Prospective Surveillance and Risk Reduction of Cancer Treatment–Related Lymphedema: Systematic Review and Meta-Analysis
Problem Identification: Secondary lymphedema is a chronic condition that may result from cancer-related treatments. Evidence is emerging on prospective surveillance and risk reduction.
Literature Search: Databases were systematically searched through April 1, 2019, for comparative studies evaluating interventions aiming to prevent lymphedema in patients with cancer.
Data Evaluation: A random-effects model was used to perform meta-analysis, when appropriate.
Synthesis: A total of 26 studies (4,095 patients) were included, with 23 providing data sufficient for meta-analysis. Surveillance programs increased the likelihood of detecting lymphedema. Physiotherapy, exercise programs, and delayed exercise reduced the incidence of lymphedema.
Implications for Research: Future research should standardize (a) evidence-based interventions to reduce the development of lymphedema and increase the likelihood of early detection and (b) outcome measures to build a body of evidence that leads to practice change.
Supplemental material can be found at https://onf.ons.org/supplementary-material-systematic-review-cancer-treatment-related-lymphedema
Jump to a section
Secondary lymphedema, or lymphedema that is caused by injury or damage to the lymphatic system, is a chronic, progressive, and debilitating condition that is often attributable to cancer treatments, such as surgery, particularly involving lymph nodes; radiation therapy; and chemotherapy. It often presents as fluid accumulation in the interstitial tissue spaces as a result of the injured or damaged lymphatic system being unable to process the fluid as it once did. This fluid accumulation can progress to swelling of the arm, breast, shoulder, neck, torso, or lower extremities (Cheville et al., 2003). Secondary lymphedema is often diagnosed following treatment for breast cancer, but it is also frequently diagnosed in patients after treatment for melanoma or gynecologic or head and neck cancers. Current guidelines support education and baseline measurements with prospective assessment for early diagnosis and treatment of secondary lymphedema (Gradishar & Salerno, 2016), but the specifics of what comprises effective prospective surveillance are not well described.
Early detection of secondary lymphedema leads to improved patient outcomes and decreases the risk of lymphedema progressing to a persistent stage. Prospective surveillance is a model to identify physical changes that can lead to lymphedema so that interventions can begin early. For example, prospective surveillance for women with breast cancer involves education, support, empowerment, monitoring, and management of the physical and psychological effects of cancer treatment (Koelmeyer et al., 2019). Evidence is emerging that the detection of subclinical lymphedema through prospective surveillance and early interventions has the potential to reduce secondary lymphedema symptoms and the condition’s progression to persistent, clinical lymphedema (Koelmeyer et al., 2019; Soran et al., 2014). Patients who are at high risk for lymphedema after their treatments for cancer are important targets of these programs. A study by Whitworth et al. (2018) using a structured surveillance protocol for high-risk women with breast cancer found that only 3% of patients progressed to unresolved lymphedema.
Oncology healthcare professionals should play an active role in identifying patients at risk for developing lymphedema by employing evidence-based strategies for surveillance and risk reduction. In this systematic review, the effects of interventions for prospective surveillance and risk reduction on the development of lymphedema are evaluated. This systematic review was performed to support the development of a clinical practice guideline by the Oncology Nursing Society (ONS).
Methods
The current authors conducted a series of systematic reviews to address the prevention and risk reduction of secondary lymphedema in patients with cancer. The systematic reviews were performed to inform the ONS Guidelines™ on secondary lymphedema. A guideline panel used these results to develop recommendations on the care of individuals with lymphedema (Armer et al., 2020). The systematic review and meta-analysis methodology are consistent with the Cochrane Handbook for Systematic Reviews of Interventions. This report is consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009).
Development of Research Questions
The guideline panel, in collaboration with methodologists, developed and prioritized symptom management questions according to the Patient, Intervention, Comparator, and Outcome (PICO) framework (Guyatt, Oxman, Kunz, et al., 2011) (see Table S1 in the Appendix). The panel identified as many as seven outcomes for each PICO question to consider in the review of the evidence. Across many of the PICO questions, several of the same outcomes were deemed critical for review.
Data Sources and Searches
A comprehensive search of databases—including MEDLINE®; Embase®; Cochrane Central Register of Controlled Trials; Cochrane Database of Systematic Reviews; Scopus®; and epub ahead of print, in-process, and other non-indexed citations—was conducted from database inception to April 1, 2019, by a medical reference librarian with input from the guideline panel and the methodologists. Reference mining from relevant systematic reviews, conference proceedings, and clinical trial registries was applied to identify additional studies.
Selection Criteria
Randomized and nonrandomized comparative studies were included if they studied the prevention of lymphedema in patients with cancer who had had surgery, chemotherapy, or radiation therapy and were at risk for lymphedema. Noncomparative studies, abstracts, reviews, and panel suggestions or guidelines were excluded. Studies not published in English were excluded.
The studies were screened by two independent reviewers and any discrepancies were resolved by consensus through discussion with a third reviewer.
Data Extraction and Risk-of-Bias Assessment
The data extraction and methodologic quality assessment were performed by two independent reviewers. The authors developed standardized data extraction forms that included baseline characteristics, intervention and comparison description, duration, primary outcomes, and other relevant outcomes. To assess the risk of bias, items from the Newcastle-Ottawa scale (Wells et al., 2017) were used for nonrandomized studies, whereas the Cochrane Collaboration risk-of-bias tool (Higgins & Green, 2011) was used for randomized controlled trials (RCTs).
Outcome Measures
The outcomes were measured at the point of the termination of the interventions or the earliest point reported after the interventions, because this most closely represents the indefinite use of interventions to prevent and manage lymphedema in practice. The primary outcomes are the number of patients who developed lymphedema, limb volume change, and percent volume change. Other relevant secondary outcomes, including change in physical activity, grip strength, and range of motion, were also extracted.
Data Synthesis and Analysis
Meta-analysis was conducted, when appropriate, using the random-effects model. For binary outcomes, relative risk (RR) and 95% confidence interval (CI) were calculated. For continuous outcomes, weighted mean differences (WMDs) and 95% CI in studies reporting outcomes with the same scale were calculated, as were standardized mean differences (SMDs) and 95% CI in studies reporting outcomes with different scales. Outcomes that cannot be pooled were summarized narratively.
Subgroup analysis was performed based on the types of interventions. For statistical heterogeneity, I2 was used, with values greater than 50% indicating substantial heterogeneity (Higgins, 2003). Publication bias evaluation and additional subgroup and sensitivity analyses were not feasible because few studies (fewer than 10) were incorporated in any of the subgroups. All statistical analyses were performed with Stata, version 15.1.
Grading the Certainty of Evidence
The GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach was applied to rate the certainty of evidence (CoE). Evidence from RCTs starts at high initial certainty, whereas evidence from nonrandomized studies starts at low initial certainty. The certainty in the body of evidence was then rated down for concerns because of risk of bias, inconsistency (e.g., heterogeneity), indirectness, imprecision, or publication bias (Guyatt, Oxman, Akl, et al., 2011).
Results
The authors identified 1,122 unique articles from the database search, and 211 full-text articles were screened for inclusion. In total, 30 articles, which include 26 unique studies (19 RCTs) with 4,095 patients, were included in the review. One study examined patients with melanoma or urogenital cancers, and all other studies examined patients with breast cancer. The flow chart of study selection is shown in Figure 1. 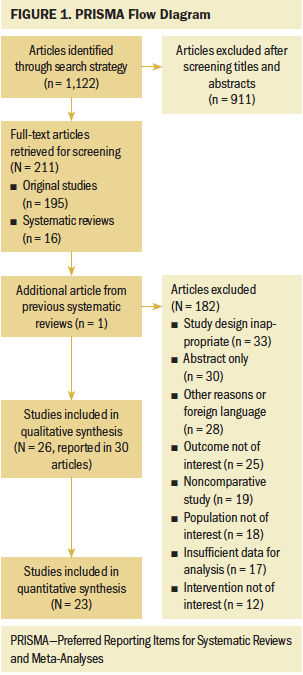
Baseline Characteristics and Risk of Bias
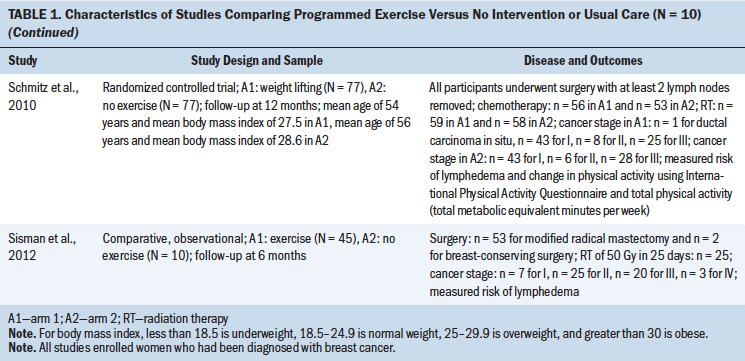
Characteristics of the included studies are summarized in the supplemental material (see Tables S2 and S4–S11 in the Appendix). One study reported interventions comparing progressive resistance training versus usual care; one compared compression versus no compression; four compared manual lymphatic drainage (MLD) versus no MLD; two compared MLD versus exercise; nine compared exercise versus no exercise; two compared delayed exercise versus early exercise; two compared physiotherapy versus no physiotherapy; and five compared surveillance, education, or clinical care versus no intervention or standard care. The studies included in the meta-analysis are shown in Table 1, with CoE of the studies that compared programmed exercise versus no intervention or usual care depicted in Table 2. 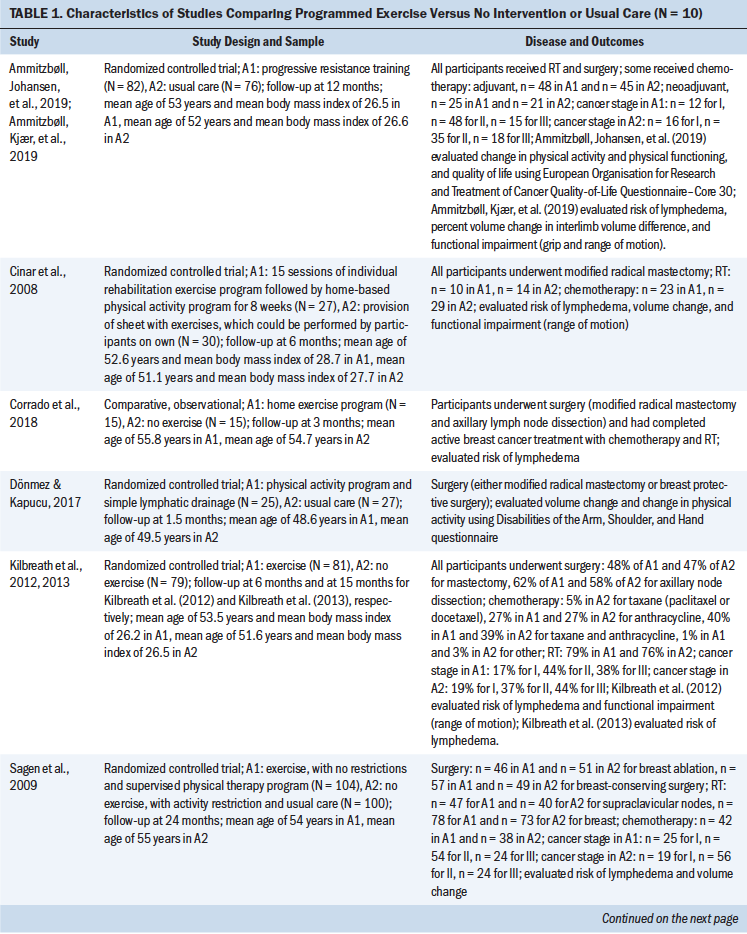
The overall risk of bias of the randomized trials was considered to be moderate because about half of the trials did not describe clear or adequate allocation concealment or random sequence generation. The majority of the randomized trials and the nonrandomized studies did not report blinding outcome assessors, participants, or personnel. However, blinding in this condition is challenging (see Figures S5 and S6 in the Appendix). 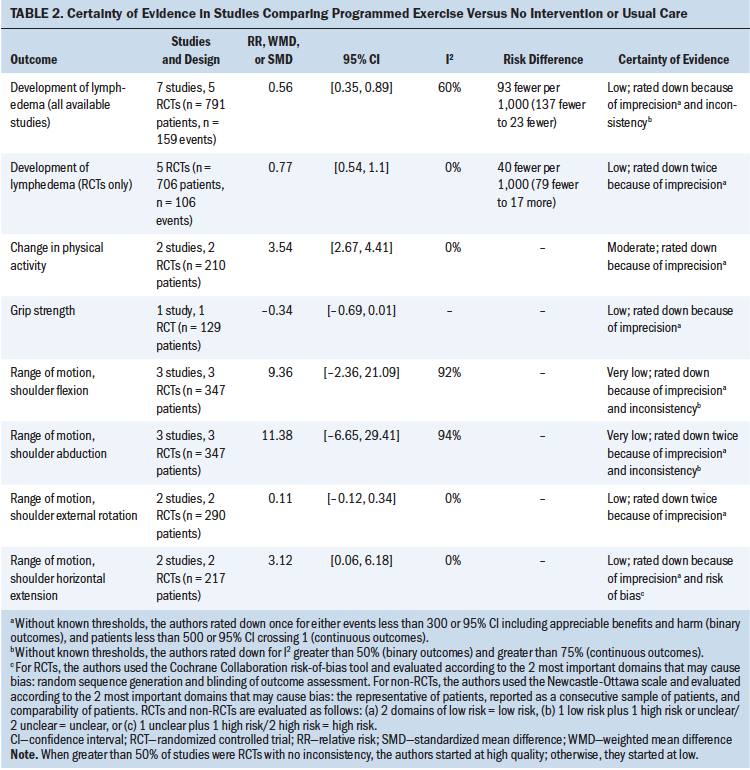
Risk of Lymphedema
The results of subgroup meta-analysis by comparisons and the CoE are summarized in Table 3, Figures S1–S4, and Table S3. Among various interventions, physiotherapy, exercise programs, and delayed exercise programs (one to two weeks following surgery) reduced the development of lymphedema (RR = 0.31, 95% CI [0.15, 0.64], I2 = 0.0%, CoE = moderate; RR = 0.56, 95% CI [0.35, 0.89], I2 = 59.8%, CoE = low; RR = 0.47, 95% CI [0.23, 0.96], CoE = low, respectively) in patients with cancer who were at risk for lymphedema after surgery and/or radiation therapy. Surveillance programs increased the likelihood of detection of lymphedema according to a single study (RR = 2.06, 95% CI [1.54, 2.76], CoE = very low) (Yang et al., 2016). The results did not indicate significant improvement associated with compression, MLD, and preoperative lymphoscintigraphy. 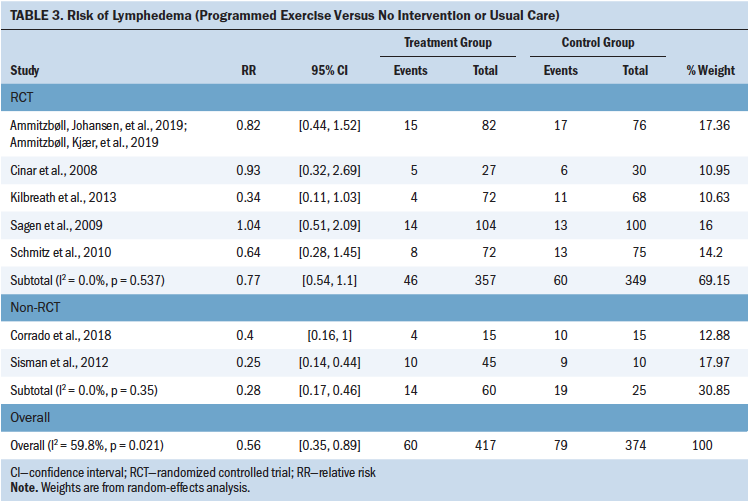
Change in Physical Activity
This outcome was informed by seven studies: five RCTs and two nonrandomized studies (Ammitzbøll, Johansen, et al., 2019; Cho et al., 2016; Devoogdt et al., 2018; Dönmez & Kapucu, 2017; Sato et al., 2014; Singh et al., 2013; Temur & Kapucu, 2019). Sample sizes ranged from 52 to 160 participants, and all included patients with breast cancer who had received surgery with or without radiation therapy or chemotherapy. Types of physical activity included home-based programs, physical therapy, and progressive resistance training. Compared with no intervention or usual care, programmed exercise with or without MLD significantly improved physical function (SMD = 3.54, 95% CI [2.67, 4.41], I2 = 0.0%, CoE = moderate). Other interventions did not show statistically significant effect.
Grip Strength
This outcome was informed by three studies (Ammitzbøll, Johansen, et al., 2019; Bendz & Fagevik Olsén, 2002; Todd et al., 2008). Sample sizes ranged from 116 to 205 and included patients with breast cancer who had received surgery with or without radiation therapy or chemotherapy. Interventions included progressive resistance training and either early or delayed arm and shoulder exercises. The current authors did not find a statistically significant difference in grip strength in two analyzed intervention groups (programmed exercise versus no intervention or usual care, and delayed versus early exercise).
Range of Motion
Only one RCT (Bendz & Fagevik Olsén, 2002) showed that delayed exercise was more effective in improving shoulder flexion (WMD = –5, 95% CI [–8.44, –1.56], CoE = low), compared with early exercise. In terms of shoulder abduction, external rotation, and horizontal extension, the current authors did not find significant benefits associated with any intervention. However, MLD and programmed exercise demonstrated worsening of shoulder external rotation (SMD = 0.48, 95% CI [0.17, 0.8], CoE = low) and horizontal extension (WMD = 3.12, 95% CI [0.06, 6.18], I2 = 0.0%, CoE = low).
Other Outcomes
Two studies reported the risk of transient versus persistent lymphedema (Devoogdt et al., 2018; Yang et al., 2016). Devoogdt et al. (2018) reported the cumulative incidence of lymphedema using four definitions at different time points following surgery to explore the effectiveness of MLD versus no MLD. They compared the RR before and after the exclusion of patients with transient lymphedema and found no difference. In another prospective study of a surveillance program for lymphedema management, Yang et al. (2016) found that among the 203 patients who developed secondary lymphedema during the follow-up period, 131 (65%) resolved or improved after six months (defined as having reversible lymphedema), with 101 in the surveillance group and 30 in the control group. This study suggests that a surveillance program for lymphedema management may improve lymphedema prevention when compared to standard of care and that patients may be evaluated in a lymphedema clinic within one month of surgery with visits every three months for the first year (Yang et al., 2016). No studies reported missed work, cost for surveillance, or the number of patients referred to lymphedema specialists.
Discussion
This systematic review focused on prospective surveillance and risk reduction and broadly found that all interventions have some benefit over doing nothing. The certainty in the evidence is variable, but evidence shows that actively doing something is better than pursuing no treatment.
Several interventions show moderate to substantial reduction in the development of lymphedema. Programmed exercise, with or without MLD, had moderate benefits to delay the development of lymphedema when compared to standard of care. MLD alone also had moderate benefits to delay the development of lymphedema when compared to no MLD. Physiotherapy, with or without MLD, was found to have a significant reduction in the development of lymphedema when compared to no physiotherapy. Delayed exercise had a moderate reduction in the development of lymphedema compared to early exercise. Surveillance led to a twofold increase in detection of lymphedema compared to no surveillance.
Strengths and Limitations
The certainty of evidence is limited by heterogeneity of included patients and the interventions used to prevent cancer treatment–related lymphedema. The number of eligible studies for each intervention or outcome was low. Therefore, confidence in the meta-analytic estimates is low. The strengths of this review include following a rigorous and transparent methodology for the identification of eligible studies. The current authors included randomized and nonrandomized studies, which can increase the risk of bias; however, this was necessary to identify the best available evidence at the present time.
Relation to Other Studies
This review identified that physical therapy, exercise, and delayed exercise after surgery led to a reduction in the development of lymphedema, which is consistent with current research. A systematic review of the preventive effect of exercise on secondary lymphedema (Baumann et al., 2018) concluded that progressive strength training, physiotherapy, physical therapy, and/or kinesiotherapy are safe and can prevent lymphedema after treatment for cancer. The growing synthesis of evidence on the benefits of exercise in the prevention of lymphedema should be a call to action for clinicians to incorporate this evidence into practice.
The current review also identified that prospective surveillance led to an increase in the detection of lymphedema. This surveillance, beginning with a preoperative assessment, has the potential to detect subclinical lymphedema and allows for early intervention. Prospective surveillance models for monitoring and assessment may include circumferential arm measurement, perometry, bioimpedance, exercise, compression garments, and referral for complete decongestive therapy (Ostby et al., 2014). Despite the known benefit of screening and measurement of lymphedema, criteria for when to refer for treatment are not universal (Bernas, 2013).
Implications for Practice
This systematic review provides a rationale for preemptive surveillance and risk reduction strategies that can be initiated by oncology nurses and healthcare professionals. Therefore, an important goal of survivorship care is to employ interventions to slow the development of lymphedema or to mitigate the progression to more severe grades of lymphedema. The most common cause of secondary lymphedema in the United States is treatment for cancer (Executive Committee, 2016; Rockson et al., 2019). Patients who undergo surgical treatment for cancer that involves the removal of lymph nodes are at risk for lymphedema, although other treatments, such as radiation therapy and chemotherapy, can also increase risk. Such patients would be an ideal target for early intervention.
Despite the prevalence and significance of secondary lymphedema, important gaps in knowledge persist. The emergence, incidence, and prevalence of cancer treatment–related lymphedema are not fully understood (Executive Committee, 2016; Ostby et al., 2014; Rockson et al., 2019). The current research lacks use of standard scales to measure and document lymphedema and its outcomes (e.g., quality of life). Research has identified that one method of measurement, landmark-based simulated circumferential measurement, may be the preferred method for limb volume assessment in screening programs. Future research, including prospective screening trials that directly compare techniques to quantify upper extremity volume, will be clinically meaningful (Sun et al., 2018). Rigorously designed RCTs with standard interventions and outcome measurements will provide guidance to clinicians and patients in the survivorship period following breast cancer treatment.
Conclusion
Early diagnosis of lymphedema may mitigate symptoms and slow or halt progression of the condition to an irreversible stage. This systematic review identified evidence-based interventions to reduce the development of lymphedema and increase the likelihood of early detection.
About the Author(s)
Jingyi Francess Ding, MD, and Bashar Hasan, MD, are both postdoctoral research fellows in the Evidence-Based Practice Research Program and in the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, both at the Mayo Clinic in Rochester, MN; Konstantinos Malandris, MD, is a doctor in the Clinical Research and Evidence-Based Medicine Unit at Aristotle University of Thessaloniki in Greece; Magdoleen H. Farah, MBBS, is a postdoctoral research fellow in the Evidence-Based Practice Research Program and in the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, both at the Mayo Clinic; Apostolos Manolopoulos, MD, MSc, is a postdoctoral research fellow in Clinical Research and Evidence-Based Medicine Unit at Aristotle University of Thessaloniki; Pamela K. Ginex, EdD, RN, OCN®, is the senior manager of evidence-based practice and inquiry at the Oncology Nursing Society in Pittsburgh, PA; Allison B. Anbari, PhD, RN, is an assistant research professor in the Sinclair School of Nursing at the University of Missouri in Columbia; Tarek Nayfeh, MD, is a postdoctoral research fellow and Moutie Rami Rajjoub is a research assistant, both in the Evidence-Based Practice Research Program and in the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, both at the Mayo Clinic; Raed Benkhadra, MD, is a postdoctoral research fellow in the Department of Internal Medicine at Allegheny General Hospital in Pittsburgh; Larry J. Prokop, MLS, is a librarian at the Mayo Clinic; Rebecca L. Morgan, PhD, MPH, is an assistant professor in the Department of Health Research Methods, Evidence, and Impact at McMaster University in Hamilton, Ontario, Canada; and M. Hassan Murad, MD, MPH, is a professor of medicine in the Division of Preventive, Occupational, and Aerospace Medicine at the Mayo Clinic. Development of this review was wholly funded by the Oncology Nursing Society, a nonprofit organization that represents oncology nurses. No honoraria were provided. Ding, Hasan, Malandris, Farah, Ginex, Nayfeh, Morgan, and Murad contributed to the conceptualization and design. Ding, Hasan, Malandris, Farah, Manolopoulos, Anbari, Nayfeh, Rajjoub, Benkhadra, Prokop, and Murad contributed to the data collection. Ding, Hasan, and Murad provided statistical support. Ding, Hasan, Ginex, Anbari, Morgan, and Murad provided the analysis. Ding, Hasan, Farah, Ginex, Anbari, Prokop, Morgan, and Murad contributed to the manuscript preparation. Murad can be reached at murad.mohammad@mayo.edu, with copy to ONFEditor@ons.org. (Submitted April 2020. Accepted June 18, 2020.)




