Pilot Feasibility Study Examining Pupillary Response During Driving Simulation as a Measure of Cognitive Load in Breast Cancer Survivors
Objectives: To test the feasibility of adding driving simulation tasks to measure visuospatial ability and processing speed to an existing neurocognitive battery for breast cancer survivors (BCSs).
Sample & Setting: 38 BCSs and 17 healthy controls from a cross-sectional pilot study conducted at the University of Kansas Medical Center.
Methods & Variables: Exploratory substudy measuring pupillary response, visuospatial ability, and processing speed during two 10-minute driving simulations (with or without n-back testing) in a sample of BCSs with self-reported cognitive complaints and healthy controls.
Results: Feasibility of measurement of pupillary response during driving simulation was demonstrated. No between-group differences were noted for pupillary response during driving simulation. BCSs had greater visuospatial ability and processing speed performance difficulties than healthy controls during driving simulation without n-back testing and slower n-back response time.
Implications for Nursing: Preliminary evidence showed a possible link between cancer/treatment on visuospatial ability and processing speed in BCSs.
Jump to a section
Breast cancer survivors (BCSs) frequently experience changes in cognitive function attributed to the disease and treatment. Proposed mechanisms for these cognitive changes include damage to neuroprogenitor cells, increased production and release of peripheral and central proinflammatory cytokines, malfunction of DNA repair mechanisms, and oxidative stress (Asher & Myers, 2015; Janelsins et al., 2014). BCSs have reported difficulty across a number of cognitive domains, such as short-term memory, attention and concentration, processing speed, and executive function, including the ability to multitask (task switching) (Ahles et al., 2012; Asher & Myers, 2015; Wefel et al., 2011). However, other studies have failed to note objective cognitive changes or dysfunction after treatment. BCSs’ performance on standard neurocognitive tests frequently do not correlate with their self-report of cognitive changes. Some qualitative research results have included participants’ concerns related to operating a vehicle, difficulty driving to familiar locations, and accident near-misses (Myers, 2012; Player et al., 2014). Standard neurocognitive testing may not be sensitive to the level of cognitive effort expended by BCSs to achieve performance that is within normal limits (Hermelink et al., 2010).
Kahneman’s (1973) theory of attention and effort defines cognitive effort (also referred to as cognitive workload) as the mental effort or amount of attention and resources allocated to perform a task. Cancer survivors frequently report having to work harder to accomplish cognitive tasks they used to perform without difficulty prior to their diagnosis and treatment. The excess expenditure of cognitive effort correlates well with self-report and likely comprises a complementary construct to the behavioral assessment of cognitive function in cancer survivors (Hermelink et al., 2010; Myers et al., 2018). Neuroimaging research has demonstrated changes in brain activation commensurate with cognitive effort in cancer survivors reporting cognitive changes (Holohan et al., 2013). These changes (e.g., hyperactivation, hypoactivation) are postulated to be task-dependent and more sensitive to cognitive effort expenditure than neurocognitive task performance. Great interest exists in the investigation of novel, less expensive, and clinically accessible methods to assess cognitive effort instead of relying on complex and costly neuroimaging. One promising area of research is the investigation of pupillary response as a measure of cognitive effort.
Pupillary response, specifically dilation, is an autonomic and reflexive response to the expenditure of cognitive effort (Beatty, 1982; Eckstein et al., 2017; Marshall, 2007). The sympathetic and parasympathetic nervous systems control pupillary dilation. During sustained cognitive tasks, parasympathetic fibers are inhibited as a result of locus coeruleus activation and inhibitory signaling to the Edinger–Westphal nucleus. This parasympathetic inhibition relaxes the iris sphincter muscle, resulting in pupil dilation (Eckstein et al., 2017; Murphy et al., 2014). The changes in pupil diameter during neurocognitive testing can be converted into the index of cognitive activity (ICA), a scaled measure of cognitive effort ranging from 0 to 1. The ICA is determined from the estimation of the average number of abrupt discontinuities in pupil size per second from the application of wavelet decomposition and signal processing of the algorithms of wavelet analysis. The ICA is differentiated from pupillary response related to light accommodation through signal smoothing. Pupillary response has been studied in younger adults (Kahya, Wood, et al., 2018), older adults (Beatty, 1982; Wang et al., 2016), adults with Parkinson’s disease (Kahya, Moon, et al., 2018; Ranchet, Morgan, et al., 2017; Ranchet, Orlosky, et al., 2017), adults at risk for Alzheimer disease (Granholm et al., 2017), and preliminarily in BCSs (Myers et al., 2018). Pupillary response as a measure of cognitive effort is advantageous in a number of ways, including portability of the equipment and lack of need for enclosed space assessment as compared to neuroimaging. The procedure is noninvasive and provides a real-time assessment of cognitive effort without increasing the time needed for neurocognitive testing. Disadvantages include potential inability to accurately scan pupils of individuals with anomalies, such as cataracts or strabismus, and the need for individuals to keep their eyes open while concentrating on cognitive recall tasks.
The current authors conducted a pilot study (referred to as the parent study) to assess pupillary response as a measure of cognitive effort in BCSs (Myers et al., 2018). Pupillary response was compared during a 60-minute battery of neurocognitive tests for working memory, sustained attention, and verbal fluency in BCSs and healthy controls. In this article, the authors report the use of pupillary response during driving simulation in a subsample from the parent study. Even abbreviated neurocognitive testing batteries can be burdensome for patients and clinicians. In addition, neurocognitive tests typically are conducted in a somewhat artificial environment (e.g., a quiet room without other stimuli or interruptions) and, therefore, may not provide an ecologically valid representation of the cognitive tasks of everyday life. The authors sought to enhance the neurocognitive battery to include tests that would be more representative of participants’ daily cognitive challenges. Driving simulation offers the opportunity to evaluate performance in specific cognitive domains while participating in challenging, ecologically valid simulations of real-life activities and ensuring the participants’ safety (Akinwuntan et al., 2012; Vardaki et al., 2016). The visual aspect of driving simulation pairs well with the pupillary response measurement of cognitive effort (Chan et al., 2010) in the cognitive domains known to be affected by cancer and cancer therapy (Wefel et al., 2011). Driving simulation also was of interest given BCSs’ reported issues with vehicle operation and driving based on qualitative studies by Myers (2012) and Player et al. (2014). To the authors’ knowledge, the current study is the first to incorporate driving simulation into neurocognitive assessment. Visuospatial ability is required to maintain lane position, follow a lead vehicle at a consistent distance, and avoid obstacles. Processing speed is necessary to respond to unexpected events (e.g., obstacle avoidance) and changes in the driving environment (e.g., speed limits). To further simulate the cognitive load involved for complex multitasking and executive function, the authors combined driving simulation with simultaneous testing of working memory and sustained attention.
The purpose of this exploratory substudy was to test the feasibility of enhancing an existing neurocognitive battery (Myers et al., 2018) by adding driving simulation tasks to measure visuospatial ability and processing speed for BCSs with self-reported cognitive complaints. The primary aim was to evaluate feasibility based on participant acceptability, tolerance, and data completeness. A second aim was to compare cognitive effort (pupillary response index of cognitive activity) between BCSs and healthy controls during driving simulation with and without the additional cognitive demand from a simultaneous task of working memory and sustained attention (n-back task). Finally, the third aim was to compare performance on driving simulation tasks measuring visuospatial ability (center line cross, speed limit exceedance, out-of-lane time) and processing speed (time in seconds to apply break at accident, collisions) between BCSs and healthy controls.
Methods
Following institutional review board approval by the University of Kansas Medical Center Human Subjects Committee in Kansas City, all participants (N = 46) who completed a cross-sectional parent pilot study conducted at the University of Kansas Medical Center to investigate pupillary response as a measure of cognitive effort in BCSs were invited to take part in this substudy to have an additional assessment during driving simulation (Myers et al., 2018). The parent study eligibility requirements included women aged 40–65 years who were diagnosed with stage I–III breast cancer, were within three months to six years of having completed chemotherapy (and radiation therapy, if received), were currently receiving endocrine therapy, and had complaints of cognitive dysfunction. Healthy controls were required to be within the same age range and education levels as the BCSs. Women (BCSs or healthy controls) with a history of severe clinical depression, Alzheimer disease, dementia, or other conditions that would significantly affect cognitive function were excluded. Substudy participation involved informed consent for one additional study visit to take part in the driving simulation.
Instruments
N-back task: The n-back task (2-back) neurocognitive test was designed to assess working memory and sustained attention and can be administered with visual or auditory stimuli (GonÇalves & Mansur, 2009; Owen et al., 2005). Psychometric analyses have produced mixed results for the n-back task validity with other clinical measures of working memory; reliability coefficients range from as low as 0.09 to 0.55 (Redick & Lindsey, 2013). However, the n-back task is the most commonly used test for research examining cognitive load and the neural basis of working memory processes (Jacola et al., 2014; Mencarelli et al., 2019; Miller et al., 2009; Owen et al., 2005; Yaple et al., 2019). The n-back task involves a series of letters presented at a consistent pace (five seconds apart). Participants must indicate when a presented letter is n number of letters back from its previous presentation. The 2-back form of this test was selected for administration in conjunction with driving simulation because of the desired level of difficulty (as compared to the 0-, 1-, and 3-back formats). The auditory form of this test was administered for this study with 17 targets and 56 distractors over 6 minutes of presentation time during the second 10-minute driving simulation. Percent accuracy and n-back response time were calculated.
Simulator Sickness Questionnaire: A very small percentage of individuals (2%–8%) may experience simulator sickness symptoms (a form of motion sickness) during driving simulation, particularly when the simulation involves multiple curves and stops (Akinwuntan et al., 2005, 2014). The Simulator Sickness Questionnaire (SSQ) is the most widely used measure of simulator sickness (Balk et al., 2013). Confirmatory factor analyses for use with driving simulation confirmed three factors of nausea, oculomotor, and disorientation (factor loadings = 0.3–0.76) (Balk et al., 2013). The SSQ is comprised of 16 items ranked by participants from 0 (none) to 3 (severe) and is predictive of participant dropout based on simulator sickness symptoms (sensitivity = 80.6%, specificity = 88.6%) (Balk et al., 2013; Kennedy et al., 1993; Kim et al., 2017). The SSQ administration time is about three to five minutes.
Procedures
Study participants were seated comfortably at the driving simulator and positioned within 60–80 cm from the eye-tracking camera. Calibration ensured correct head positioning to capture pupillary measurements when participants were gazing at the monitor. Pupillary response was recorded at 60 Hz with the FX3, an eye-tracking camera that was calibrated with EyeWorks™ software.
Driving simulation was conducted with a portable driving simulator (PDS). This desktop model is a low-fidelity simulator, and images were generated with STISIM Drive® software and displayed on a 23-inch computer screen. A Logitech® steering wheel and pedals are integrated to the simulator system. Car engine and ambient traffic sounds are simulated with a sound bar system to provide an immersive experience. Driving simulation was initiated with a one-minute straight road phase session during which participants were oriented to the PDS accelerator, brakes, video monitor, and steering wheel. Two 10-minute driving simulation scenarios were developed for the study. Simulation 1 involved a car-following task. Instructions were delivered via prerecorded audio. A lead vehicle was displayed driving on the right side of a four-lane road at an average speed of 60 miles per hour. This lead vehicle changed speed at an increase or decrease of 10 feet per second in a sinusoidal wave form pattern. Participants were instructed to follow the lead vehicle, keeping a consistent distance behind the vehicle while adapting to the constantly changing speed. The test evaluated visuospatial ability in an applied setting. Outcome measures included speed limit time and distance, and lane positioning in relationship to the center line and road edge. An unexpected event (accident) appeared at the end of the scenario. Participants needed to quickly apply the brakes (processing speed) to avoid a collision. Simulation 2 was identical. However, a greater cognitive load was imposed by requiring participants to simultaneously complete the n-back (2-back) task during the car-following phase of the second scenario. Participants completed the SSQ following simulation 1 to confirm the absence of symptoms prior to proceeding with simulation 2.
Written instructions displayed on the PDS monitor and prerecorded audio instructions were employed to explain the n-back task to study participants. A practice test was conducted prior to the initiation of simulation 2 to familiarize participants to the task and answer any questions. Auditory delivery of a series of 73 letters was prerecorded and played over six minutes during simulation 2. Participants were instructed to pull the lever mounted on the steering wheel when any letter played was the same as the letter played two steps earlier.
Data Processing
Pupillary response was calculated from the raw pupil size signal extracted with the EyeWorks software. Wavelet decomposition was applied with the software to estimate the average number of abrupt discontinuities in pupil size per second. The pupillary response value was transformed to the ICA (range = 0–1) (Marshall, 2007). Mean and standard deviation ICA values for the participants’ left and right eyes were calculated for the car-following task in simulation 1 (without the simultaneous n-back task) and simulation 2 (with the simultaneous n-back task). Mean and standard deviation ICA values also were calculated for the unexpected event (accident) phase of both driving simulations. Data cleaning eliminated any missing ICA values because of eye blinks or position changes prior to data analyses. Driving performance metrics were extracted for each participants’ two driving simulations.
Statistical Analyses
Descriptive statistics were calculated for the participants’ demographics. Two-sample Student’s t tests were conducted for normally distributed continuous variables. When assumptions of normality were violated, the Wilcoxon rank-sum test was used. Effect sizes (Cohen’s d) were calculated for driving simulation and n-back performance measures. Chi-square testing was used for comparing categorical variables between the two groups. Fisher’s exact test was used when the tables were sparse, with more than 20% expected cell counts less than 5. Within-group differences for pupillary response (as indexed by ICA) and driving performance were calculated for both groups with the Wilcoxon signed-rank test. Between-group differences in change scores were tested with the Wilcoxon rank-sum test. Between-group differences in menopausal status were not associated with the study outcomes, so the analyses were conducted with the full sample. Participants with greater than 50% missing ICA data for any particular performance variable were excluded from the analyses for that variable. The significance level was set at 0.05 for each test. No control for multiple tests was considered for this exploratory study.
Results
Forty-six participants from the parent study (Myers et al., 2018) were invited to take part in the driving simulation assessment. Thirty-eight consented (21 BCSs and 17 healthy controls). The majority were Caucasian, married, well educated, and employed full-time (see Table 1). The only significant difference between the two groups was menopausal status. About one-third of the healthy controls were premenopausal despite no difference in mean age (53 years for both groups). None of the participants exhibited symptoms on the SSQ that warranted cessation of driving simulation. Missing data were minimal. Only two participants (one BCS and one healthy control) had more than 50% missing data for the left or right eye with any performance variable. 
No between-group difference was noted for pupillary response during driving simulation 1 (without n-back task) or simulation 2 (with n-back task) (see Table 2). Some of the estimated effect sizes (Cohen’s d) were small to moderate (greater than 0.4), indicating that BCSs’ performance was worse than healthy controls during simulation 1 for collisions (d = 0.52), speeding tickets (d = 0.41), and out-of-lane time percentage (d = 0.52), as well as slower processing speed for the n-back task in simulation 2 (d = 0.52) (see Table 3). Healthy controls demonstrated worse performance for out-of-lane distance (p = 0.0452, d = –0.68) during simulation 2. 
Significant within-group differences were noted for BCSs (see Table 4). BCSs’ performance improved for collisions (p = 0.037) and out-of-lane time percentage (p = 0.029) during simulation 2. BCSs exhibited reduced pupillary response for both eyes (as indexed by ICA) during the car-following task with simultaneous n-back testing in simulation 2 as compared to the car-following task without concurrent n-back testing in simulation 1 (p = 0.048 for left eye, p = 0.044 for right eye).
Two between-group differences in change scores were demonstrated. BCSs performed better than healthy controls for out-of-lane time percentage (p = 0.048) and center line crossings (p = 0.046). No between-group differences in change scores were found for n-back task accuracy. 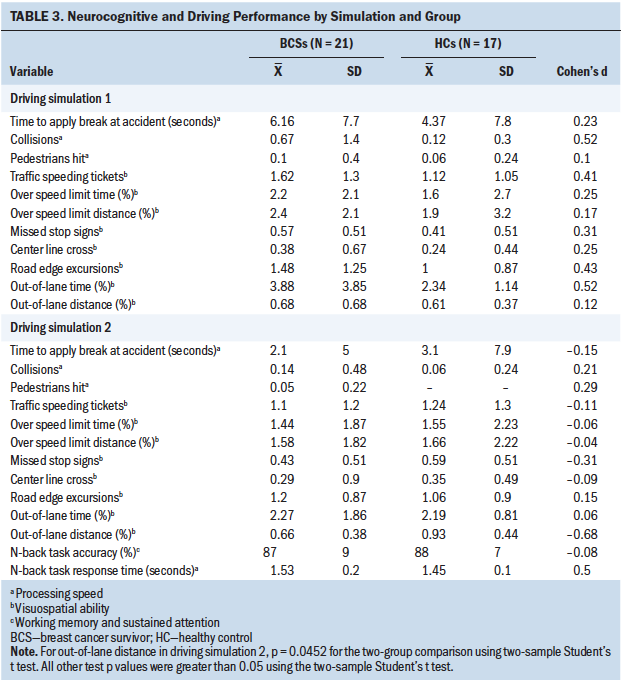
Discussion
Driving simulation was acceptable to BCSs (91% from the parent study were retained for the substudy), tolerable (0% simulator sickness noted), and feasible for data completeness (only two participants’ data were excluded from the analysis for any performance value). Despite the between-group differences in cognitive effort expenditure (as indexed by pupillary response/ICA) for BCSs and healthy controls during neurocognitive testing demonstrated in the parent study (Myers et al., 2018), none were noted during the driving simulation assessments, even when the cognitive load was increased by adding the simultaneous administration of the n-back task. In addition, within-group pupillary response was significantly reduced for BCSs when the n-back task was administered during simulation 2.
No significant between-group differences were noted for visuospatial and processing speed performance during driving simulation; however, effect size indicated difficulty for BCSs during simulation 1 for collisions, speeding, and poor lane position. These difficulties within the cognitive domains for processing speed and visuospatial ability are consistent with the evidence from qualitative work in which BCSs reported issues with vehicle operation and driving (Myers, 2012; Player et al., 2014). Further investigation with a larger sample is needed to validate the results compared to healthy controls. Driving simulation performance for BCSs improved for most outcomes during simulation 2. BCSs’ visuospatial performance improved more than healthy controls for two of the simulation outcomes (out-of-lane time percentage and center line crossings). BCSs performed as well as healthy controls for accuracy on the n-back task but with slower processing speed, as indicated by a moderate effect size. BCSs’ improvement during the second simulation may be because of practice effect and their ability to compensate for increases in cognitive load. The small drop in sample size from the original pilot study may have affected the power to determine between-group differences for pupillary response. The significant within-group reduction in pupillary response during the simultaneous driving simulation and n-back testing may signal development of cognitive fatigue when the cognitive load was increased. 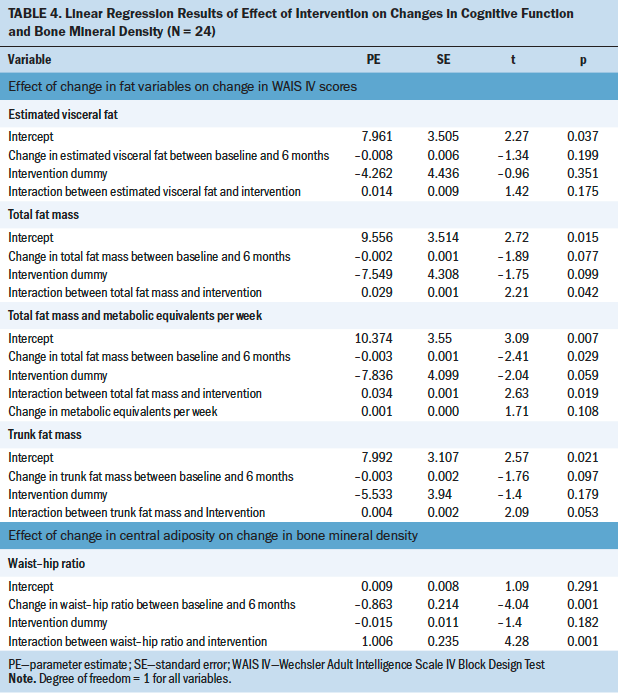
Limitations and Strengths
Cross-sectional study design and small sample size limit the generalization of findings. Participants were tested with the visual form of the n-back task (0-, 1-, and 2-back) during the parent study. Subsequent testing with the auditory 2-back test may have induced a practice effect. However, the minimum amount of time between testing was six months, so a practice effect on this neurocognitive test is unlikely.
The driving scenarios were developed specifically to evaluate visuospatial ability and processing speed. The authors used the addition of the n-back task to escalate the cognitive demand (task difficulty) and necessity for task switching. Given the improvements noted between simulations 1 and 2 for the BCS group, the level of difficulty of the driving scenarios may have been too simple for this population. Development of more complex scenarios for future studies will include additional auditory and visual distractors (e.g., wind sounds, areas of fog, increased ambient traffic), more unexpected events (e.g., animals crossing the highway), and additional tasks to simultaneously complete while driving beyond maintaining a consistent distance behind a lead vehicle with varying speeds on relatively straight roads.
The authors noted minimal missing data. Missing ICA data are attributed to individual differences in eye blinks, saccadic eye movements, and head position during scanning. The cutoff point of 50% for inclusion in the analyses of any performance value is conservative compared to other studies (Evans et al., 2017; Hershman et al., 2018; Massar et al., 2018; Reddy et al., 2018).
Implications for Nursing
These preliminary findings in combination with previously published qualitative research support oncology nurses opening and maintaining a dialogue with BCSs regarding issues they may be experiencing in their daily activities related to processing speed or visuospatial ability, such as driving. 
Conclusion
These study results provide preliminary evidence for the use of driving simulation to enhance the neurocognitive battery designed to measure cognitive effort in BCSs. Measurement of pupillary response during the driving simulation scenarios was achieved, and no symptoms of simulator sickness were exhibited. The results provide further evidence for the impact of cancer and cancer therapy on visuospatial ability that may translate to difficulty with driving performance (as evidenced by BCSs’ difficulty with collisions, speeding tickets, and out-of-lane time during the driving simulation). Further research is needed with more complex driving scenarios and a larger sample size to determine the appropriate level of difficulty for driving simulation to correlate with pupillary response in the BCS population. The ability to detect the increased cognitive effort necessary to compensate and function at a normal level is key to the validation of cognitive deficits in the BCS population. This information may be helpful for identifying accommodations needed to facilitate return to work. This validation also may be helpful to improve survivors’ loved ones’ understanding of the phenomenon, particularly when there is no outward sign of toxicity.
About the Author(s)
Jamie S. Myers, PhD, RN, AOCNS®, is a research associate professor in the School of Nursing at the University of Kansas in Kansas City; Nesreen Alissa, MS, is a research assistant in the School of Medicine at the University of Maryland in Baltimore; Melissa Mitchell, MD, PhD, is an associate professor in the Division of Radiation Oncology at the University of Texas MD Anderson Cancer Center in Houston; Junqiang Dai, MS, is a senior research analyst, Jianghua He, PhD, is an associate professor in the Department of Biostatistics and Data Science, and Sanghee Moon, BS, is a graduate research assistant in the Department of Physical Therapy and Rehabilitation Science, all at the University of Kansas Medical Center in Kansas City; Anne O’Dea, MD, is an associate professor in the Division of Medical Oncology and Jennifer Klemp, PhD, MPH, is an associate professor in the Division of Clinical Oncology and the Director of Cancer Survivorship, both at the University of Kansas Cancer Center in Kansas City; and Monica Kurylo, PhD, ABPP, is a professor in the Department of Psychiatry and Behavioral Sciences and Rehabilitation Medicine, Abiodun Akinwuntan, PhD, MBA, MPH, is a professor and Dean of the School of Health Professions, and Hannes Devos, PhD, is an assistant professor in the Department of Physical Therapy and Rehabilitation Science, all at the University of Kansas Medical Center. The study was funded by the University of Kansas Cancer Center Pilot Project Award and supported, in part, by a National Institutes of Health Clinical and Translational Science Award grant (UL1 TR002366) awarded to the University of Kansas. Akinwuntan and Devos co-invented the portable driving simulator used in the current study. Myers, Mitchell, O’Dea, Klemp, Kurylo, Akinwuntan, and Devos contributed to the conceptualization and design. Myers and Alissa completed the data collection. Alissa, Dai, and He provided statistical support. Myers, Alissa, Dai, He, Moon, O’Dea, Klemp, Akinwuntan, and Devos provided the analysis. Myers, Mitchell, O’Dea, Klemp, Kurylo, Akinwuntan, and Devos contributed to the manuscript preparation. Myers can be reached at jmyers@kumc.edu, with copy to ONFEditor@ons.org. (Submitted July 2019. Accepted September 10, 2019.)
References
Ahles, T.A., Root, J.C., & Ryan, E.L. (2012). Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. Journal of Clincal Oncology, 30(30), 3675–3686. https://doi.org/10.1200/jco.2012.43.0116
Akinwuntan, A.E., De Weerdt, W., Feys, H., Pauwels, J., Baten, G., Arno, P., & Kiekens, C. (2005). Effect of simulator training on driving after stroke: A randomized controlled trial. Neurology, 65(6), 843–850. https://doi.org/10.1212/01.wnl.0000171749.71919.fa
Akinwuntan, A.E., Devos, H., Baker, K., Phillips, K., Kumar, V., Smith, S., & Williams, M.J. (2014). Improvement of driving skills in persons with relapsing-remitting multiple sclerosis: A pilot study. Archives of Physical Medicine and Rehabilitation, 95(3), 531–537. https://doi.org/10.1016/j.apmr.2013.08.294
Akinwuntan, A.E., Wachtel, J., & Rosen, P.N. (2012). Driving simulation for evaluation and rehabilitation of driving after stroke. Journal of Stroke and Cerebrovascular Diseases, 21(6), 478–486. https://doi.org/10.1016/j.jstrokecerebrovasdis.2010.12.001
Asher, A., & Myers, J.S. (2015). The effect of cancer treatment on cognitive function. Clinical Advances in Hematology and Oncology, 13(7), 441–450.
Balk, S.A., Bertola, M.A., & Inman, V.W. (2013, June 19). Simulator sickness questionnaire: Twenty years later [Paper presentation]. Seventh International Driving Symposium on Human Factors in Driver Assessment, Training and Vehicle Design, Bolton Landing, New York, United States. https://doi.org/10.17077/drivingassessment.1498
Beatty, J. (1982). Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin, 91(2), 276–292. https://doi.org/10.1037/0033-2909.91.2.276
Chan, E., Pradhan, A.K., Pollatsek, A., Knodler, M.A., & Fisher, D.L. (2010). Are driving simulators effective tools for evaluating novice drivers’ hazard anticipation, speed management, and attention maintenance skills. Transportation Research Part F: Traffic Psychology and Behavior, 13(5), 343–353. https://doi.org/10.1016/j.trf.2010.04.001
Eckstein, M.K., Guerra-Carrillo, B., Miller Singley, A.T., & Bunge, S.A. (2017). Beyond eye gaze: What else can eyetracking reveal about cognition and cognitive development? Developmental Cognitive Neuroscience, 25, 69–91. https://doi.org/10.1016/j.dcn.2016.11.001
Evans, S., Dowell, N.G., Tabet, N., King, S.L., Hutton, S.B., & Rusted, J.M. (2017). Disrupted neural activity patterns to novelty and effort in young adult APOE-e4 carriers performing a subsequent memory task. Brain and Behavior, 7(2), e00612. https://doi.org/10.1002/brb3.612
Gonçalves, V.T., & Mansur, L.L. (2009). N-Back auditory test performance in normal individuals. Dementia and Neuropsychologia, 3(2), 114–117. https://doi.org/10.1590/s1980-57642009dn30200008
Granholm, E.L., Panizzon, M.S., Elman, J.A., Jak, A.J., Hauger, R.L., Bondi, M.W., . . . Kremen, W.S. (2017). Pupillary responses as a biomarker of early risk for Alzheimer’s disease. Journal of Alzheimer’s Disease, 56(4), 1419–1428. https://doi.org/10.3233/jad-161078
Hermelink, K., Küchenhoff, H., Untch, M., Bauerfeind, I., Lux, M.P., Bühner, M., . . . Münzel, K. (2010). Two different sides of ‘chemobrain’: Determinants and nondeterminants of self-perceived cognitive dysfunction in a propspective, randomized, multicenter study. Psycho-Oncology, 19(12), 1321–1328. https://doi.org/10.1002/pon.1695
Hershman, R., Henik, A., & Cohen, N. (2018). A novel blink detection method based on pupillometry noise. Behavior Research Methods, 50(1), 107–114. https://doi.org/10.3758/s13428-017-1008-1
Holohan, K.N., Von Ah, D., McDonald, B.C., & Saykin, A.J. (2013). Neuroimaging, cancer, and cognition: State of the knowledge. Seminars in Oncology Nursing, 29(4), 280–287. https://doi.org/10.1016/j.soncn.2013.08.008
Jacola, L.M., Willard, V.W., Ashford, J.M., Ogg, R.J., Scoggins, M.A., Jones, M.M., . . . Conklin, H.M. (2014). Clinical utility of the n-back task in functional neuroimaging studies of working memory. Journal of Clinical and Experimental Neuropsychology, 36(8), 875–886. https://doi.org/10.1080/13803395.2014.953039
Janelsins, M.C., Kesler, S.R., Ahles, T.A., & Morrow, G.R. (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry, 26(1), 102–113. https://doi.org/10.3109/09540261.2013.864260
Kahneman, D. (1973). Attention and effort. Prentice Hall.
Kahya, M., Moon, S., Lyons, K.E., Pahwa, R., Akinwuntan, A.E., & Devos, H. (2018). Pupillary response to cognitive demand in Parkinson’s Disease: A pilot study. Frontiers in Aging Neuroscience, 10, 90. https://doi.org/10.3389/fnagi.2018.00090
Kahya, M., Wood, T.A., Sosnoff, J.J., & Devos, H. (2018). Increased postural demand is associated with greater cognitive workload in healthy young adults: A pupillometry study. Frontiers in Human Neuroscience, 12, 288. https://doi.org/10.3389/fnhum.2018.00288
Kennedy, R.S., Lane, N.E., Berbaum, K.S., & Lilienthal, M.G. (1993). Simulator sickness questionnaire: An enhanced method for quantifying simulator sickness. International Journal of Aviation Psychology, 3(3), 203–220. https://doi.org/10.1207/s15327108ijap0303_3
Kim, A., Darakjian, N., & Finley, J.M. (2017). Walking in fully immersive virtual environments: An evaluation of potential adverse effects in older adults and individuals with Parkinson’s disease. Journal of NeuroEngineering and Rehabilitation, 14(1), 16. https://doi.org/10.1186/s12984-017-0225-2
Marshall, S.P. (2007). Identifying cognitive state from eye metrics. Aviation, Space, and Environmental Medicine, 78(5, Suppl.), B165–B175.
Massar, S.A.A., Lim, J., Sasmita, K., & Chee, M.W.L. (2019). Sleep deprivation increases the costs of attentional effort: Performance, preference and pupil size. Neuropsychologia, 123, 169–177. https://doi.org/10.1016/j.neuropsychologia.2018.03.032
Mencarelli, L., Neri, F., Momi, D., Menardi, A., Rossi, S., Rossi, A., & Santarnecchi, E. (2019). Stimuli, presentation modality, and load-specific brain activity patterns during n-back task. Human Brain Mapping, 40(13), 3810–3831. https://doi.org/10.1002/hbm.24633
Miller, K.M., Price, C.C., Okun, M.S., Montijo, H., & Bowers, D. (2009). Is the n-back task a valid neuropsychological measure for assessing working memory? Archives of Clinical Neuropsychology, 24(7), 711–717. https://doi.org/10.1093/arclin/acp063
Murphy, P.R., O’Connell, R.G., O’Sullivan, M., Robertson, I.H., & Balsters, J.H. (2014). Pupil diameter covaries with BOLD activity in human locus coeruleus. Human Brain Mapping, 35(8), 4140–4154. https://doi.org/10.1002/hbm.22466
Myers, J.S. (2012). Chemotherapy-related cognitive impairment: The breast cancer experience. Oncology Nursing Forum, 39, E31–E40. https://doi.org/10.1188/12.ONF.E31-E40
Myers, J.S., Kahya, M., Mitchell, M., Dai, J., He, J., Moon, S., . . . Devos, H. (2018). Pupillary response: Cognitive effort for breast cancer survivors. Supportive Care in Cancer, 27(3), 1121–1128. https://doi.org/10.1007/s00520-018-4401-0
Owen, A.M., McMillan, K.M., Laird, A.R., & Bullmore, E. (2005). n-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. https://doi.org/10.1002/hbm.20131
Player, L., Mackenzie, L., Willis, K., & Loh, S.Y. (2014). Women’s experiences of cognitive changes or ‘chemobrain’ following treatment for breast cancer: A role for occupational therapy? Australian Occupational Therapy Journal, 61(4), 230–240. https://doi.org/10.1111/1440-1630.12113
Ranchet, M., Morgan, J.C., Akinwuntan, A.E., & Devos, H. (2017). Cognitive workload across the spectrum of cognitive impairments: A systematic review of psychological measures. Neuroscience and Biobehavioral Reviews, 80, 516–537. https://doi.org/10.1016/j.neubiorev.2017.07.001
Ranchet, M., Orlosky, J., Morgan, J., Qadir, S., Akinwuntan, A.E., & Devos, H. (2017). Pupillary response to cognitive workload during saccadic tasks in Parkinson’s disease. Behavioral Brain Research, 327, 162–166. https://doi.org/10.1016/j.bbr.2017.03.043
Reddy, L.F., Reavis, E.A., Wynn, J.K., & Green, M.F. (2018). Pupillary responses to a cognitive effort task in schizophrenia. Schizophrenia Research, 199, 53–57. https://doi.org/10.1016/j.schres.2018.03.005
Redick, T.S., & Lindsey, D.R. (2013). Complex span and n-back measures of working memory: A meta-analysis. Psychonomic Bulletin and Review, 20(6), 1102–1113. https://doi.org/ 10.3758/s13423-013-0453-9
Vardaki, S., Devos, H., Beratis, I., Yannis, G., & Papageorgiou, S.G. (2016). Exploring the association between working memory and driving performance in Parkinson’s disease. Traffic Injury Prevetion, 17(4), 359–366. https://doi.org/10.1080/15389588.2015.1091926
Wang, C.A., McInnis, H., Brien, D.C., Pari, G., & Munoz, D.P. (2016). Disruption of pupil size modulation correlates with voluntary motor preparation deficits in Parkinson’s disease. Neuropsychologia, 80, 176–184. https://doi.org/10.1016/j.neuropsychologia.2015.11.019
Wefel, J.S., Vardy, J., Ahles, T.A., & Schagen, S.B. (2011). International cognition and cancer task force reommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncology, 12(7), 703–708. https://doi.org/10.1016/s1470-2045(10)70294-1
Yaple, Z.A., Stevens, W.D., & Arsalidou, M. (2019). Meta-analyses of the n-back working memory task: fMRI evidence of age-related changes in prefrontal cortex involvement across the adult lifespan. NeuroImage, 196, 16–31. https://doi.org/10.1016/j.neuroimage.2019.03.074




