director of psychodiagnostics evaluation for medical interventions in the Department of Psychiatry and Behavioral Sciences at the University of Kansas Medical Center in Kansas City; Kadie M. Harry, PhD, is a postdoctoral fellow in the University of Kansas Health System in Mission; Jacob Marszalek, PhD, is an associate professor in the College of Arts and Sciences, and Mary-Joy O. Marsh, BA, is a research assistant and student and Elizabeth J. Wilson, MA, is a graduate student, both in the Department of Psychology, all at the University of Missouri–Kansas City. No financial relationships to disclose. Eways, Bennett, Hamilton, and Harry contributed to the conceptualization and design. Eways, Hamilton, Harry, and Marsh completed the data collection. Eways, Bennett, Marszalek, and Marsh provided statistical support. Eways, Harry, and Marsh provided the analysis. Eways, Bennett, Hamilton, Harry, Marsh, and Wilson contributed to the manuscript preparation. Eways can be reached at kaloneways@mail.umkc.edu, with copy to ONFEditor@ons.org. (Submitted June 2019. Accepted August 2, 2019.)
References
Abramson, L.Y., Seligman, M.E., & Teasdale, J.D. (1978). Learned helplessness in humans: Critique and reformulation. Journal of Abnormal Psychology, 87, 49–74. https://doi.org/10.1037/0021-843X.87.1.49
Atkin, C.K., Smith, S.W., McFeters, C., & Ferguson, V. (2008). A comprehensive analysis of breast cancer news coverage in leading media outlets focusing on environmental risks and prevention. Journal of Health Communication, 13, 3–19. https://doi.org/10.1080/10810730701806912
Bennett, K. (2018). Causal attributions and social judgments. In T.D. Nelson (Ed.), Getting grounded in social psychology: The essential literature for beginning researchers (1st ed., pp. 79–104). New York, NY: Routledge.
Bennett, K.K., Compas, B.E., Beckjord, E., & Glinder, J.G. (2005). Self-blame and distress among women with newly diagnosed breast cancer. Journal of Behavioral Medicine, 28, 313–323. https://doi.org/10.1007/s10865-005-9000-0
Bennett, K.K., Howarter, A.D., & Clark, J.M. (2013). Self-blame attributions, control appraisals and distress among cardiac rehabilitation patients. Psychology and Health, 28, 637–652. https://doi.org/10.1080/08870446.2012.743128
Broadbent, E., Ellis, C.J., Thomas, J., Gamble, G., & Petrie, K.J. (2009). Further development of an illness perception intervention for myocardial infarction patients: A randomized controlled trial. Journal of Psychosomatic Research, 67, 17–23. https://doi.org/10.1016/j.jpsychores.2008.12.001
Cella, D., Riley, W., Stone, A., Rothrock, N., Reeve, B., Yount, S., . . . Hays, R. (2010). Initial adult health item banks and first wave testing of the Patient-Reported Outcomes Measurement Information System (PROMIS) network: 2005–2008. Journal of Clinical Epidemiology, 63, 1179–1194. https://doi.org/10.1016/j.jclinepi.2010.04.011
Chambers, S.K., Girgis, A., Occhipinti, S., Hutchison, S., Turner, J., McDowell, M., . . . Dunn, J.C. (2014). A randomized trial comparing two low-intensity psychological interventions for distressed patients with cancer and their caregivers [Online exclusive]. Oncology Nursing Forum, 41, E256–E266. https://doi.org/10.1188/14.ONF.E256-E266
Clark, L.A., & Watson, D. (1995). Constructing validity: Basic issues in objective scale development. Psychological Assessment, 7, 309–319. https://doi.org/10.1037/1040-3590.7.3.309
Cole, J.C. (2008). How to deal with missing data: Conceptual overview and details for implementing two modern methods. In J.W. Osborne (Ed.), Best practices in quantitative methods (pp. 214–238). Los Angeles, CA: Sage.
Costello, A.B., & Osborne, J.W. (2005). Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Practical Assessment, Research and Evaluation, 10, 1–9.
Fiske, S.T., & Taylor, S.E. (2017). Social cognition: From brains to culture (3rd ed.). Thousand Oaks, CA: Sage.
Friedman, L.C., Romero, C., Elledge, R., Chang, J., Kalidas, M., Dulay, M.F., . . . Osborne, C.K. (2007). Attribution of blame, self-forgiving attitude and psychological adjustment in women with breast cancer. Journal of Behavioral Medicine, 30, 351–357. https://doi.org/10.1007/s10865-007-9108-5
Harry, K.M., Bennett, K.K., Clark, J.M.R., Howarter, A.D., & Eways, K.R. (2015). Self-blame attributions and cardiac symptom experiences in cardiac rehabilitation patients: A preliminary study. North American Journal of Psychology, 17, 541–533.
Harry, K.M., Bennett, K.K., Marszalek, J.M., Eways, K.R., Clark, J.M., Smith, A.J., . . . Wilson, E.J. (2018). Scale development and psychometric properties of the cardiac self-blame attributions scale in patients with cardiovascular disease. Health Psychology Open, 5. https://doi.org/10.1177/2055102918786865
Hayes, S.C., Pistorello, J., & Levin, M.E. (2012). Acceptance and commitment therapy as a unified model of behavior change. Counseling Psychologist, 40, 976–1002. https://doi.org/10.1177/0011000012460836
Hoeppner, B.B., Kelly, J.F., Urbanoski, K.A., & Slaymaker, V. (2011). Comparative utility of a single-item versus multiple-item measure of self-efficacy in predicting relapse among young adults. Journal of Substance Abuse Treatment, 41, 305–312. https://doi.org/10.1016/j.jsat.2011.04.005
Janoff-Bulman, R. (1979). Characterological versus behavioral self-blame: Inquiries into depression and rape. Journal of Personality and Social Psychology, 37, 1798–1809. https://doi.org/10.1037/0022-3514.37.10.1798
Jensen, R.E., Potosky, A.L., Moinpour, C.M., Lobo, T., Cella, D., Hahn, E.A., . . . Reeve, B.B. (2017). United States population-based estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. Journal of Clinical Oncology, 35, 1913–1920. https://doi.org/10.1200/JCO.2016.71.4410
Jensen, R.E., Potosky, A.L., Reeve, B.B., Hahn, E., Cella, D., Fries, J., . . . Moinpour, C.M. (2015). Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Quality of Life Research, 24, 2333–2344. https://doi.org/10.1007/s11136-015-0992-9
Lebel, S., Feldstain, A., McCallum, M., Beattie, S., Irish, J., Bezjak, A., & Devins, G.M. (2013). Do behavioural self-blame and stigma predict positive health changes in survivors of lung or head and neck cancers? Psychology and Health, 28, 1066–1081. https://doi.org/10.1080/08870446.2013.781602
Lee, H., Lim, Y., Yoo, M.S., & Kim, Y. (2011). Effects of a nurse-led cognitive-behavior therapy on fatigue and quality of life of patients with breast cancer undergoing radiotherapy: An exploratory study. Cancer Nursing, 34, E22–E30. https://doi.org/10.1097/NCC.0b013e31820d1734
Lobchuk, M.M., Murdoch, T., McClement, S.E., & McPherson, C. (2008). A dyadic affair: Who is to blame for causing and controlling the patient’s lung cancer? Cancer Nursing, 31, 435–443. https://doi.org/10.1097/01.NCC.0000339253.68324.19
Maasland, D.H., van den Brandt, P.A., Kremer, B., Goldbohm, R.A., & Schouten, L.J. (2014). Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: Results from the Netherlands Cohort Study. BMC Cancer, 14, 187. https://doi.org/10.1186/1471-2407-14-187
Moorey, S., Cort, E., Kapari, M., Monroe, B., Hansford, P., Mannix, K., . . . Hotopf, M. (2009). A cluster randomized controlled trial of cognitive behaviour therapy for common mental disorders in patients with advanced cancer. Psychological Medicine, 39, 713–723. https://doi.org/10.1017/S0033291708004169
Plaufcan, M.R., Wamboldt, F.S., & Holm, K.E. (2012). Behavioral and characterological self-blame in chronic obstructive pulmonary disease. Journal of Psychosomatic Research, 72, 78–83. https://doi.org/10.1016/j.jpsychores.2011.10.004
Rubenstein, L.M., Freed, R.D., Shapero, B.G., Fauber, R.L., & Alloy, L.B. (2016). Cognitive attributions in depression: Bridging the gap between research and clinical practice. Journal of Psychotherapy Integration, 26, 103–115. https://doi.org/10.1037/int0000030
Shiovitz, S., & Korde, L.A. (2015). Genetics of breast cancer: A topic in evolution. Annals of Oncology, 26, 1291–1299. https://doi.org/10.1093/annonc/mdv022
Taylor, S.E. (1983). Adjustment to threatening events: A theory of cognitive adaptation. American Psychologist, 38, 1161–1173. https://doi.org/10.1037/0003-066X.38.11.1161
Voth, J., & Sirois, F.M. (2009). The role of self-blame and responsibility in adjustment to inflammatory bowel disease. Rehabilitation Psychology, 54, 99–108. https://doi.org/10.1037/a0014739
Wakefield, M.A., Loken, B., & Hornik, R.C. (2010). Use of mass media campaigns to change health behaviour. Lancet, 376, 1261–1271. https://doi.org/10.1016/S0140-6736(10)60809-4
Wallston, K.A. (2005). The validity of the Multidimensional Health Locus of Control scales. Journal of Health Psychology, 10, 623–631. https://doi.org/10.1177/1359105305055304
Wallston, K.A., Stein, M.J., & Smith, C.A. (1994). Form C of the MHLC Scales: A condition-specific measure of locus of control. Journal of Personality Assessment, 63, 534–553. https://doi.org/10.1207/s15327752jpa6303_10


