Association Between Physical Activity Levels and Chemotherapy-Induced Peripheral Neuropathy Severity in Cancer Survivors
Objectives: To evaluate for differences in demographic and clinical characteristics, as well as subjective and objective measures of chemotherapy-induced peripheral neuropathy (CIPN), among different exercise groups.
Sample & Setting: Cancer survivors (N = 290) were recruited from throughout the San Francisco Bay Area.
Methods & Variables: Based on the recommended 150 minutes or more of exercise per week, survivors were classified into the no exercise (NoEx), less exercise (LessEx), or recommended exercise (RecEx) group. Survivors completed self-report questionnaires and underwent sensory and balance testing.
Results: Compared to the RecEx group, survivors in the NoEx group had less education, were less likely to be married/partnered, had a lower household income, had a higher level of comorbidity, and had poorer functional status. No differences were found among the groups in CIPN duration; pain intensity scores; or changes in light touch, cold, and pain sensations.
Implications for Nursing: Clinicians can recommend walking as a therapeutic option for survivors with CIPN and refer them to physical therapy.
Jump to a section
Chemotherapy-induced peripheral neuropathy (CIPN) occurs in 30%–50% of cancer survivors (Kerckhove et al., 2017; Vollmers et al., 2018), has negative effects on patient outcomes (Chan et al., 2019; Kneis et al., 2019), and is associated with an increased risk of falls (Kneis et al., 2019). Although duloxetine is the only drug recommended to decrease CIPN pain (Hershman et al., 2014), a growing body of evidence suggests that regular physical activity is a safe and low-cost intervention to decrease the severity of CIPN symptoms (Andersen Hammond et al., 2019; Streckmann et al., 2014). The mechanisms that underlie the efficacy of exercise are not completely understood, but findings from preclinical studies suggest that physical exercise can decrease levels of proinflammatory cytokines and neurotrophins, increase GABAergic inhibition, increase the upregulation of analgesic factors, activate the descending serotonin inhibitory pathway, and increase the release of endogenous opioids (Andersen Hammond et al., 2019; Cooper et al., 2016; Kami et al., 2017).
In terms of clinical research, only three studies have evaluated the effects of exercise on CIPN symptoms in cancer survivors (Kneis et al., 2019; McCrary et al., 2019; Wonders et al., 2013). In one study (Wonders et al., 2013), breast cancer survivors with CIPN (n = 20) were asked to follow a 10-week home-based exercise program that included walking and resistance exercises. The number of survivors who reported unpleasant skin sensations, abnormal sensitivity to touch, and sudden bursts of pain decreased following the intervention. In the second 12-week study that compared endurance and balance training (n = 18) to only endurance training (n = 19) (Kneis et al., 2019), although no between-group differences were found in functional performance, both groups reported decreases in sensory, motor, and autonomic scores on the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–CIPN 20-Item Scale (CIPN20) (Postma et al., 2005). In another study that evaluated the effects of an eight-week multimodal exercise intervention on CIPN symptoms and functional deficits in 29 survivors with CIPN (McCrary et al., 2019), significant improvements in dynamic balance and CIPN symptoms were found following the intervention. Although sample sizes were small and the exercise interventions were diverse, these findings provide preliminary evidence of the beneficial effects of exercise for CIPN.
Another approach to determine the efficacy of exercise is to evaluate for differences in CIPN characteristics in survivors who self-report that they do or do not meet the minimum recommended levels of physical exercise (i.e., 150 minutes or more per week) (Office of Disease Prevention and Health Promotion, 2018). In the first of two studies that used this approach (Wonders & Drury, 2012), of the 134 women with breast cancer and CIPN, only 15.6% reported meeting the physical exercise recommendation. Of note, only 15% of the patients who exercised reported experiencing pain, compared to 72% of the sedentary patients. In another study of colorectal cancer survivors (Mols et al., 2015), although a definitive diagnosis of CIPN was not confirmed, in survivors who received chemotherapy (n = 506), not meeting the recommended level of physical activity was associated with increased rates of CIPN symptoms.
Although these two studies suggested positive relationships between self-reported levels of physical activity and decreases in CIPN symptoms (Mols et al., 2015; Wonders & Drury, 2012), neither study used both subjective and objective measures of CIPN. Therefore, the current study aimed to evaluate for differences in demographic and clinical characteristics, subjective and objective measures of CIPN, and measures of balance among a sample of 290 cancer survivors with CIPN who were classified into one of three exercise groups using the recommendation for physical activity from the Office of Disease Prevention and Health Promotion’s (2018) Healthy People 2020 report. The authors hypothesized that lower levels of exercise would be associated with worse scores on subjective and objective measures of CIPN and more balance problems.
Methods
Sample and Setting
The current analysis is part of a larger study, funded by the National Cancer Institute, that evaluated CIPN in cancer survivors. The methods for the parent study were described in detail by Miaskowski et al. (2017). Survivors were recruited from throughout the San Francisco Bay Area. Those with CIPN met the following inclusion criteria:
• Aged 18 years or older
• Had received a platinum and/or a taxane compound
• Had completed their course of chemotherapy three months or more prior to enrollment
• Had changes in sensation and/or pain in their feet and/or hands of three months’ duration or more following the completion of chemotherapy
• Had a rating of 3 or more on a 0–10 numeric rating scale (NRS) for any one of the following sensations from the Pain Quality Assessment Scale (PQAS)(Jensen et al., 2006): numb, tender, shooting, sensitive, electrical, tingling, radiating, throbbing, cramping, itchy, or unpleasant
• If they had pain associated with CIPN, had an average pain intensity score in their feet and/or hands of 3 or greater on a 0–10 NRS
• Had a Karnofsky Performance Status (KPS) score of 50 or greater
• Were able to read, write, and understand English
Survivors were excluded if they had peripheral vascular disease, vitamin B12 deficiency, thyroid dysfunction, HIV neuropathy, another painful condition that was difficult for them to distinguish from their CIPN, a hereditary sensory or autonomic neuropathy, and/or a hereditary mitochondrial disorder. Of the 1,450 survivors who were screened, 754 were enrolled and 623 (423 with CIPN and 200 without CIPN) completed the self-report questionnaires and the study visit. For this analysis, complete data on regular exercise were available from 290 survivors with CIPN.
Procedure
Research nurses screened and consented the survivors via telephone, sent the self-report questionnaires and asked the survivors to complete them prior to their study visit, and scheduled an in-person assessment. At the assessment, written informed consent was obtained, questionnaires were reviewed for completeness, and objective measurements were done.
Measures
Demographic and clinical characteristics: Survivors provided information on demographic characteristics and completed the Alcohol Use Disorders Identification Test (AUDIT) (Babor et al., 2001), the KPS scale (Karnofsky, 1977), and the Self-Administered Comorbidity Questionnaire (SCQ) (Sangha et al., 2003). The AUDIT assesses alcohol consumption, alcohol dependence, and the consequences of alcohol abuse in the past 12 months (Babor et al., 2001). The SCQ consists of 13 common medical conditions that are simplified into language that can be understood without any prior medical knowledge. Patients were asked to indicate if they had the condition, if they received treatment for it, and if it limited their activities. A patient can receive a maximum of three points for each condition. The total SCQ score can range from 0–39 (Sangha et al., 2003).
Evaluation of regular exercise: Survivors completed a six-item exercise questionnaire that asked them to report whether they exercised on a regular basis, what types of physical activity they engaged in at the present time (e.g., walking, swimming), how many days per week they exercised, how many times per day they exercised, and the duration and intensity of each session. Based on responses to this questionnaire, the following three exercise groups were created: no exercise (NoEx), less than 150 minutes per week (LessEx), and 150 or more minutes per week (RecEx).
Pain questionnaires: Separate assessments were done for pain intensity and quality ratings for the hands and feet. A detailed CIPN history was obtained using a questionnaire from the authors’ previous (Langford et al., 2015; Posternak et al., 2016) and ongoing studies. Information was obtained on the date of pain onset and its interference level with function. Average and worst pain intensity during the past 24 hours was assessed using an NRS ranging from 0 (no pain) to 10 (worst pain imaginable) (Downie et al., 1978).
The 20-item PQAS was used to assess the qualities associated with CIPN (Jensen et al., 2005, 2006). Sixteen items evaluated the magnitude of the different pain quality descriptors (e.g., sharp, hot, aching, cold), measured on an NRS ranging from 0 to 10. Four items evaluated global and spatial pain qualities. In studies of various types of neuropathic pain, the PQAS has well-established validity and reliability (Jensen et al., 2005, 2006).
Sensation: Light touch was evaluated with Semmes-Weinstein monofilaments (Bell-Krotoski, 2002). A Tip Therm® rod was used to evaluate cold sensation (Papanas & Ziegler, 2011; Viswanathan et al., 2002). A Neurotip™ evaluated pain sensation (Papanas & Ziegler, 2011). A biothesiometer assessed vibration threshold (Garrow & Boulton, 2006). For all measures of sensation, the upper and lower extremities on the dominant side were tested.
Balance: To assess balance, self-report questions from the Chemotherapy-Induced Peripheral Neuropathy Assessment Tool were used (Tofthagen et al., 2011). Objective measures of balance were the Timed Up and Go (TUG) test (Mathias et al., 1986) and the Fullerton Advanced Balance (FAB) test (Hernandez & Rose, 2008; Rose et al., 2006).
Data Analysis
Data were analyzed using IBM SPSS Statistics, version 23.0. For survivors’ demographic and clinical characteristics, descriptive statistics and frequency distributions were calculated. For the four measures of sensation (light touch, cold, pain, and vibration), composite scores were created over all sites that were tested on the dominant upper and lower extremities. The number of sites with loss of each sensation was summed for light touch, cold, and pain. The mean score across the sites was calculated for vibration.
The three exercise groups were created using the survivors’ responses to the exercise questionnaire. Survivors who responded “no” to the question about whether they exercised on a regular basis were assigned to the NoEX group. The remaining two groups (survivors who exercised less than 150 minutes per week [LessEx] and survivors who exercised for the recommended 150 minutes or more per week [RecEx]) were assigned based on a calculation of the number of times they exercised per week, the number of times per day that they exercised, and the duration of the exercise sessions.
Differences among the three exercise groups in demographic and clinical characteristics, as well as subjective and objective measures of CIPN, were evaluated using analyses of variance, chi-square analyses, or Kruskal–Wallis tests. For the Bonferroni-corrected post-hoc contrasts, a p value of less than 0.0167 (i.e., 0.05/3) was considered statistically significant.
Results
Classification of the Exercise Groups
Of the 290 survivors with CIPN who completed the exercise questionnaire, 21% were classified in the NoEx group, 45% in the LessEx group, and 35% in the RecEx group. As shown in Figures 1–4, compared to the RecEx group, patients in the LessEx group exercised for fewer minutes per session and for fewer total minutes per week and participated in less intense exercise and for fewer days per week (all, p < 0.001). Figure 5 illustrates the differences in the percentages of patients in the two exercise groups who engaged in different types of exercise. 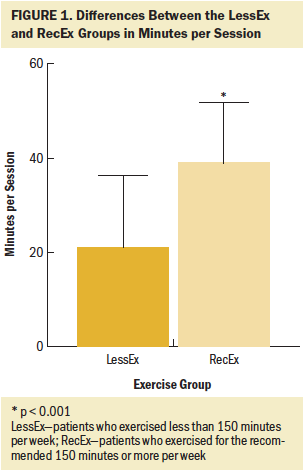
Differences in Demographic and Clinical Characteristics
As shown in Table 1, compared with the RecEx group, survivors in the NoEx group had completed significantly fewer years of education and were less likely to be married or partnered. In addition, compared to the other two exercise groups, survivors in the NoEx group had significantly lower annual household incomes. 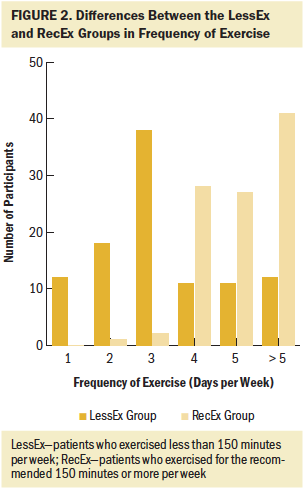
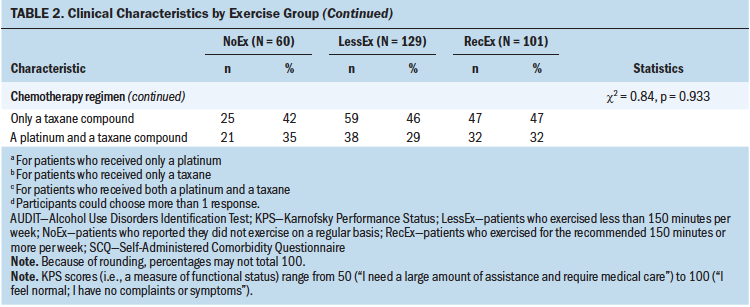
In terms of clinical characteristics (see Table 2), compared to the other two groups, survivors in the NoEx group had a significantly lower KPS score and a higher body mass index (BMI). In addition, compared to the RecEx group, survivors in the NoEx group had a significantly higher number of comorbidities and a higher SCQ score. 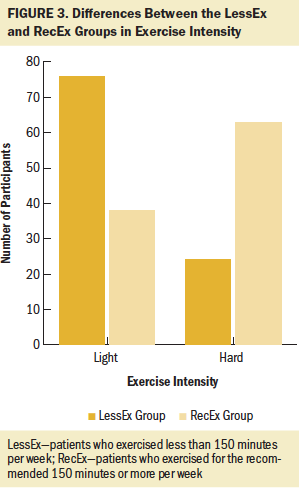
Differences in Self-Reported Pain Characteristics
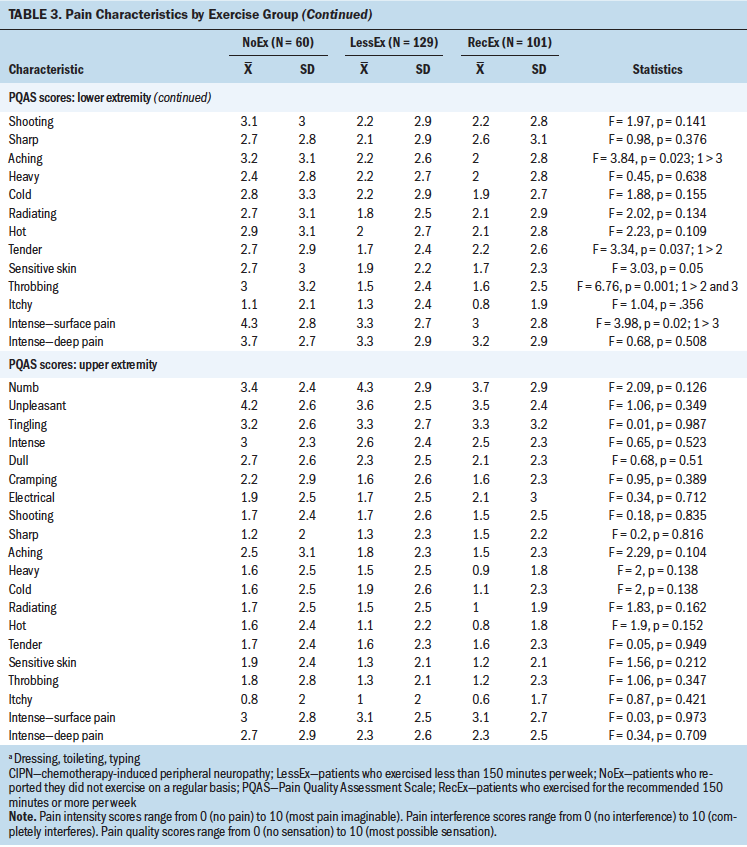
In terms of pain characteristics, no differences were found among the exercise groups in terms of duration of CIPN and pain intensity scores. However, in terms of interference scores in the lower extremities, compared to the RecEx group, survivors in the NoEx group reported significantly higher interference scores for balance, walking ability, normal work, sleep, and overall interference. In addition, compared to the other two groups, survivors in the NoEx group reported significantly higher interference scores for enjoyment of life. In terms of pain interference scores in the upper extremities, compared to the RecEx group, survivors in the NoEx group reported significantly higher interference scores for enjoyment of life and sleep. 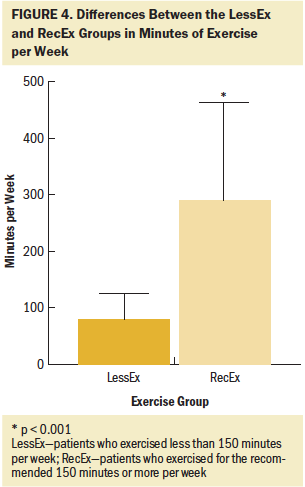
Table 3 summarizes the differences among the three exercise groups in pain quality scores in the upper and lower extremities. In terms of the lower extremities, compared to the RecEx group, survivors in the NoEx group reported significantly higher PQAS scores for the following qualities: unpleasant, intense, aching, throbbing, and intense surface pain. Compared with the LessEx group, the NoEx group reported significantly higher PQAS scores in the lower extremities for throbbing and tender qualities. For the upper extremities, no significant differences were found among the three groups for any of the PQAS scores. 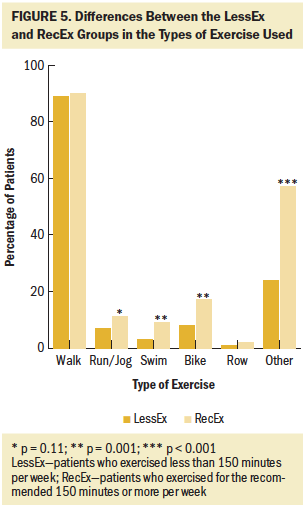
Differences in Objective Measures of Sensation
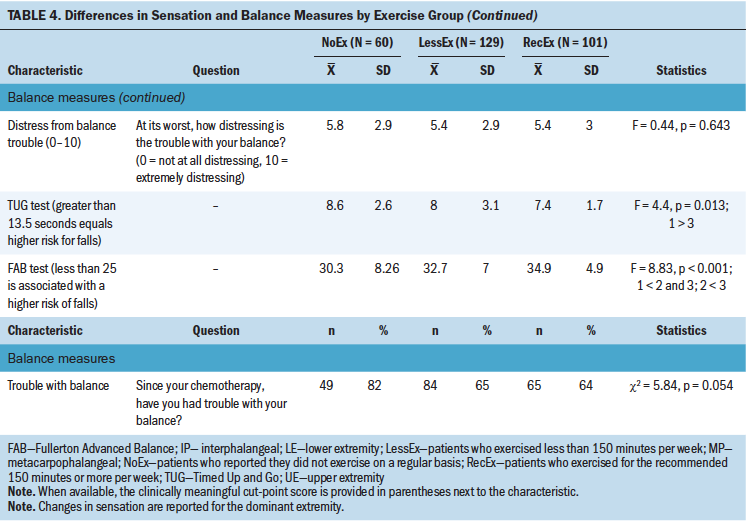
As shown in Table 4, compared to the RecEx group, survivors in the NoEx group had significantly higher vibration scores in the lower extremities. No statistically significant differences were found among the three exercise groups for any of the other objective measures of sensation. 
Differences in Balance
In terms of objective measures of balance, compared with the RecEx group, survivors in the NoEx group had significantly higher TUG scores. In addition, compared to the other two groups, survivors in the NoEx group had significantly lower FAB scores. Compared to survivors in the RecEx groups, survivors in the LessEx group had significantly lower FAB scores. 
Discussion
This study is the first to evaluate for differences in demographic and clinical characteristics, subjective and objective measures of CIPN, and measures of balance among cancer survivors with CIPN who were categorized using self-reported levels of exercise. Consistent with prior reports (Kneis et al., 2019; McCrary et al., 2019; Mols et al., 2015; Wonders et al., 2013), the current findings support the authors’ hypothesis that lower levels of exercise would be associated with worse scores for both subjective and objective measures of CIPN, as well as more problems with balance. Compared to the survivors who met the Healthy People 2020 exercise recommendation (Office of Disease Prevention and Health Promotion, 2018), survivors in the NoEx group had worse scores for the majority of the measures. 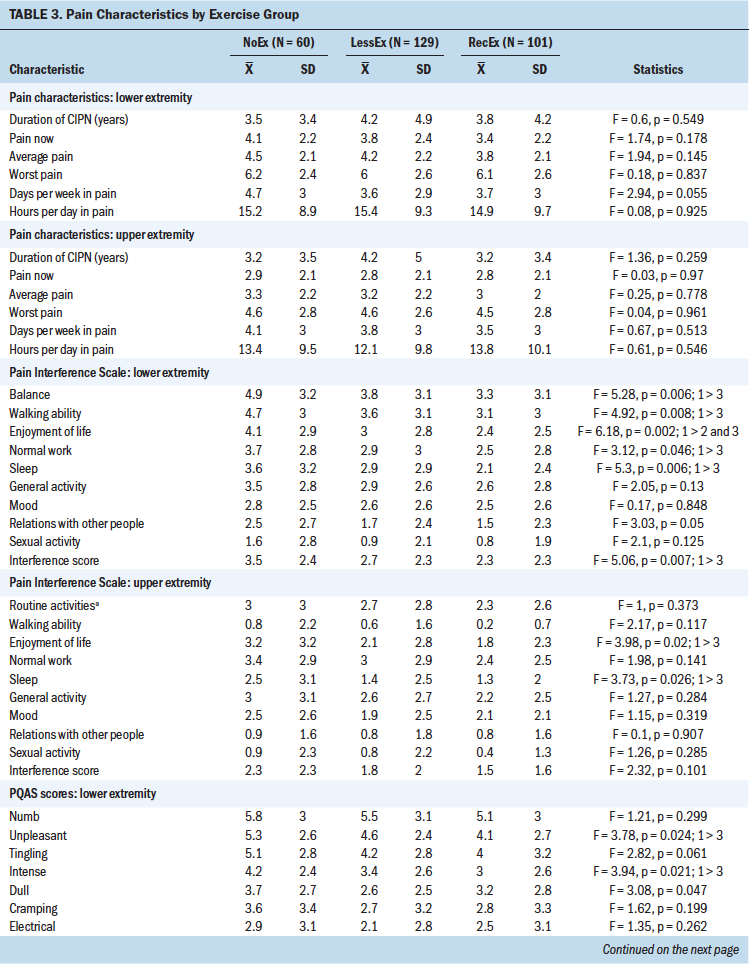
Given that the majority of the differences were found between the NoEx and the RecEx groups, it is possible that some level of physical activity is beneficial to survivors with CIPN. However, when the authors evaluated, using data from the LessEx and RecEx groups, the relationships between the total number of minutes of exercise and worst pain intensity, pain interference, TUG, and FAB scores, no dose–response effect was found for worst pain or pain interference. For TUG (r = –0.174, p = 0.008) and FAB (r = 0.191, p = 0.004) scores, although statistically significant, the correlations were extremely small. The actual amount of exercise that is sufficient to decrease CIPN signs and symptoms and improve balance remains to be determined. 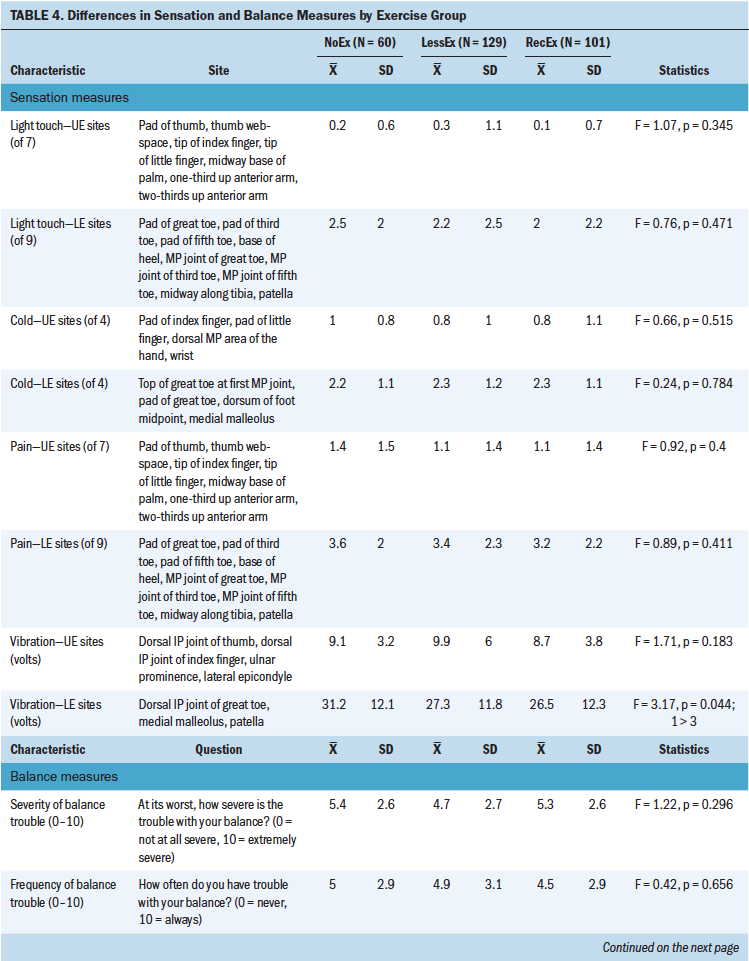
In the current study, although 35% of the survivors reported that they exercised for 150 minutes or more per week, this percentage is lower than the 88% reported in a Dutch registry study (Mols et al., 2015), but higher than the 16% reported by breast cancer survivors with CIPN in the United States (Wonders & Drury, 2012). The current findings are consistent with previous studies of cancer survivors that found that self-reported rates of physical activity ranged from 30% to 37% (Beasley et al., 2012; Pinto & Ciccolo, 2011).
Compared to the RecEx group, survivors in the NoEx group had fewer years of education, were less likely to be married or partnered, and reported a lower annual household income. Although these characteristics were not evaluated in previous CIPN studies (Kneis et al., 2019; Mols et al., 2015; Wonders et al., 2013), evidence from the general population suggests that these same demographic characteristics are associated with lower levels of exercise (Bird et al., 2015). In several studies (AuYoung et al., 2016; Clifford et al., 2018; Hirschey et al., 2020), lacking someone to help motivate an individual to exercise and not having sufficient resources to join a gym or exercise group, as well as lack of time, were cited as significant barriers to increasing physical activity.
Consistent with previous studies, compared to the other two exercise groups, survivors in the NoEx group had a higher BMI (Beasley et al., 2012; Blanchard et al., 2010) and poorer functional status (Su et al., 2014). However, for all exercise groups, survivors’ BMIs were in the overweight range (Centers for Disease Control and Prevention, 2016). In terms of functional status, the decrement found between the RecEx and NoEx groups represents not only a statistically significant but also a clinically meaningful decrease in KPS score (Cohen’s d = 0.7). In addition, and consistent with reports from the general population (Pedersen & Saltin, 2015; Roberts et al., 2015), compared to the RecEx group, survivors in the NoEx group had a worse comorbidity profile. Although no differences were found among the exercise groups in the occurrence rates for specific comorbidities, as noted in reports regarding the need to tailor exercise regimens for various chronic conditions (Hayes et al., 2019; Pedersen & Saltin, 2015), clinicians need to identify survivors who warrant referrals to physical therapy. These therapists can develop exercise interventions that accommodate not only the deficits associated with CIPN, but also those that are required for other chronic conditions. Of note, no differences were found among the exercise groups in the types of chemotherapy regimens and doses of neurotoxic drugs the survivors received.
This study is the first detailed examination of the associations between a comprehensive set of subjective measures of CIPN (i.e., duration, intensity, qualities, and interference) and self-reported levels of exercise. It is difficult to compare findings across previous studies of self-reported exercise (Mols et al., 2015; Wonders & Drury, 2012) because of the variability in the subjective measures that were used. Although the authors found no differences among the exercise groups in the duration of CIPN and the severity of upper and lower extremity pain, these findings require confirmation because none of the previous studies reported on differences in either of these characteristics. Findings regarding the effects of exercise on pain intensity in patients with cancer (Syrjala et al., 2014) and patients with chronic pain (Geneen et al., 2017; Naugle et al., 2012) are inconsistent. For example, in two studies of patients with cancer, one found a decrease (Griffith et al., 2009) and the other found no change (Cho et al., 2012) in pain intensity. In the two reviews of the effects of exercise on chronic noncancer pain (Geneen et al., 2017; Naugle et al., 2012), the authors suggested that the effects of exercise on pain intensity were inconsistent and were dependent on the type of exercise and the type of chronic pain evaluated.
Although no differences were found among the exercise groups in any of the pain quality scores in the upper extremities, which is consistent with previous reports (Duregon et al., 2018; Kneis et al., 2019; Mols et al., 2015), compared to the RecEX group, survivors in the NoEx group reported higher scores for unpleasant, intense, aching, and throbbing sensations. The lack of effect of exercise on pain qualities in the hands may be partially explained by the fact that the most common type of exercise in the current study was walking, which is not likely to have an effect on symptoms in the upper extremities.
Compared to the RecEX group, survivors in the NoEX group reported higher pain interference scores in their lower extremities for sleep, enjoyment of life, normal work, walking ability, and balance and in their upper extremities for enjoyment of life and sleep. Although prior studies reported significant improvements in CIPN20 scores associated with exercise (Kneis et al., 2019; Mols et al., 2015), no studies have used the interference items from the Brief Pain Inventory (BPI). The authors adapted one of the BPI items to assess the effects of CIPN on upper extremity function (i.e., interference with routine activities like dressing, toileting, and typing), but additional research is needed using items that are more specific to hand- and arm-related activities (e.g., manipulation of small objects), as well as exercises that are tailored to the upper extremities.
Of all objective measures of sensation that were evaluated, vibration was the only one that demonstrated significant between-group differences. Compared to the RecEx group, survivors in the NoEx group had higher vibration thresholds in the lower extremities. These findings are consistent with a pilot study that found significant improvements in vibration thresholds but no differences in light touch sensation in patients with cancer randomized to either sensorimotor training or whole-body vibration training (Streckmann et al., 2019).
Although from 64% (RecEx) to 82% (NoEx) of the survivors reported balance problems that were moderately severe, frequent, and distressing, no differences were found among the exercise groups in their self-reports of balance problems. However, compared to the RecEX group, survivors in the NoEx group had worse scores on the TUG and FAB tests. These results are consistent with the findings from a systematic review on the effects of exercise in patients with CIPN undergoing active treatment (Duregon et al., 2018), as well as findings in the geriatric literature that demonstrate that exercise decreases the risk of falls (Guirguis-Blake et al., 2018; Sherrington et al., 2017).
Limitations
Several limitations warrant consideration. In this study, because only survivors who had received platinum- and/or taxane-containing regimens were evaluated, the authors cannot determine whether these findings generalize to survivors who received other types of neurotoxic chemotherapy. The cross-sectional nature of this study limits the authors’ ability to determine causal associations between various CIPN characteristics and levels of physical activity. Although levels of exercise prior to and during chemotherapy were not evaluated, findings from one study suggest that level of physical activity prior to a cancer diagnosis is a strong predictor of activity as many as 10 years postdiagnosis (Mason et al., 2013). Self-reported levels of exercise, rather than objective measures of exercise, are susceptible to recall and social desirability biases (Brenner & DeLamater, 2016), but self-reported physical activity is moderately correlated with data obtained using an accelerometer (Limb et al., 2019; Nelson et al., 2019; Sloane et al., 2009).
Implications for Nursing
Despite these limitations, these findings suggest that the lack of regular exercise in cancer survivors is associated with worse scores on subjective and objective measures of CIPN and objective balance scores. With the projected increase in the number of cancer survivors in the United States to 20 million by 2026 (Miller et al., 2019), as well as the lack of effective treatments for numbness, tingling, and pain associated with CIPN (Hershman et al., 2014), it is critical to evaluate cost-effective and readily available strategies (e.g., walking) to improve CIPN symptoms and balance problems. A growing body of evidence suggests that patients and survivors can safely engage in moderate amounts of exercise during and after cancer treatment (Schmitz et al., 2019; Segal et al., 2017; Stout et al., 2017). Based on these real-world findings, as well as the findings from a limited number of studies on the efficacy of exercise for CIPN symptoms and balance problems that suggest benefits (Andersen Hammond et al., 2019; Kneis et al., 2019; McCrary et al., 2019), clinicians can recommend walking as a therapeutic option, as well as provide referrals to physical therapy for additional strength and balance training.
Conclusion
Prospective longitudinal studies are needed to determine the optimal dose and types of exercise that are necessary to prevent and treat CIPN symptoms and balance problems. In addition, preclinical and clinical studies are warranted to determine the mechanisms that underlie the therapeutic benefits of exercise for CIPN.
About the Author(s)
Anna Wilcoxon, RN, MS, is a triage RN at the Pacific Hematology Oncology Association and a master’s student in the School of Nursing at the University of California, San Francisco; Kord M. Kober, PhD, is an assistant professor and Carol Viele, RN, MS, is an associate clinical professor, both in the School of Nursing, Kimberly Topp, PT, PhD, is a professor and chair emeritus, Betty Smoot, PT, DPTSc, is an associate professor, Gary Abrams, MD, is a professor of neurology, and Margaret Chesney, PhD, is a professor of medicine, all in the School of Medicine, and Steven M. Paul, PhD, is a research data analyst in the School of Nursing, all at the University of California, San Francisco; Yvette P. Conley, PhD, is a professor in the School of Nursing at the University of Pittsburgh in Pennsylvania; and Jon D. Levine, MD, PhD, is a professor in the School of Medicine and Christine Miaskowski, RN, PhD, is a professor in the School of Nursing, both at the University of California, San Francisco. This study was funded by the National Cancer Institute (CA151692). Miaskowski is supported by a grant from the American Cancer Society. This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through University of California, San Francisco–Clinical and Translational Science Institute Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Recruitment was facilitated by the Dr. Susan Love Foundation for Breast Cancer Research’s Love Research Army. Kober, Topp, Chesney, Levine, and Miaskowski contributed to the conceptualization and design. Kober, Abrams, and Miaskowski completed the data collection. Kober, Paul, and Miaskowski provided statistical support. Wilcoxon, Kober, Chesney, Conley, and Miaskowski provided analysis. Wilcoxon, Kober, Viele, Topp, Smoot, Abrams, Chesney, Conley, Levine, and Miaskowski contributed to the manuscript preparation. Miaskowski can be reached at chris.miaskowski@ucsf.edu, with copy to ONFEditor@ons.org. (Submitted May 2020. Accepted July 7, 2020.)




