Frailty in Patients With Hematologic Malignancies and Those Undergoing Transplantation: A Scoping Review
Problem Identification: Although frailty is an important parameter in treatment planning and in predicting prognosis and overall survival among patients with hematologic malignancies and recipients of hematopoietic stem cell transplantation, frailty assessment tools are not standardized in clinical care settings. The purpose of this article is to provide an overview of the literature on frailty assessment tools in these patient populations.
Literature Search: A systematic search of CINAHL®, Embase®, MEDLINE®, PubMed®, and Web of Science was performed using keywords and controlled vocabulary for the concepts “bone marrow transplants,” “hematologic neoplasms,” and “frailty.”
Data Evaluation: Extracted data included study type, diagnosis, transplantation status, frailty tools used, and outcome measures.
Synthesis: A systematic search resulted in 24 studies that met the inclusion criteria. There were significant differences in how various groups define and assess frailty.
Implications for Practice: Addressing the lack of standardized frailty assessments will assist healthcare providers to routinely integrate frailty measures in clinical assessments to identify those at risk for poor outcomes, improving personalized care.
Jump to a section
Frailty is considered “a medical syndrome with multiple causes and contributors that is characterized by diminished strength and endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death” (Morley et al., 2013, p. 392). The progression from being healthy to frail is often silent and typically manifests in the clinical setting as an unexpected decline in an individual’s physical and cognitive state (Searle & Rockwood, 2015; Song et al., 2014); however, there is no clear consensus on how to operationalize the measurement of physical frailty as a vulnerable state (Pel-Littel et al., 2009). Frailty is a common clinical syndrome in older adults that increases the risk for poor health outcomes, including hospitalization, falls, and increased mortality (Perna et al., 2017). Although common in aging populations, the condition of frailty is increasingly being recognized as having a significant impact on clinical outcomes in multiple diseases and disorders such as cancer, diabetes, and heart failure (El Assar et al., 2019; Walston et al., 2018). As such, a number of specific tools have been developed to measure frailty, either as a separate construct or as one of several comorbidities in the context of a particular health state. For example, in the field of hematologic cancers and hematopoietic stem cell transplantation (HSCT), frailty has been defined, studied, and assessed in various ways. The purpose of this scoping review is to chart the state of the science for frailty assessment and describe frailty screening tools used in patients with hematologic cancers, with a focus on recipients of HSCT.
Hematologic malignancies are cancers that are initiated in the blood-forming cells of the body, such as the bone marrow and cells of the immune system. Some examples of blood cancers are leukemias, lymphomas, and multiple myeloma. HSCT is a process by which abnormal blood-forming stem cells are replaced by healthy cells, potentially serving as a curative treatment for hematologic cancers, myelodysplastic syndrome, and other blood disorders. Type of malignancy, disease severity, and donor availability determine whether an individual receives an autologous or an allogeneic transplantation. The patient’s own cells are used in an autologous transplantation, whereas in allogeneic HSCT, cells are harvested from a donor. The disease process and other factors (e.g., patient age, existing comorbidities) determine the conditioning chemotherapy regimen that patients receive prior to transplantation (Phelan et al., 2020). Treatment planning, goals, and decisions regarding aggressive chemotherapy regimens for treating hematologic cancers include eligibility determinants such as chronologic age, comorbidities, and physical performance status. To proceed to HSCT, individuals with hematologic malignancy are expected to be in remission. Regardless of the transplantation process, these individuals have already been exposed to intense therapies to treat their cancer, with some receiving multiple regimens, that unfortunately increase their risk for frailty. The sequelae from distinct disease processes with unique clinical courses, combined with the potential for treatment-related toxicity, emphasize the need for additional assessments in patients with hematologic malignancies as well as patients undergoing HSCT (Abel & Klepin, 2018).
In addition, advancements in treatment modalities and reduced-intensity chemotherapy regimens have led to an increase in the number of patients eligible for HSCT. Despite increases in the number of novel agents and treatment regimens, there has been a scarcity of specific guidance for treatment decisions that integrate characteristics of the malignancy, as well as the patient’s individual characteristics, prior therapies, and frailty. This is particularly relevant among the older adult population, where the majority of blood cancers occur and frailty is more prevalent (Abel & Klepin, 2018). The rise in the number of patients eligible for HSCT requires healthcare providers to update existing assessments to include new, relevant criteria, such as frailty, as part of developing a comprehensive risk-stratification strategy. Pidala et al. (2013) found that 92% of providers predominantly consider age as a dominant driver for predicting HSCT eligibility. In cancers such as diffuse large B-cell lymphoma, myelodysplastic syndrome, and multiple myeloma, it has been suggested that older adult patients be assessed using a multifaceted approach that involves measures of comorbidities, functional and cognitive capabilities, emotional health, nutritional status, and physiologic age (based on functional and biologic factors) for personalized therapy and predictions of therapy tolerance (Abel & Klepin, 2018; Morrison et al., 2015). Although chronologic age is an easier and important criterion to assess, it is critical to understand and acknowledge that existing comorbidities, functional and cognitive decline, and other physiologic measures need to be included in HSCT preassessments. These multidimensional parameters can also aid in monitoring prognosis, predicting therapy tolerance, and providing more personalized therapy.
In individuals with cancer, frailty may indicate chemotherapy intolerance and an increased risk of postoperative complications (Handforth et al., 2015; Rosko et al., 2015). Identification of frail patients will help providers modify the treatment regimen so that it is suitable for these patients and intervene in a timely manner (e.g., with physical therapy) that could improve physical functioning and quality of life (Wiskemann et al., 2011). This scoping review systematically maps literature that has used a frailty assessment or screening tool in the context of hematologic cancers and HSCT. The goal is to compile and examine data that will serve as a precursor for identifying the most promising tool(s), both in clinical trials and in real-world clinical practice. These data will serve as a foundation to standardize assessment and guide implementation to eventually improve clinical outcomes.
Methods
Electronic Search
For this scoping review, a systematic search for published English-language literature was conducted along with a search of the reference lists of the retrieved publications to identify published articles addressing hematologic malignancies, HSCT, and frailty. The databases searched from inception through November 19, 2018, were CINAHL®, Embase®, MEDLINE®, PubMed®, and Web of Science. The search used a combination of keywords and controlled vocabulary for the concepts “bone marrow transplants,” “hematologic neoplasms,” and “frailty” that were adapted to each database. In addition, a hand search of reference lists of articles was used to discover publications not identified in the database searches. A total of 711 articles were found, with 565 left to review after deduplication. Title and abstract screening of articles resulted in the exclusion of 380 articles. The remaining 185 full articles were assessed for eligibility. During the study selection process for this scoping review, the current authors identified cohort studies that addressed frailty in hematologic malignancies and stated “HSCT” in the article abstract or body and, therefore, met the inclusion criteria for this review. A total of 24 articles was included in this scoping review. Of these, 11 articles involved recipients of HSCT, and 13 articles included studies in individuals with hematologic malignancies. The search results and study selection process are illustrated in Figure 1, which depicts the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for this scoping review (Tricco et al., 2018). 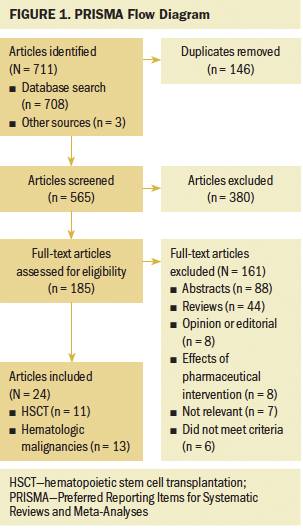
Study Selection
All references were screened, and duplicates were removed. Two of the current authors (L.M., L.S.) conducted the title and abstract review, as well as the full-text review. Any disagreements between the reviewers were resolved by consensus between the reviewers. Articles that did not involve any frailty or geriatric assessments, tools, or questionnaires were excluded. Review articles, Delphi studies, editorial opinions, expert articles, and pharmaceutical intervention studies were excluded during the selection process.
Data Collection and Analysis
The following information was extracted using a standardized data collection form: author, year of publication, diagnosis, sample population, transplantation type, and frailty assessment tool. The results were summarized in both text and tabular forms. Results were grouped in a tabular manner based on the study population: (a) patients who received an HSCT and (b) patients with hematologic malignancies. Tables 1 and 2 show characteristics of studies involving patients undergoing HSCT and with hematologic malignancies, respectively. Tables 3 and 4 detail frailty assessment tools used in HSCT and hematologic malignancy, respectively. 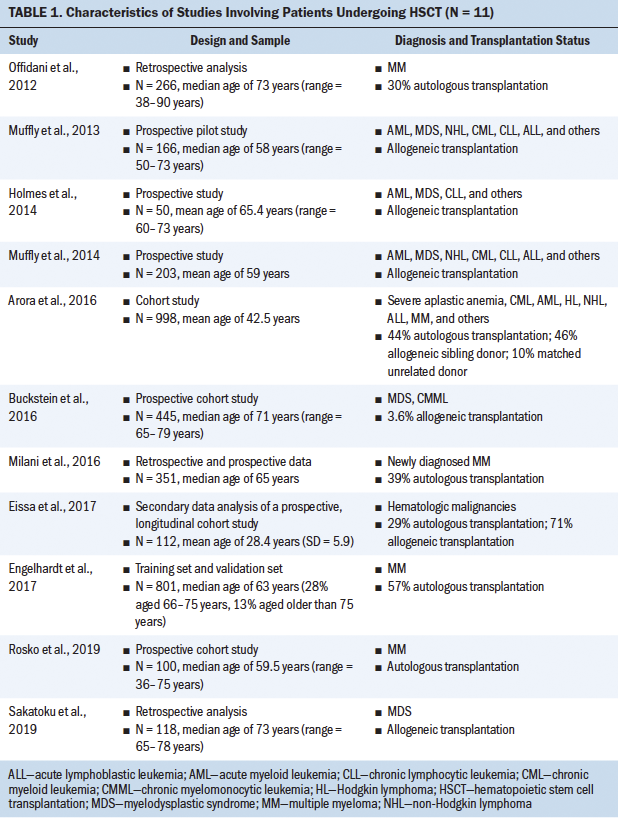
Results
Tools for Frailty Screening
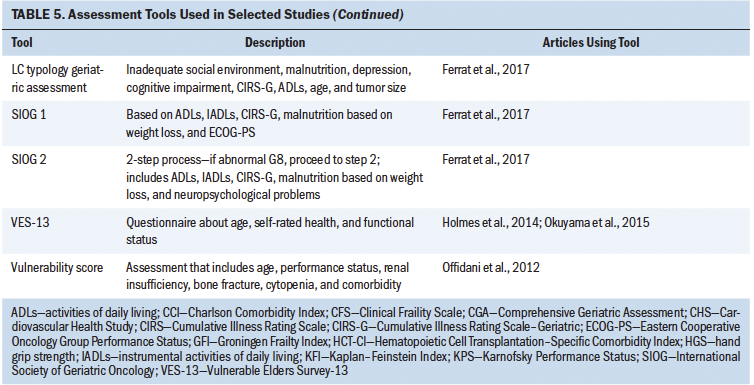
The use of frailty as a marker to better understand and stratify patients slated to receive HSCT and at-risk patients with blood cancers is slowly gathering momentum. However, given the differences in how frailty is defined, measured, and applied to treatment decision-making and eligibility in this population, there is an urgent need to identify standardized, reliable tools, as well as to implement measures in a consistent manner. Frailty assessment tools that have been used in the reviewed literature among patients undergoing HSCT and with hematologic cancer are summarized in Table 5. Twenty-four studies met the established criteria. 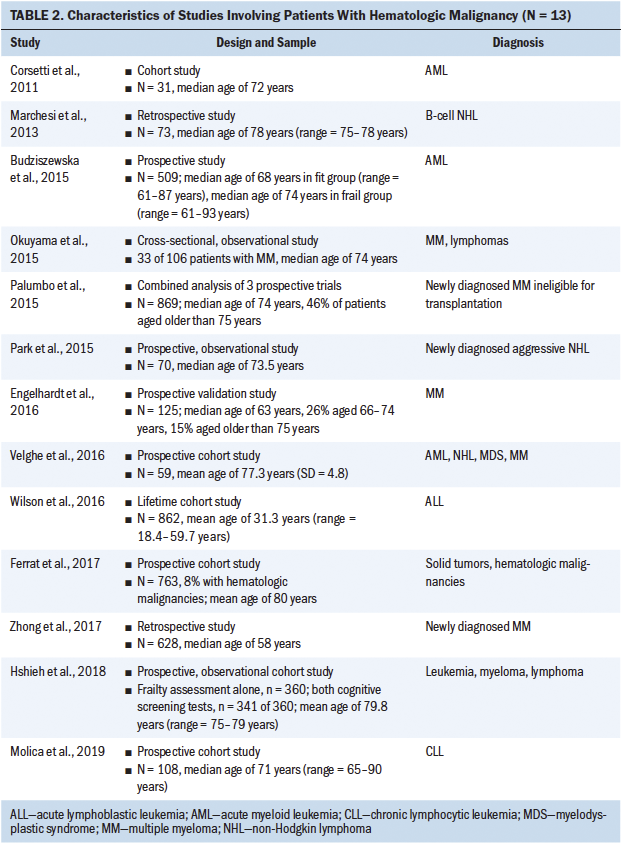
Frailty in Patients Undergoing HSCT
The current authors set out to determine how frailty involving patients undergoing HSCT was defined in the literature and the tools that were used to assess frailty in these studies. Eleven studies assessed frailty in patients undergoing HSCT, and almost half of these studies were in patients with a median age of older than 60 years. The majority of the studies used a prospective study design, followed by retrospective analysis. Frailty was assessed either by a single independent tool or by a combination of measures. Although eight different tools were used to assess frailty in these 11 studies, highlighting the heterogeneity in frailty assessments in patients undergoing HSCT, these studies indicated that frailty (as defined by each study) is associated with poor prognosis, increased mortality, decreased progression-free survival, or poor performance status. Some of the frailty tools used were Fried’s frailty phenotype (Cardiovascular Health Study [CHS] frailty index), the Clinical Frailty Scale (CFS), the Vulnerable Elders Survey-13 (VES-13), vulnerability score, the revised Myeloma Comorbidity Index (R-MCI), the International Myeloma Working Group (IMWG) score, G8, and geriatric assessments (Engelhardt et al., 2017; Holmes et al., 2014; Offidani et al., 2012; Rosko et al., 2019; Sakatoku et al., 2019). 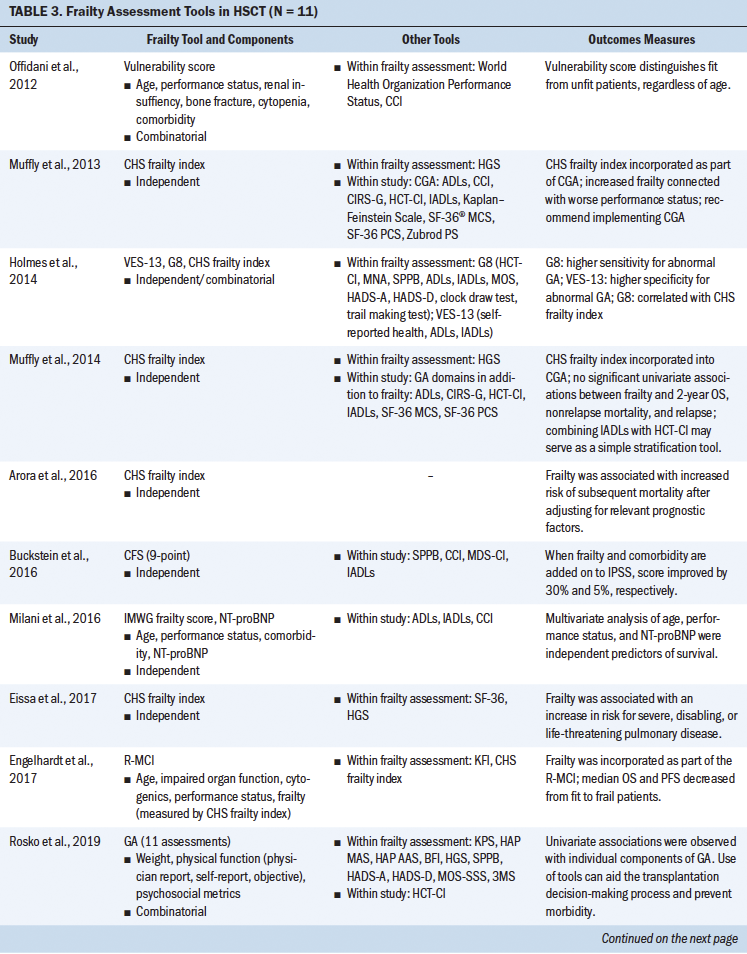
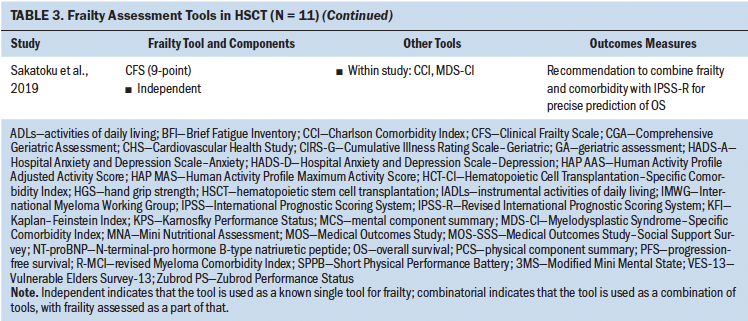
Cardiovascular Health Study Frailty Index
The most commonly used CHS frailty index has two “frailty phenotypic” categories described as the pre-frail and frail states that are each defined by the severity of the symptom cluster consisting of fatigue, decreased physical activity, weight loss, decreased grip strength, and slowed gait. This index has concurrent and predictive validity for outcomes that include incident disease, hospitalization, falls, disability, and mortality in community-dwelling older adults (Fried et al., 2001). Although this scale has been widely used, there is heterogeneity in how specific components in this scale are measured. For instance, weight loss is measured as either unintentional weight loss in the past year or low lean muscle mass. Scores on the CHS frailty index in non–older adult patients undergoing HSCT (aged 18–64 years) approached those seen among older adults and showed a four-fold higher prevalence than those of their siblings, also suggesting that frailty in patients undergoing HSCT can occur across the lifespan, irrespective of age (Arora et al., 2016). Increased frailty has been associated with worse performance status; increased risk of severe, disabling, or life-threatening pulmonary disease; and increased risk of mortality (Arora et al., 2016; Eissa et al., 2017; Muffly et al., 2014). This tool has been used independently or incorporated as a part of geriatric assessments and the R-MCI (the R-MCI includes age, frailty, and cytogenetics) (Domm et al., 2014). 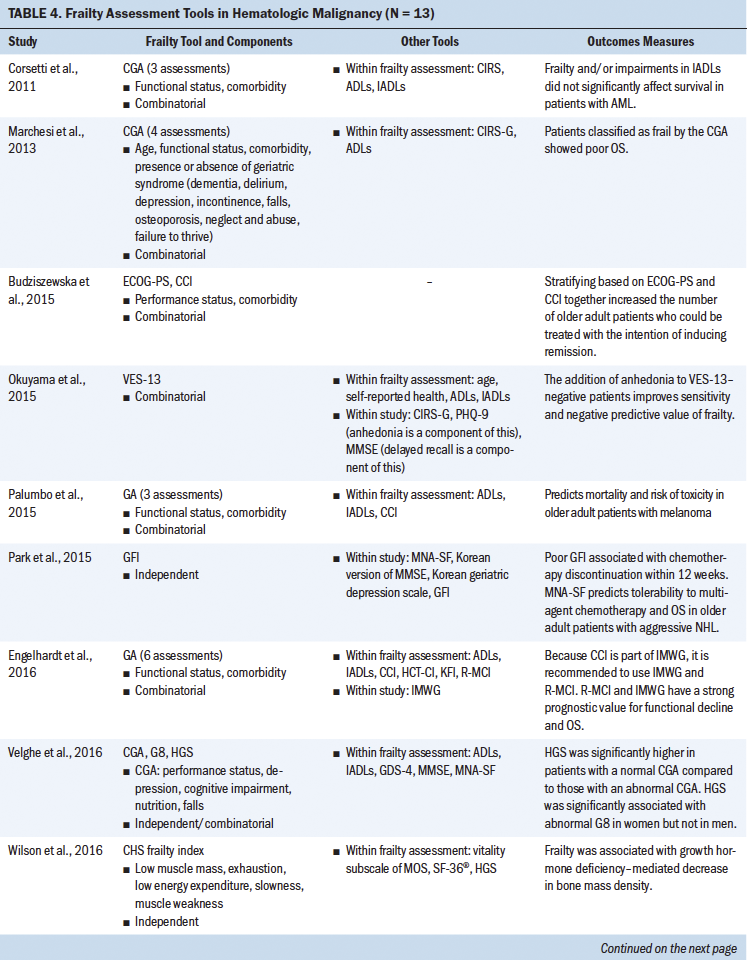
Comprehensive Geriatric Assessment
In 2005, an International Society of Geriatric Oncology taskforce recommended use of the Comprehensive Geriatric Assessment (CGA) for older adults with cancer to improve functional status and survival. Aging in older adults is associated with significant decline in physical performance, leading to disability, loss of independence, and adverse clinical outcomes. The CGA assesses multiple domains, such as functional and mental health status, comorbidities, social support, and environmental and functional limitations; each domain is evaluated by various tools. However, frailty as a construct is not specifically assessed as a domain within the CGA. Although it is considered the gold standard for identifying older adults at high risk for adverse outcomes, the CGA has not been extensively evaluated or routinely used for patients undergoing transplantation (Extermann & Hurria, 2007; Muffly et al., 2014). This could be because of the perception that patients undergoing HSCT are typically younger than the traditional older adult population or the notion that only “fit” older adults undergo transplantation. 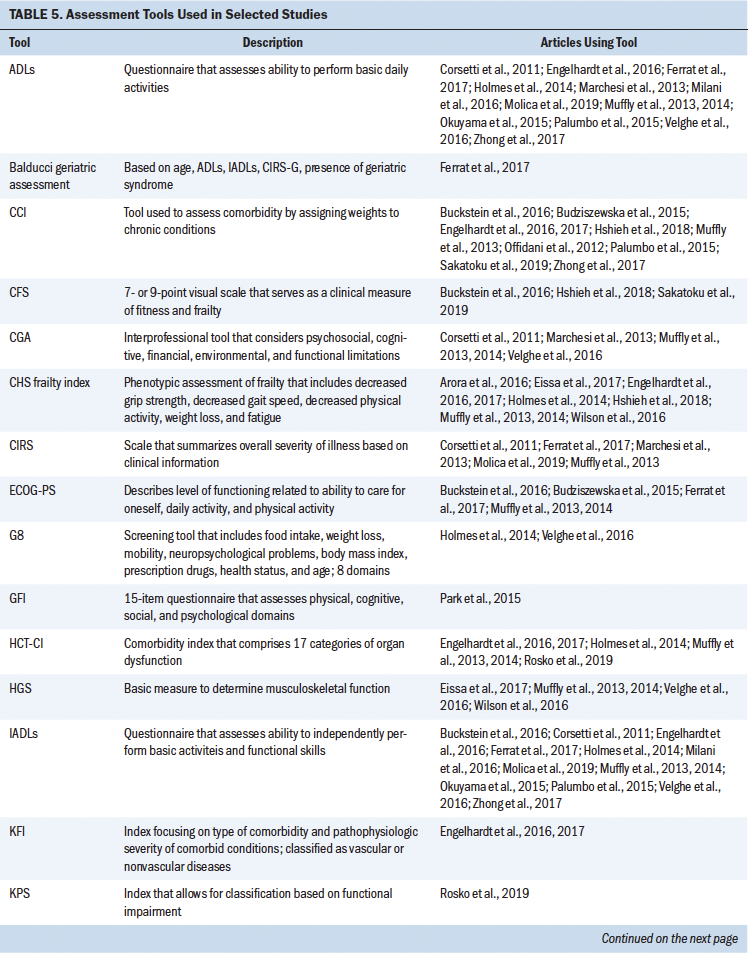
Nevertheless, a prospective pilot study of the CGA administered prior to allogeneic HSCT was conducted, with the intent of determining the prevalence and severity of comorbidities, vulnerabilities, and feasibility of the CGA in transplantation-eligible older adults. In the pilot study, use of the CHS frailty index score as a component of the CGA showed associations with performance status. The study recommended use of the CGA in older recipients of HSCT prior to transplantation, but also suggested functional and comorbidity assessments as simple risk stratification tools (Muffly et al., 2013, 2014).
Geriatric Assessments
Multiple studies have considered the use of select domains of the CGA to assess frailty and refer to them as geriatric assessments; however, there are variations in components that constitute these geriatric assessments. Some include functional status, comorbidities, and age, whereas others include additional assessments of cognition and psychosocial dysfunction (Engelhardt et al., 2016; Ferrat et al., 2017; Marchesi et al., 2013; Palumbo et al., 2015; Velghe et al., 2016; Zhong et al., 2017). The time-intensive nature of the CGA is probably one of the reasons for studies using selective, fewer, and shorter assessments for geriatric assessment, instead of the more time-consuming and extensive measures in the CGA. Some of the tools used as a part of geriatric assessments include the VES-13, G8, and Karnofsky Performance Status (KPS) scale.
Tools such as the VES-13 and G8 have been analyzed to determine their respective predictive ability for abnormal geriatric assessment or frailty. The VES-13 is a 13-item survey that includes age, as well as self-rated health and functional status, and is scored from 0 to 10, with higher scores indicating worse or more extensive frailty (Saliba et al., 2001). The G8 consists of eight domains: food intake, weight loss, body mass index, mobility, neuropsychological problems, prescription drugs, health status, and age. In recipients of allogeneic HSCT, the G8 correlates with the CHS frailty index and showed a higher sensitivity (69.7%), whereas the VES-13 had higher specificity (100%) (Holmes et al., 2014).
A study by Muffly et al. (2013) showed that 83% of patients who had performance status scores indicating good health fell into either the frail or pre-frail category based on the CHS frailty index (incorporated as part of the CGA). Similarly, KPS allows patients to be classified based on their functional impairment; it also demonstrates that patients can have a KPS score of greater than 80 (indicating good functional status) but also have an abnormal geriatric assessment (Crooks et al., 1991; Holmes et al., 2014). These studies clearly indicate that individual screening tools (e.g., World Health Organization Peformance Status, KPS) may not be able to comprehensively assess frailty and overall health.
These results also show that although shorter geriatric assessment tools are available, they do have certain drawbacks. Using a consistent combination of tools may be important to accurately determine comorbidities, vulnerability, and frailty. It is important to acknowledge these discrepancies and come to a uniform consensus on shorter tools to be used in this specific population, if needed. At this time, there is a lack of evidence to strongly recommend a relatively shorter assessment tool that is sensitive and specific.
Frailty and Comorbidities
Although frailty and comorbidities coexist, they have been identified as distinct entities. Measures of frailty often include the presence of comorbidities, potentially hindering an absolute measure of frailty. Comorbidity has been defined as any distinct additional health condition that has existed or may occur during the clinical course of a patient with a primary disease (Feinstein, 1970). Some studies in patients undergoing HSCT have adopted a combinatorial approach involving performance status, frailty, and comorbidity assessments for stratifying at-risk patients. The Charlson Comorbidity Index (CCI) is calculated by assigning weights to chronic conditions; this tool has been widely used in various chronic conditions (Charlson et al., 1987; Extermann, 2000). Offidani et al. (2012) conducted a study involving a vulnerability score that combined both performance status and the CCI. This score proved to better predict the survival of patients with multiple myeloma versus a comparison of patients using performance status or the CCI alone. Importantly, Offidani et al.’s (2012) results suggest that this vulnerability score can identify two key groups of patients: (a) those who can tolerate effective therapy and (b) those who may experience nonmyeloma-related mortality, indicating great promise for risk stratification.
The Myelodysplastic Syndrome–Specific Comorbidity Index (MDS-CI) is another dynamic index that is used to predict the outcome of patients with myelodysplastic syndrome (Buckstein et al., 2016). In a study by Buckstein et al. (2016), the predictive value of a prognosis scoring system improved when frailty and comorbidity measures were added. Adding the CFS and the CCI/MDS-CI comorbidity tools increased the predictive value of the scoring system by 30% and 5%, respectively, leading to a more precise prediction of overall survival (Buckstein et al., 2016; Sakatoku et al., 2019).
With the intention of establishing comorbidity scores in patients undergoing HSCT, Sorror et al. (2005) introduced a new simple index, the Hematopoietic Cell Transplantation–Specific Comorbidity Index (HCT-CI), that provides valid and reliable scoring for patients pretransplantation. HCT-CI scores consider 17 categories of organ dysfunction that have the potential to predict nonrelapse mortality and survival. This tool is used in HSCT preassessments (Sorror et al., 2005). The HCT-CI has been incorporated within geriatric assessments and in combination with functional status assessments, and has served as an improved stratification tool (Engelhardt et al., 2017; Holmes et al., 2014; Muffly et al., 2013, 2014; Rosko et al., 2019).
In summary, comorbidity tools (e.g., CCI, MDS-CI, HCT-CI) emphasize the importance of assessing patients for comorbidities but also affirm that the concomitant use of independent frailty assessments (e.g., performance status, CFS, vulnerability score) add to eligibility screening, stratification of at-risk patients, and the predictive value of outcomes. However, in carrying out future research, it is essential that there be agreement on how both comorbidities and frailty are defined and assessed.
Although this article primarily focuses on frailty assessment in adults undergoing HSCT, it is important to acknowledge the incidence of frailty in childhood survivors of HSCT. A study among adult survivors of childhood hematologic cancers demonstrated a four-fold increase in the incidence of frailty using the CHS frailty index in recipients of HSCT compared to survivors treated with conventional therapy (Eissa et al., 2017). Overall, there is a need to assess frailty, identify the appropriate tool, and demonstrate the value of combining tools for frailty and comorbidity in this patient population.
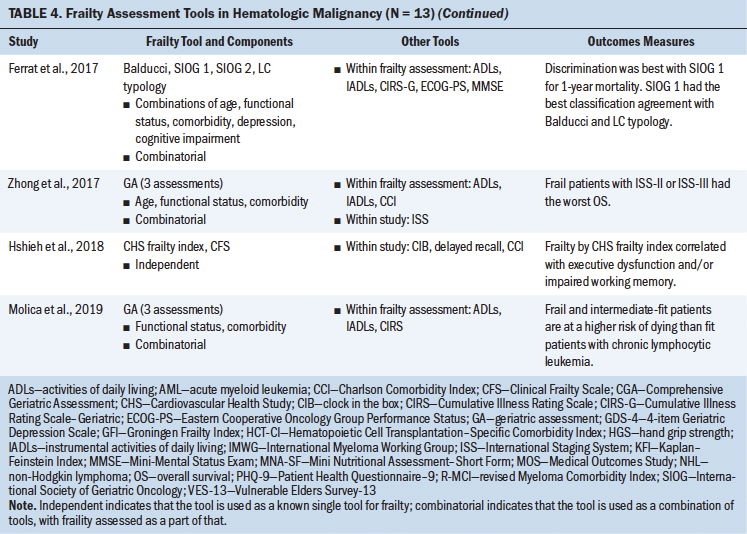
Frailty in Patients With Hematologic Malignancies
During the study selection process for this scoping review, the current authors identified cohort studies that addressed frailty in hematologic malignancies and listed “HSCT” in the article abstract or body and, therefore, met the inclusion criteria for this review. Of the 13 studies grouped under hematologic malignancies, 3 involved patients with a diagnosis of multiple myeloma and 2 were in patients being treated for lymphomas; the remaining studies looked at patients with leukemias and other hematologic cancers. The current authors reviewed tools that were used to assess frailty among patients with hematologic malignancies. Some studies proposed the use of prescreening tools that measure features of frailty, such as hand grip strength and the Mini Nutritional Assessment–Short Form (Park et al., 2015; Velghe et al., 2016). In a study by Velghe et al. (2016), decreased hand grip strength showed significant association with abnormal G8 scores in women but not in men. These prescreening tools show associations with abnormal geriatric assessments and have a predictive ability of overall survival in these patients. In some instances, tools such as the CHS frailty index and CFS were implemented in patients with leukemia and myeloma, showing associations of frailty with outcomes such as decreased bone mass density and impaired cognitive function (Hshieh et al., 2018; Wilson et al., 2016). There were several studies reviewed for the current article that indicated the full use of the CGA; however, further exploration revealed that only select assessments had been used as part of the overall CGA (e.g., comorbidity, functional status, incontinence, falls), whereas other CGA domains were not measured. Therefore, it was difficult to interpret the utility of the CGA equally across the studies reviewed.
Use of Combinatorial Assessments
Building on individual tools, researchers have explored and initiated a combinatorial approach for assessing frailty in hematologic malignancies, similar to that performed in recipients of HSCT, as previously discussed. Review of the hematologic cancer literature showed predominant use of functional assessment, such as activities of daily living (ADLs) and instrumental ADLs (IADLs); age; and comorbidity as measures of frailty in blood cancer diagnosis (Engelhardt et al., 2016; Mellqvist, 2015). IMWG score combined age, ADLs, IADLs, and the CCI and was able to stratify based on fitness and predict the risk of mortality in patients newly diagnosed with multiple myeloma (Palumbo et al., 2015). Similar studies evaluated use of the VES-13, Eastern Cooperative Oncology Group Performance Status, the CCI, and age for stratifying individuals with high-risk blood cancer (Budziszewska et al., 2015; Milani et al., 2016; Okuyama et al., 2015). Combined use of the VES-13 and assessment of anhedonia among older patients with newly diagnosed cancer showed improved sensitivity and negative predictive value of frailty (Okuyama et al., 2015). Although it is understandable that each cancer type has its own assessment, the data provide compelling evidence to believe that combining assessment tools will help to improve stratification of high-risk patients, identification of patients for managing treatment toxicities, and the predictive power of outcomes (e.g., overall survival, disease-free survival, mortality) in these patients (Corsetti et al., 2011; Marchesi et al., 2013; Molica et al., 2019; Spina et al., 2012).
Discussion
Healthcare providers have used chronological age, various clinical parameters, and different assessments to determine eligibility for specific treatment regimens (e.g., chemotherapy, HSCT) for patients, as well as prognosis and outcomes. With the advent of novel therapeutic agents and reduced-intensity chemotherapy regimens, there has been an increase in the number of patients who are being treated; consequently, providers are increasingly being presented with the challenge of accurately determining eligibility for various treatments and procedures. Clinicians and scientists have stated repeatedly that there is a need to improve and refine existing preassessments (Tay et al., 2019). Frailty has been associated with increased toxicity, poor therapeutic response, and decreased survival for patients with hematologic malignancies (Ness et al., 2013; Sorror et al., 2005). Research shows that pre-HSCT prevalence of frailty is higher in patients requiring HSCT compared to the general geriatric population (Artz et al., 2011; Muffly et al., 2014). As the average lifespan increases, healthcare providers will encounter more patients who are aging and frail, emphasizing the need to have an established plan for stratifying patients who will be eligible for HSCT (Hamaker et al., 2012; Mohile & Magnuson, 2013). Such assessments will help in identifying those who will be able to tolerate transplantation, improve informed decision-making regarding therapy modality, and monitor response and overall quality of life by providing appropriate supportive care (Holmes et al., 2014).
The purpose of this scoping review was to systematically gather data on how frailty has been assessed in recipients of HSCT and patients with hematologic malignancies. Tools such as the CHS frailty index, CFS, VES-13, hand grip strength, G8, and CGA have been used in various settings in patients undergoing HSCT. Inconsistencies in how frailty is defined and assessed present as a barrier in applying data to larger cohorts of patients and providing guidelines. The current synthesis of existing literature demonstrates that frailty has been assessed independently using established frailty tools such as the CHS frailty index, surrogate measures such as hand grip strength, combinatorial assessments (which typically include a combination of performance status, functional status, and comorbidities), and the time- and personnel-intensive CGA.
In addition, the current authors encountered variable measures that present challenges for quantitatively evaluating and recommending tools for implementation. For example, in studies using the CHS frailty index, the physical activity component was either a self-reported question or a compendium of physical activities using metabolic rates. With geriatric assessments, the individual components that comprise the physical activity score and the evaluation methods or tools used were varied. Such variability in assessing a component of a measure makes it challenging to compare data across various clinical settings or studies. The task at hand is to determine the tool, or combination of tools, that should be used to assess frailty, with specific instructions on how to administer the assessment in a uniform manner.
Research has repeatedly shown the benefits of combining assessment tools for frailty and comorbidity to help improve healthcare providers’ ability to stratify high-risk patients, which also improves patient outcomes, such as overall survival. An array of comorbidity assessment tools are used in patients with hematologic malignancies or in recipients of HSCT (CCI, R-MCI, HCT-CI). Although these three tools are all broadly considered comorbidity indices, the CCI specifically measures chronic conditions, the HCT-CI measures organ dysfunction, and the R-MCI measures frailty (via the CHS frailty index) in addition to organ dysfunction, contributing to the overall scores. Given the compelling evidence in favor of combining tools to arrive at a more holistic and accurate measure of frailty, the current authors suggest the testing and implementation of a frailty and comorbidity assessment tool to evaluate patients, but also recommend that clinicians and researchers adhere to the same tools and combination of tools for their specific patient populations. For example, the R-MCI could be used for all patients with myeloma, whereas a combination of the HCT-CI and CHS frailty index could be used for all patients undergoing HSCT, regardless of the underlying diagnosis. Such recommendations would serve as an important first step to better stratify patients that may, ultimately, lead to the identification of patient subgroups that could benefit from planned interventions. The Composite Health Assessment Risk Model (CHARM) study conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 1704) plans to investigate a composite heath assessment model that includes comorbidity and geriatric assessment in older adults receiving allogeneic HSCT (Artz & Sorror, 2021). Results from this study have the potential to provide information that could help clinicians and researchers shape their preassessment strategies.
Frailty should be incorporated as part of preassessment and risk stratification for potential recipients of HSCT and patients with hematologic malignancies. The CHS frailty index is most commonly used across studies, but the combination of assessment tools (e.g., concurrent use of a frailty tool and comorbidity index) has been shown to have more predictive power. The specific tools that will contribute to the best cumulative frailty score remain to be determined; however, once identified, all researchers and clinicians should adhere to predetermined assessment tools to maintain consistency, which will enable early implementation of supportive therapy in this patient population. These strategies will better inform personalized treatment planning and supportive interventions (early implementation of physical therapy, occupational therapy, lifestyle counseling, and dietary guidance), as necessary (Chemaitilly & Robison, 2012; Hegde & Murthy, 2018).
Implications for Nursing
Healthcare providers, like advanced nurse practitioners (ANPs) and nurses, play a significant role in providing patient-centered care, which involves prevention and risk assessment in patients with cancer (Morgan & Tarbi, 2016). This is also applicable in patients slated to receive HSCT, recipients of HSCT, and patients diagnosed with hematologic cancers. ANPs and nurses are involved in the initial consultation with these patients; this involves determining baseline health history information, diagnosis, comorbidities, barriers and challenges (physical and emotional), and caregiver support. They are well positioned to conduct frailty assessments; in conjunction with the interprofessional transplantation team, they can help to facilitate appropriate treatment planning and provide personalized care. The involvement of APNs and nurses throughout the care continuum, established trust, effective communication, and collaboration with the transplantation team give nurses the unique ability to lead the way in assessing and addressing frailty as an essential component of treatment planning and individualized interventions in recipients of HSCT and patients diagnosed with hematologic malignancies. Based on information gathered in this scoping review, it is recommended that nurse clinicians and researchers incorporate frailty assessments as a preassessment and a risk-stratification tool, as well as conduct clinical and translational research to determine appropriate frailty assessment tools. This should be followed by consistent administration of the identified tools to assess frailty in this population. 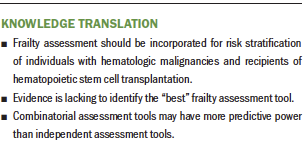
Conclusion
Frailty is an important parameter for understanding an individual’s “physiological age” and developing personalized treatment plans for patients with hematologic malignancies and recipients of HSCT. Frailty assessments are being used by healthcare providers; however, there is a need for consistency on how frailty is defined and assessed. A combinatorial approach that includes evaluation of frailty and comorbidity has shown improved predictive power of patient outcomes. The first step is to identify the tools of choice and administer them in a consistent manner. This can subsequently lead to the initiation and implementation of appropriate interventions in a timely manner that will help to improve and sustain a better quality of life in these patient populations.
About the Author(s)
Lathika Mohanraj, PhD, RN, BMTCN®, and Lana Sargent, PhD, RN, FNP-C, GNP-BC, are both assistant professors in the School of Nursing, Roy Brown, MLIS, AHIP, is an associate professor, interim co-department head of the Research and Education Department, research and education librarian in the Tompkins-McCaw Library for the Health Sciences, and liaison to the School of Nursing, and Theresa Swift-Scanlan, PhD, RN, FAAN, is an associate professor and the director of the Biobehavioral Research Laboratory in the School of Nursing, all at the Virginia Commonwealth University in Richmond. This research was supported by the Virginia Commonwealth University Presidential Research Quest Fund, awarded to Mohanraj. All authors contributed to the conceptualization and design, completed the data collection, provided the analysis, and contributed to the manuscript preparation. Mohanraj can be reached at mohanrajl@vcu.edu, with copy to ONFEditor@ons.org. (Submitted August 2020. Accepted October 10, 2020.)




