Demographic, Symptom, and Lifestyle Factors Associated With Cancer-Related Fatigue in Men With Prostate Cancer
Objectives: To identify potential demographic, symptom, and lifestyle factors associated with cancer-related fatigue (CRF) in men with prostate cancer.
Sample & Setting: Data were retrieved from men with prostate cancer across the disease trajectory who were enrolled in the Genitourinary Cancer Collaborative Registry–Prostate Cancer.
Methods & Variables: Self-reported data on demographic characteristics, lifestyle habits (smoking history, alcohol consumption, physical activity/exercise, dietary habits, and vitamins/supplements), and symptom experiences (measured using the Brief Fatigue Inventory, European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Prostate Cancer and –Bone Metastasis, and Pittsburgh Sleep Quality Index) were included in the registry.
Results: Demographic (younger age) and symptom (sleep quality, urinary, bowel, hormone-related, and sexual activity) correlates of CRF were identified. Higher levels of moderate to vigorous exercise and activities were associated with lower CRF in the sample as a whole. However, there was no association between CRF and physical activity in men with bone metastasis.
Implications for Nursing: CRF is a common and burdensome symptom among individuals with cancer and survivors. Identification of demographic, symptom, and lifestyle factors associated with CRF can enhance understanding of this symptom and contribute to early risk assessment and intervention.
Jump to a section
Cancer-related fatigue (CRF) is commonly defined as “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (National Comprehensive Cancer Network [NCCN], 2020, p. FT-1). CRF is different from fatigue caused by insufficient sleep or general tiredness in that it involves physical, mental, and emotional aspects. It is persistent and not relieved by simply getting adequate rest or sleep (Charalambous & Kouta, 2016). CRF often presents at diagnosis, worsens during treatment, and lasts into survivorship, culminating in a prolonged detrimental effect on health-related quality of life. CRF remains prevalent, poorly understood, challenging to assess, and difficult to manage (Dickinson et al., 2021; Feng et al., 2017; Randall et al., 2019).
Identification of demographic, symptom, and lifestyle factors associated with CRF is essential to enhance understanding of this challenging symptom and contribute to early risk assessment and intervention. Previous studies examining the relationship between demographic and clinical data and CRF have shown that age, body composition (fat and lean mass), and hematologic values (hemoglobin, testosterone, hematocrit, and red blood cells) have been associated with CRF in men with prostate cancer (Bandara et al., 2019; Chao et al., 2018; Feng et al., 2015; Newton et al., 2018). In addition, the influence of concurrent symptoms (e.g., urinary symptoms, pain, depression) has been associated with CRF in men with prostate cancer (Chao et al., 2018; Feng et al., 2019).
Although several studies have explored demographic and clinical factors associated with fatigue in men with prostate cancer, few have examined sociodemographic or lifestyle factors—excluding physical activity and nutrition—that are associated with CRF in men with prostate cancer. One study of men with nonmetastatic prostate cancer found that younger age contributed to increases in CRF during treatment with radiation therapy, but race, residence, working status, and smoking status did not contribute to increases in CRF (Chao et al., 2018). A second study observed that older age was associated with higher fatigue scores, whereas smoking status did not reach statistical significance in men with metastatic prostate cancer receiving docetaxel (Bergin et al., 2017). Therefore, the purpose of this exploratory study was to identify potential demographic, symptom, and lifestyle factors associated with CRF in men with prostate cancer across the disease trajectory who were enrolled in a genitourinary cancer registry.
Methods
Data for this cross-sectional, exploratory study were retrieved from the Genitourinary Cancer Collaborative Registry (GUCaRe)–Prostate Cancer. GUCaRe is a subset of the larger integrated Cancer Repository for Cancer Research (iCaRe2) registry at the Fred and Pamela Buffett Cancer Center and the University of Nebraska Medical Center (Sherman et al., 2011). Written informed consent was provided by all participants. Enrollment to the registry was initiated in 2014; all available data from 2014 to 2019 were included.
GUCaRe Questionnaire and Study Variables
The GUCaRe questionnaire consists of both core data elements from the iCaRe2 registry and genitourinary-specific elements from the GUCaRe extended questionnaire.
iCaRe2 core data elements: Data were used from the demographic, smoking history, and alcohol modules. The demographic module included age, race, ethnicity, marital status, education, employment status, and income. The smoking history module included data on smoking habits and exposure (e.g., start age, current use, total years). The alcohol module included data on consumption habits (e.g., start age, current use, total years).
GUCaRe extended questionnaire: Data were used from the physical activity/exercise level, dietary habits, and vitamins/supplements modules of the GUCaRe extended questionnaire. The physical activity/exercise level module consisted of data on time spent participating in moderate and vigorous activities, including exercise, sports, home activities, and occupation activities. The dietary habits module included data regarding habits and consumption (carbohydrates, fruits and vegetables, protein, fats, and dairy), and the vitamins/supplements module included data on patients’ usage of more than 30 different vitamins and supplements.
In addition to the existing modules, four symptom questionnaires were included in the GUCaRe questionnaire: the Brief Fatigue Inventory (BFI), the Pittsburgh Sleep Quality Index (PSQI), the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Prostate Cancer (EORTC QLQ-PR25), and the EORTC QLQ–Bone Metastases (EORTC QLQ-BM22).
Fatigue was the primary symptom of interest and was assessed using the BFI, which is a nine-item measure of fatigue severity and interference. Three questions assess fatigue severity during the past 24 hours. Six questions assess the interference of fatigue with general activity, mood, walking ability, normal work, relations with other people, and enjoyment of life. Items are individually rated on a scale ranging from 0 to 10, with higher scores indicating greater fatigue severity. The reliability and validity of the BFI is well established (Mendoza et al., 1999).
The PSQI is a 19-item measure of sleep quality during the past month. Responses from the items are used to generate a global score, as well as seven component scores on the following variables: sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, sleep medication usage, and daytime dysfunction. Each component score ranges from 0 to 3, with a total possible score ranging from 0 to 21. Higher scores indicate poor sleep quality. The reliability and validity of the PSQI is well established (Buysse et al., 1989).
The EORTC QLQ-PR25 is a 25-item questionnaire that assess quality of life in men with prostate cancer using five multi-item symptom and functional scales. The symptom scales assess urinary, bowel, and hormonal treatment–related symptoms, as well as a single item to assess urinary incontinence aid. The functional scales assess sexual activity and sexual functioning. The EORTC QLQ-PR25 questions for incontinence aid and sexual functioning both have qualifiers, meaning that the respondent completes them only if they meet certain criteria (i.e., being sexually active in the past four weeks). If a respondent does not meet the criteria to answer the questions, then the questions are skipped. Items are individually rated on a scale ranging from 1 to 4, with a total transformed score ranging from 0 to 100. Higher scores indicate higher symptoms or worse sexual activity and functioning. The validity and reliability of the EORTC QLQ-PR25 is well established (van Andel et al., 2008).
The EORTC QLQ-BM22 is a 22-item questionnaire assessing quality of life for those with bone metastasis and consists of both symptom and functional scales. The symptom scales assess painful sites and pain characteristics. The functional scales assess functional interference and psychosocial aspects. Items are individually rated on a scale ranging from 1 to 4, with a total transformed score ranging from 0 to 100. Higher scores indicate higher symptoms or higher level of functional interference and worse psychosocial aspects. The validity and reliability of the EORTC QLQ-BM22 is also well established (Chow et al., 2012).
Statistical Analyses
Descriptive statistics were used for reporting demographic, lifestyle, and symptom data for the sample. Normality of the data was assessed. Spearman’s rank correlation coefficients were used to determine associations between demographic, symptom, and lifestyle factors and fatigue. To determine whether any of these relationships varied depending on bone metastasis status, multiple linear regression models were performed (interactions). Because of a positive skew in fatigue scores, a natural log transformation was used in the regression models. Where significant interactions were observed, model predicted values were back transformed to create interaction plots. Significance was set at alpha < 0.05. Statistical analyses were conducted using IBM SPSS Statistics, version 25.0.
Results
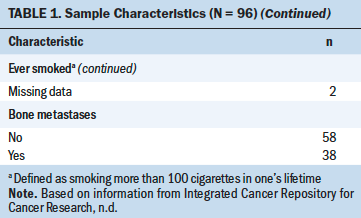
Data from 96 men were included in this sample (see Table 1). Ages ranged from 29 to 89 years, with an average of 70.6 years (SD = 9.1). Most of the participants were White (n = 94) and married (n = 70). Most participants had less than a four-year college degree (n = 56) and a household income of more than $45,000 (n = 51). Fifty-nine participants reported being current alcohol drinkers, and five participants reported being current smokers. Of the 96 participants, 38 reported having bone metastasis. The demographic characteristics of those with bone metastasis were similar to those without metastasis. No significant differences were found in age, time since diagnosis, years of alcohol consumption, daily alcohol use, smoking status, sleep disturbance, fatigue, marital status, education level, or income. 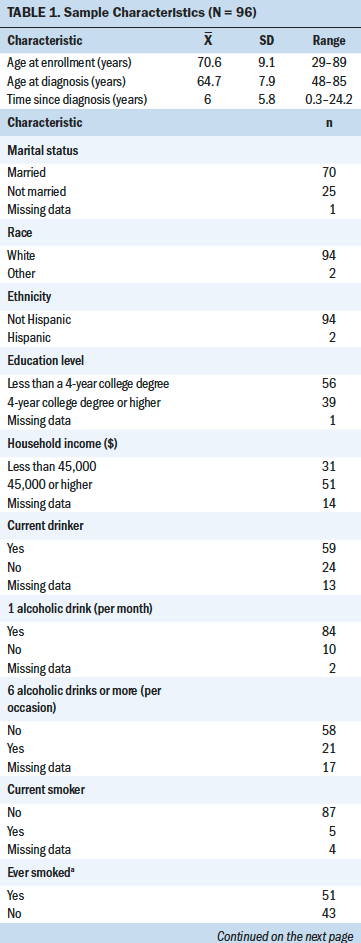
Demographic Correlations With Fatigue
Age at diagnosis was the only demographic characteristic that significantly correlated with fatigue (rs = –0.21, p < 0.05). Younger age was associated with higher fatigue. Marital status, education level, and household income were not significantly associated with fatigue (see Table 2). 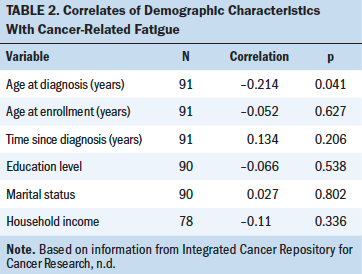
Symptom Correlations With Fatigue
Table 3 shows correlations among fatigue, sleep quality, prostate-specific quality of life, and quality of life related to bone metastasis. Fatigue was significantly correlated with total scores on the PSQI, indicating that higher fatigue was associated with poorer sleep quality. Fatigue scores were significantly associated with all scales of the EORTC QLQ-PR25, except the incontinence aid symptom scale and the sexual function scale. These results indicate that higher fatigue was associated with higher prostate-specific symptomatology, as well as worse sexual activity. 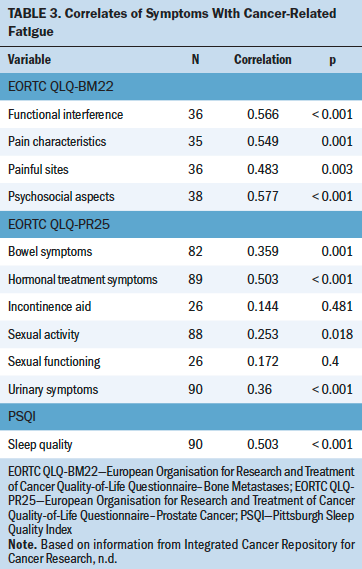
Lifestyle Correlations With Fatigue
Higher activity levels of vigorous exercise (rs = –0.4, p < 0.001), vigorous home activities (rs = –0.31, p = 0.004), and moderate home activities (rs = –0.39, p < 0.001) were significantly associated with lower levels of fatigue in the sample. There were no significant correlations between smoking, alcohol, dietary, vitamins/supplements, or other exercise habits with fatigue (see Table 4). 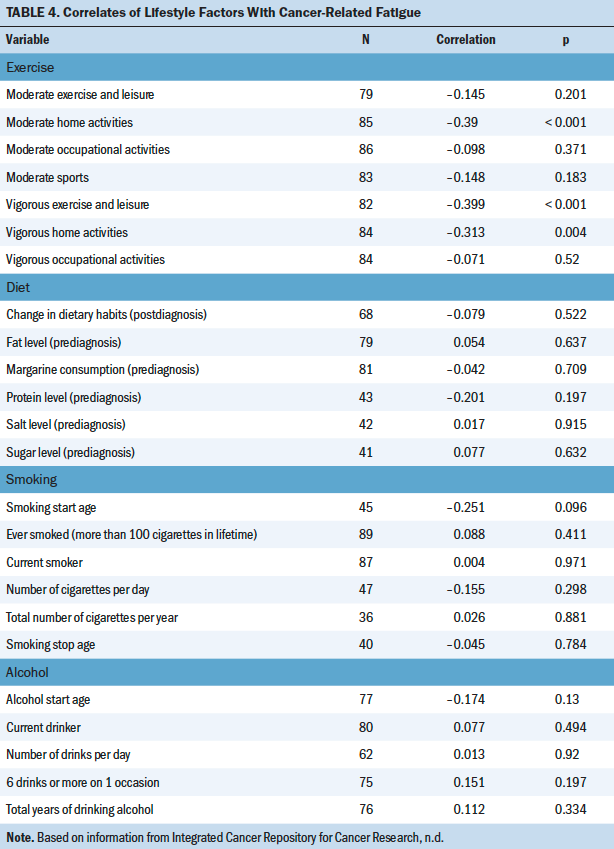
Bone Metastasis
There were only two different interactions found from multiple linear regression models between those with (n = 38) and those without (n = 58) bone metastasis. For respondents without bone metastasis, moderate exercise was significantly correlated with lower fatigue (r = –0.39, p = 0.006), as were moderate sports (r = –0.363, p = 0.01); however, no association was found for fatigue with either activity in those with bone metastasis. Fatigue was significantly correlated with all scales of the EORTC QLQ-BM22, indicating that higher fatigue correlated with higher bone metastasis–related symptomatology, higher level of functional interference, and worse psychosocial aspects.
Discussion
The purpose of this exploratory study was to identify potential demographic, symptom, and lifestyle factors associated with CRF in men with prostate cancer across the disease trajectory. Demographic (younger age) and symptom (sleep quality, urinary, bowel, hormone-related, and sexual activity) correlates of CRF were identified. The literature reporting on the relationship of age with CRF is inconsistent, particularly when comparing studies in those with localized versus advanced prostate disease (Bergin et al., 2017; Challapalli et al., 2018; Chao et al., 2018; Feng et al., 2017). Additional research is needed to determine the relationship between age and other sociodemographic factors and CRF in men with prostate cancer across the disease trajectory.
Symptoms (sleep quality, urinary, bowel, hormone-related, and sexual activity) were also correlated with CRF and aligned with observations in the literature. Sleep quality is often found to co-occur with fatigue in individuals with cancer (Charalambous et al., 2019), including those with prostate cancer (Koskderelioglu et al., 2017). Urinary, bowel, and hormone-related symptoms are common side effects observed in men with prostate cancer and have been found to be associated with CRF in this patient population (Randall et al., 2019). In addition, urinary, bowel, and hormone-related symptoms have been associated with distress, anxiety, and depressive symptoms in survivors of prostate cancer (Feng et al., 2019; Sharp et al., 2016). Limited research has explored the relationship between sexual activity and function and CRF (Higano, 2012; Zdravkovic et al., 2020). More research in this area is needed.
Associated symptoms (sleep quality, urinary, bowel, hormone-related, and sexual activity) likely arise from the primary and adjuvant treatments provided to men with prostate cancer. Urinary, bowel, and sexual activity complications often are a result of tissue damage within the radiation field. Similarly, hormone-related and sexual activity symptoms can develop from the use of adjuvant treatments. These treatment-related complications likely interfere with sleep quality and other activities of daily living, which may synergistically contribute to CRF. Therefore, the use of self-management interventions targeting these correlated symptoms, such as behavioral sleep interventions, might provide corresponding relief of CRF (Feng et al., 2019).
Regarding lifestyle factors associated with CRF, it was observed that higher levels of moderate to vigorous exercise and activities were associated with lower CRF in survivors of prostate cancer without bone metastasis. Exercise is the predominant management strategy currently recommended by supportive care professional organizations for CRF (Mitchell et al., 2017; NCCN, 2020). Although exercise is highly recommended, more research regarding the type, frequency, duration, and intensity is needed to provide clinicians with a practical guideline for prescribing this intervention (Bernardo & Becker, 2017; Brown et al., 2012). In addition, the use of multicomponent management strategies, such as mind–body interventions, may be more effective at alleviating symptomatology by mitigating not only CRF but also co-occurring and/or associated symptoms (Feng et al., 2019; McQuade et al., 2017).
An important finding from this study was that physical activity was not associated with CRF in men with bone metastasis. Much of the existing literature on exercise has excluded those with advanced disease, resulting in limited understanding of its benefits for this clinical population (Knowlton et al., 2020). Previous studies that have investigated physical activity in those with advanced cancer showed that physical activity had no effect on fatigue. The prevalence of fatigue was similar between those with advanced cancer who adhered to the American College of Sports Medicine (ACSM) exercise guidelines and those who did not adhere to the guidelines (Knowlton et al., 2020). Similarly, in a study by Oldervoll et al. (2011), individuals with advanced cancer randomized to a physical exercise group had no difference in physical fatigue scores compared to those in the usual care group. More research is needed to better understand the efficacy of physical activity for CRF in those with advanced cancer and to investigate whether different approaches to fatigue management (e.g., mind–body) might be more beneficial for those with advanced disease.
Limitations
Strengths of this study include the design, specifically the use of a cancer registry and validated symptom assessments. The inclusion of men across the disease trajectory, including those with metastatic disease, provides a unique perspective to understanding CRF. The limitations of this study include the cross-sectional nature of the registry, being restricted to the data contained in the registry, the small sample size, and lack of racial and ethnic diversity in the sample. A larger sample with more racial and ethnic diversity might have different factors associated with CRF that would be important to identify for personalized education and management.
Implications for Nursing
Identifying potential demographic, symptom, and lifestyle factors associated with CRF has clinical and research relevance. Knowledge of demographic, symptom, and lifestyle correlates of CRF may help clinicians to identify patients at risk for CRF onset or intensification. These at-risk patients may benefit from early education and intervention to prevent or mitigate CRF. The findings also support the belief that survivors of prostate cancer without metastasis may benefit more from vigorous exercise (e.g., jogging or running, fast bicycling, circuit weight training, aerobic dance, martial arts, jumping rope, swimming) and moderate to vigorous home activities (e.g., mowing the lawn, general lawn and garden maintenance, vacuuming) to mitigate CRF. This aligns with the ACSM guidelines that state that individuals should strive for 90–150 minutes of moderate aerobic activity during the week, with the inclusion of resistance training (ACSM, 2019; Bower, 2014; Knowlton et al., 2020). These guidelines should be considered when providing education about CRF management in the clinical setting. However, caution needs to be taken in those with advanced or metastatic disease when prescribing exercise (NCCN, 2020), particularly because the findings from this study demonstrated that physical activity was not associated with CRF in men with bone metastasis.
The findings of the current study also support the need for development and testing of individualized, tailored interventions that support the ACSM guidelines and account for the disease trajectory and stage. Of note, additional research is needed in those with advanced and metastatic prostate cancer, particularly to the bone. Enhanced understanding of barriers and facilitators to exercise interventions is needed in survivors of cancer across the disease trajectory (Knowlton et al., 2020). Research to improve CRF assessment and management, particularly in relation to exercise, is vital to enhance the lives of men with prostate cancer across the disease trajectory. 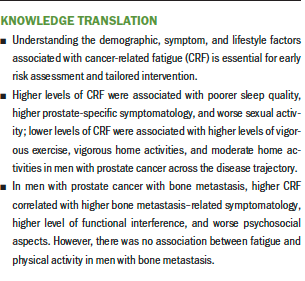
Conclusion
This study identified demographic, symptom, and lifestyle factors associated with CRF in men with prostate cancer. Importantly, this study highlighted the potential benefit of moderate to vigorous physical activity for CRF in survivors of prostate cancer without metastasis. However, the findings also highlighted that physical activity may not be beneficial for CRF for men with bone metastasis, underscoring the important need for CRF intervention research across the cancer disease trajectory. Understanding the demographic, symptom, and lifestyle factors associated with CRF can aid in the development and delivery of tailored education and management strategies to reduce this common and distressing symptom.
About the Author(s)
Kristin Dickinson, PhD, RN, OCN®, is an assistant professor, Andrew Lim, BSN, RN, is a graduate research assistant, and Kevin A. Kupzyk, PhD, is an assistant professor, all in the College of Nursing at the University of Nebraska Medical Center in Omaha. The collection of data used in this study was supported by the Integrated Cancer Repository for Cancer Research, which was developed and maintained by the Cancer Research Informatics Office and the Clinical Trials Office at the Fred and Pamela Buffett Cancer Center and is funded by a National Cancer Institute Cancer Center Support Grant under award number P30CA036727. Dickinson contributed to the conceptualization and design and completed the data collection. Dickinson and Kupzyk provided statistical support. All authors provided the analysis and contributed to the manuscript preparation. Dickinson can be reached at kristin.dickinson@unmc.edu, with copy to ONFEditor@ons.org. (Submitted November 2020. Accepted January 28, 2021.)




