Impact of Hyperglycemia and Age on Outcomes in Patients With Acute Myeloid Leukemia
Purpose/Objectives: To examine the prevalence and impact of hyperglycemia on health outcomes (number of neutropenic days, infection, and hospital length of stay) in patients hospitalized for acute myeloid leukemia (AML) receiving initial induction therapy.
Design: Retrospective, descriptive study.
Setting: A large urban hospital in Indianapolis, Indiana.
Sample: 103 patients with AML and a subset of 41 patients aged 65 years or older.
Methods: Demographics and medical information were extracted from electronic health records. Serum-fasting blood glucose was used to assess glycemic status. The association of hyperglycemia with the health outcomes was analyzed. A subset of patients aged 65 years or older was also analyzed.
Main Research Variables: Hyperglycemia, age, and health outcomes in patients with AML.
Findings: Forty patients experienced hyperglycemia during initial induction for AML. In the larger sample, no associations were noted between hyperglycemia and health outcomes. A significant relationship (p = 0.022) was noted between hyperglycemia and infection in patients aged 65 years or older. Patients aged 65 years or older had 5.6 times the risk of developing infection as those aged younger than 65 years. Although not statistically significant, patients aged 65 years or older with hyperglycemia had 2.5 more days of neutropenia and 1.5 days longer hospital length of stay.
Conclusions: This study provides preliminary evidence that hyperglycemia is prevalent during initial induction for AML and may have harmful consequences, particularly for patients aged 65 years or older. More research is needed to determine clinically significant levels of hyperglycemia and their impact on health outcomes.
Implications for Nursing: Oncology nurses can assess and proactively collaborate with members of the healthcare team to implement strategies to prevent or mitigate the harmful consequences of hyperglycemia.
Jump to a section
Acute myeloid leukemia (AML), a hematologic cancer, is the most common type of acute leukemia in adults, particularly among older adults (O’Donnell et al., 2012). The incidence of AML increases with age (Rodak, Fritsma, & Keohane, 2011), with the median age being 67 years at diagnosis (O’Donnell et al., 2012). The diagnosis and treatment of AML are associated with acuity and with symptom and side effect profile (O’Donnell et al., 2012). One side effect, hyperglycemia, has been shown to be detrimental in critical care and general medical-surgical patients (Richardson & Pollack, 2005), but is not well understood in patients with cancer.
Hyperglycemia, a disorder of glucose metabolism, is clinically defined as blood glucose of 126 mg/dl or greater (American Diabetes Association [ADA], 2015). Hyperglycemia is common in critical care and hospitalized patients, with about 32% of adult patients incurring it during hospitalization (Swanson, Potter, Kongable, & Cook, 2011). However, much less is known regarding the prevalence of hyperglycemia in patients with cancer. No studies have examined the prevalence of hyperglycemia as defined by the ADA in patients with AML. Ali et al. (2007) examined hyperglycemia in patients with AML, using different thresholds for hyperglycemia, and found that 91% of 283 patients with AML had at least one blood glucose level greater than 110 mg/dl, and 52% had at least one blood glucose level greater than 150 mg/dl during the course of treatment. These findings suggest that hyperglycemia may be a common problem in patients with AML; however, more research is needed.
Hyperglycemia has also been shown to have a detrimental impact on health outcomes. In critically ill and general medical-surgical patients, researchers have demonstrated an association between hyperglycemia and increased incidence of infection and sepsis (Benfield, Jensen, & Nordestgaard, 2007; Mraovic et al., 2010), longer hospital length of stay (HLOS) (Carr, 2001; Krinsley, 2003), and increased morbidity and mortality (Kreutziger, Schlaepfer, Wenzel, & Constantinescu, 2009; Krinsley, 2003; Umpierrez et al., 2002; Van den Berghe et al., 2001).
Among patients with cancer, emerging experimental and clinical evidence suggests that hyperglycemia may affect health outcomes. Researchers have found that hyperglycemia may alter response to treatment (Biernacka et al., 2013; Ma et al., 2014; Trédan, Galmarini, Patel, & Tannock, 2007; Zeng et al., 2010) and result in other poor health outcomes (e.g., infection, mortality, longer HLOS) in patients with cancer (Storey & Von Ah, 2012). However, the significance of hyperglycemia for outcomes in patients with AML is largely unknown and has yet to be fully explored. To date, only three studies have been conducted to examine the impact of hyperglycemia on health outcomes in patients with AML (Ali et al., 2007; Matias Cdo, Lima, Teixeira, Souto, & Magalhães, 2013; Storey & Von Ah, 2015).
Only one study has examined the impact of hyperglycemia on neutropenic days, and it found that patients with hyperglycemia were more likely (odds ratio [OR] = 1.6, p < 0.01) to experience neutropenia (Storey & Von Ah, 2015). The influence of hyperglycemia on number of neutropenic days is important to study. Patients with AML are immunocompromised and are susceptible to infections. The presence of hyperglycemia has been shown to decrease the activity of white blood cells (Collier, Dossett, May, & Diaz, 2008; Price & Knight, 2009), which could contribute to longer neutropenic periods and greater risk of subsequent infections for patients with AML during induction.
In a sample of 283 patients with AML, Ali et al. (2007) found that hyperglycemia increased the odds of developing infection and sepsis (OR = 1.15, p < 0.005), severe sepsis (OR = 1.24, p < 0.001), or severe sepsis with respiratory failure (OR = 2.04, p < 0.001), and it was linked with increases in hospital mortality (p < 0.001). Matias Cdo et al. (2013) noted increased odds for developing a complicated infection (OR = 3.97, p < 0.001) and death (OR = 3.55, p < 0.001) in those with hyperglycemia, compared to those without hyperglycemia. In contrast, Storey and Von Ah (2015) did not find a relationship between hyperglycemia and infection in a pilot study of 42 patients with leukemia, which included patients with AML. However, they did find that the patients with hyperglycemia had longer HLOS (2 days versus 15 days, p = 0) than patients with normoglycemia (Storey & Von Ah, 2015). Together, these findings suggest that hyperglycemia has a detrimental impact on health outcomes in patients with AML.
However, these studies failed to examine the impact of age, which is a risk factor for hyperglycemia (Hammer, Motzer, Voss, & Berry, 2010) and AML (Rodak et al., 2011), on health outcomes (Payandeh, Aeinfar, & Aeinfar, 2012). A lifetime of cellular exposure to low-grade inflammation and high levels of oxidative stress creates a milieu that facilitates the development of hyperglycemia, diabetes (Onitilo et al., 2012), and cancer (Fulop et al., 2010). Alterations in cellular signaling and response accompany aging, decreasing the ability of the immune system to conduct surveillance and immunoediting activities (Fulop et al., 2010). Overall, research is needed to examine the relationship of hyperglycemia to outcomes in patients with AML and to compare these outcomes between older and younger patients.
The purpose of this study was to examine the prevalence of hyperglycemia and its impact on health outcomes in patients with AML, including older versus younger patients. Specific aims were to: (a) identify the prevalence of hyperglycemia in patients hospitalized for induction therapy for the treatment of AML; (b) examine the impact of hyperglycemia (mean fasting blood glucose [FBG] = 126 mg/dl or greater) on number of neutropenic days, infection, and HLOS; and (c) examine the impact of hyperglycemia on health outcomes (number of neutropenic days, infection, and HLOS) in a subset of the sample who were aged 65 years or older.
Methods
This study used a retrospective, descriptive design. Patients were treated at an 800-bed tertiary hospital in the Midwest from January 1, 2006, to April 30, 2014, and met the following inclusion criteria: (a) diagnosed with AML, (b) received the 7 + 3 chemotherapy regimen for initial induction (seven days of continuous IV cytarabine [DepoCyt®] infusion, during which an anthracycline is administered for three days), (c) were aged 18 years or older, and (d) had serum FBG test results during hospital admission. Patients with AML receiving reinduction or consolidation therapy or foregoing treatment for hospice were excluded from the study. The initial induction phase is a critical time period for patients with AML. The response to treatment during initial induction can determine the need for subsequent reinductions.
Data Collection
This study was approved by the Indiana University Institutional Review Board. Consecutive electronic health records (EHRs) were reviewed. Data were extracted and transcribed to a data collection tool developed by the investigators to track daily laboratory values and major study outcomes. All data were entered into an encrypted electronic database with access limited to study personnel.
Measures
Demographic and medical information was collected from the EHR to describe the sample. Demographic information included age, gender, and ethnicity. Medical information included height, weight, body mass index, diagnosis of diabetes mellitus (“no” or “yes”), and comorbidities.
Hyperglycemia was defined as a mean number of FBG values 126 mg/dl or greater during the induction period. Only serum FBG values analyzed in the hospital laboratory were collected. Frequencies were used to determine the percentage of patients with AML in the hyperglycemia category. Prevalence was calculated based on previous studies (Hardy, Nowacki, Bertin, & Weil, 2010), with the mean of the total number of occurrences of FBG values at 126 mg/dl or greater divided by the mean of the total number of FBG tests for all patients taken during the induction period.
Health outcomes (neutropenic days, infection, HLOS) were evaluated from the day of hospital admission for induction therapy to the date of reinduction, discharge, or hospital death.
Neutropenic days: The number of neutropenic days was defined as the number of days the absolute neutrophil count (ANC) was less than 500 cells/mm3 from date of initiation of induction chemotherapy to reinduction chemotherapy, discharge from the hospital, or death. An ANC of less than 500 cells/mm3 is clinically considered neutropenia, and precautions are set in place to protect the patient from infection.
Infection: Infection was defined as urinary tract infection (UTI); Clostridium difficile (C. diff); and positive bacterial, viral, or fungal cultures. UTI and C. diff were defined based on elevated colony counts per laboratory urinalysis and polymerase chain reaction, respectively. Septicemia was identified through laboratory results and validated by an infection prevention specialist using the criteria established by the Centers for Disease Control and Prevention (2015). Sepsis was defined when two or more criteria for systemic inflammatory response syndrome were present (Kaukonen, Bailey, Pilcher, Cooper, & Bellomo, 2015; Martin, 2012) and validated by an infection prevention specialist. The presence of infection was extracted as a dichotomous variable (“no” or “yes”) from the EHR and based on laboratory results or physician documentation.
Hospital length of stay: HLOS was recorded as the number of days from the date of admission for induction therapy to date of reinduction, discharge from hospital, or death.
Comorbidities, or co-occurring illnesses, were determined by reviewing admission health and physical forms and discharge summaries. Comorbidities were documented using the Charlson Comorbidity Index (CCI) (Charlson, Pompei, Ales, & Mackenzie, 1987). The researchers used online CCI calculators to determine the CCI score for all study participants and recorded each score on the data collection tool.
Data Analysis
Data analysis was performed using SPSS®, version 22.0. The level of statistical significance for all analyses was set at p ≤ 0.05. Two-sided statistical tests were performed because significant differences in either direction were of interest. Descriptive statistics were used to describe the sample.
Continuous variables (age, neutropenic days, actual body mass index, and HLOS) were analyzed as arithmetic mean, with standard deviation (SD) or median and interquartile range dependent on the parametric nature of the data. Frequencies and percentages were used to describe the categorical variables (gender, diabetic status, presence of hyperglycemia, infection, septicemia, sepsis, and comorbidities). Nonparametric data (age, neutropenic days, and HLOS) were compared using the Mann–Whitney U test. Fisher’s exact testing was used to compare nominal data (gender, infection, septicemia, and sepsis) between patients aged younger than 65 years and patients aged 65 years or older who had hyperglycemia.
Results
Data were abstracted from 103 adult patients who had received the standard chemotherapy for initial induction for AML from January 2006 to April 30, 2014. Initial induction therapy includes seven days of continuous IV cytarabine (also known as Ara-C) and the administration of an anthracycline (either idarubicin [Idamycin®] or daunorubicin [Cerubidine®]) for the first three days (O’Donnell et al., 2012; Tefferi & Letendre, 2012). A total of 2,429 FBG laboratory values were collected within the induction period, which, on average, consisted of a duration of 37 days (range = 2–55 days).
Sample Characteristics
The majority of patients were Caucasian and male, and they had a mean age of 59.3 years (SD = 14.7 years). Patients were, on average, pre-obese (25–29.99 kg/m2) according to classification criteria from the World Health Organization (2015). The majority of patients in the sample did not have a documented diagnosis of diabetes mellitus; however, 40 (39%) experienced hyperglycemia during the induction period. Table 1 summarizes patient demographic and medical characteristics for the larger sample (N = 103). No differences were noted between the larger sample and the subset aged 65 years or older (n = 41).
In the subset aged 65 years or older, the mean age was 73 years (SD = 6.49 years). These patients were also, on average, pre-obese. Patients aged 65 years or older had greater CCI scores than those who were aged younger than 65 years. The majority of patients in the subset did not have a documented diagnosis of diabetes; however, 16 experienced hyperglycemia during the induction period.
The mean number of days of hyperglycemia in the larger sample was 6.25 (range = 0–48). The mean number of FBG tests was 23.6 per hospital induction period, and the prevalence rate of hyperglycemia was 27%. Among patients aged 65 years or older, hyperglycemia was more prevalent. The mean number of days of hyperglycemia for the older group was 9.4 (range = 0–46). The mean number of FBG tests was 25.4 per hospital induction period, and the prevalence rate of hyperglycemia was 37%.
Impact of Hyperglycemia on Health Outcomes
The authors examined the health outcomes in patients experiencing hyperglycemia during induction for AML. In addition, they studied these outcomes in a subset of patients with hyperglycemia who were aged 65 years or older. The health outcomes included the number of neutropenic days, infection, and HLOS.
Neutropenic days: No association was found between hyperglycemia and number of neutropenic days in those aged younger than 65 years or those aged 65 years or older. Although not statistically significant, of note is that those aged 65 years or older whose mean FBG was 126 mg/dl or greater experienced 2.5 more days of neutropenia than those aged younger than 65 years with a comparable FBG.
Infection: The primary types of infections were UTI, C. diff, and positive viral and fungal cultures. However, other miscellaneous documented infectious conditions were included, such as abscesses, stomatitis, severe mucositis, colitis, gastritis, cellulitis, tonsillitis, typhlitis, otitis, parotitis, and pneumonia. Because of the low number of cases of septicemia and sepsis, all infections were analyzed and reported as a composite finding.
Hyperglycemia was not associated with infection in the larger sample of patients with AML. However, among the subset of 41 patients aged 65 years or older, a significant association was noted among those with hyperglycemia and development of infection (p = 0.022, OR = 5.6, 95% confidence interval [CI] [1.43, 22.2]).
Hospital length of stay: No significant association was found between hyperglycemia and HLOS for patients in the larger sample and the subset aged 65 years or older. Although not statistically significant, those aged 65 years or older whose mean FBG was 126 mg/dl or greater did have a 1.5 day longer HLOS for induction than those aged younger than 65 years with a comparable mean FBG.
Discussion
The purpose of this study was to examine the prevalence and impact of hyperglycemia and of hyperglycemia plus older age to health outcomes (neutropenic days, infection, and HLOS) in patients with AML, including those of patients aged younger than 65 years versus those aged 65 years or older. To the authors’ knowledge, this is the first study to comprehensively examine the prevalence of hyperglycemia and its impact on health outcomes in patients with AML during the induction period, as well as to explore these relationships in older adult cancer survivors, who make up 60% of patients with cancer (National Cancer Institute, 2014). Similar to other studies, the incidence of hyperglycemia was quite high (39%), with the prevalence rate (total number of occurrences during the induction period) being 27% and 37% for the larger and aged 65 years or older groups, respectively. ADA (2015) recommends that hyperglycemia be promptly identified and treated. The study findings for incidence and prevalence should alert clinicians to the need for monitoring and treatment of hyperglycemia in hospitalized patients with AML.
In the overall sample, hyperglycemia was not associated with neutropenic days, infection, or HLOS. In the subset sample of patients aged 65 years or older, hyperglycemia was not significantly associated with neutropenic days or HLOS, although those aged 65 years or older with hyperglycemia did have more neutropenic days than those aged younger than 65 years with hyperglycemia. These findings are similar to those of Karnchanasorn, Malamug, Jin, Karanes, and Chiu (2012), who failed to find a significant relationship between hyperglycemia and neutropenic days in patients receiving a bone marrow transplantation (BMT). Conversely, two studies demonstrated a significant association between hyperglycemia and a greater number of neutropenic days in, respectively, patients undergoing BMT and patients with various leukemia diagnoses (Sheean, Freels, Helton, & Braunshweig, 2006; Storey & Von Ah, 2015).
Although these findings were not statistically significant in the current study, they are clinically meaningful. Patients with AML experience profound bone marrow suppression as a result of the disease process and treatment. The influence of hyperglycemia on the white blood cells can prolong suppression by blunting white blood cell activity and delaying recovery (Collier et al., 2008; Price & Knight, 2009). This, in turn, increases the vulnerability to infection in an already highly susceptible patient population. Additional days of neutropenia may result in increased risk for infection, longer HLOS, and increased healthcare costs. More research is needed to explore the impact of hyperglycemia on the number of neutropenic days.
Hyperglycemia was not associated with infection in the total sample. However, a significant relationship was found in patients aged 65 years or older between hyperglycemia and the development of infection. Fuji et al. (2007) studied 112 patients undergoing BMT who were categorized as having normoglycemia or mild, moderate, or severe hyperglycemia and found no difference in overall infection rates among the groups. In addition, Storey and Von Ah (2015), in their pilot analysis, did not find a significant relationship between hyperglycemia and infection in patients with various types of leukemia.
In contrast, Weiser et al. (2004) noted, in 287 patients with acute lymphoblastic leukemia (ALL), that those with hyperglycemia were 40% (p = 0.016) more likely to develop a complicated infection (pneumonia or fungal). Matias Cdo et al. (2013) similarly noted that hyperglycemia increased the risk of complicated infections (sepsis, respiratory, or renal failure; OR = 3.97; 95% CI [2.08, 7.57]; p < 0.001) but did not increase the risk of fungal infections in patients with ALL and AML undergoing induction therapy. When assessing a glucose control intervention in patients undergoing BMT, Fuji et al. (2009) noted a significantly higher incidence of documented infection among those with hyperglycemia. Likewise, among 1,175 patients undergoing BMT, hyperglycemia was noted to increase the risk of infection (Hammer et al., 2009). To the authors’ knowledge, this was the first study to examine the relationship of hyperglycemia to infection in an older subset of patients with AML, and more studies are needed to confirm the findings.
In the current study, HLOS was 1.5 days longer for those aged 65 years or older with hyperglycemia. Soysal et al. (2012) found a nonsignificant increase of one day longer HLOS among patients with hyperglycemia. Although this finding was not statistically significant, it may be clinically meaningful in caring for patients with AML. The impact of hyperglycemia on induction HLOS in patients with AML has not been well studied. In patients undergoing BMT (including patients with leukemia), HLOS among those with hyperglycemia (150 mg/dl or greater) were longer (mean = 17 days, SD = 6 days versus mean = 14 days, SD = 4 days, p = 0.0001) than for those without hyperglycemia (Karnchanasorn et al., 2012). Garg, Bhutani, Alyea, and Pendergrass (2007) noted a significant increase in HLOS (24 days versus 16 days) in 126 patients undergoing BMT with hyperglycemia. Similarly, Storey and Von Ah (2015) noted that patients with leukemia who had hyperglycemia had longer HLOS (19.6 days versus 5.4 days; p = 0.02) than those with normoglycemia.
The management of glucose not only improves outcomes for patients but also has ramifications for hospitals. The cascading effect of hyperglycemia, infection, and longer HLOS for already at-risk patients with AML, particularly those aged 65 years or older, can have negative financial consequences for hospitals.
Limitations
Findings of this study must be reviewed in light of certain study limitations. The study size was small, limiting generalizability. The retrospective study design may have limited information as it related to prediabetes, undiagnosed diabetes, or glucose-intolerant individuals. Clinical acuity may have been an important factor that could have influenced health outcomes, but this was difficult to measure when abstracting data from charts. In addition, the ability to tolerate activity, nutritional support, and pharmacologic treatments, such as corticosteroids, antibiotics, insulin, or hypoglycemic agents, was not assessed. These factors are likely to influence blood glucose levels and moderate their impact on glycemic status and subsequent outcomes. These variables, or yet unknown factors that were not extrapolated from the data, may have confounded the results. Additional studies should include and account or control for these factors.
Strengths of the study included a specific time period within the trajectory of treatment for AML. Standard treatment regimens and similarities in the expected symptom profiles and HLOS among this patient group during this time period reduced variability. The use of only one abstractor of data facilitated quality control and consistency in its collection. An additional strength of this study was the use of the CCI to control for the influence, number, and severity of comorbidities on the health outcomes.
Implications for Nursing
This study provides important information regarding the prevalence of hyperglycemia and its relationship to the health outcomes of vulnerable hospitalized patients with AML during initial induction therapy. Nurses knowledgeable about the prevalence and ramifications of hyperglycemia can proactively identify, assess, and intercede on behalf of these patients. The findings from this study will facilitate nurses in the development of a patient-specific plan of care that integrates the knowledge of disease process, cancer therapy, and changes in diet and activity on blood glucose. Oncology nurses play an important role in the prompt identification of hyperglycemia and can collaborate with members of the multidisciplinary healthcare team to implement strategies to prevent or mitigate the harmful consequences of hyperglycemia.
Future research should include the analysis of actual blood glucose measurements or aggregated mean blood glucose, or examining various thresholds for hyperglycemia as it relates to the impact on health outcomes rather than categorizing hyperglycemia only as a dichotomous variable. Assessment of the onset, duration, and number of occurrences of hyperglycemia during the induction period could facilitate the identification of times when patients are most likely to experience hyperglycemia and to which interventions can be targeted.
Conclusion
This study provides preliminary evidence that hyperglycemia is prevalent during induction for AML and may have harmful consequences for the hospitalized patient with AML, particularly in those aged 65 years and older. The limited number of studies on this important topic reveals gaps in knowledge about the full effects of hyperglycemia on the health outcomes of hospitalized patients with AML. More research is needed to elucidate clinically significant levels of hyperglycemia and its impact on health outcomes to inform interventions to mitigate symptoms and improve quality of life for hospitalized patients with AML.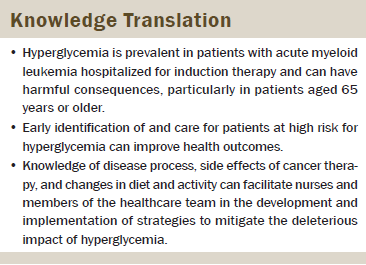
References
Ali, N.A., O’Brien, J.M., Jr., Blum, W., Byrd, J.C., Klisovic, R.B., Marcucci, G., . . . Grever, M.R. (2007). Hyperglycemia in patients with acute myeloid leukemia is associated with increased hospital mortality. Cancer, 110, 96–102. doi:10.1002/cncr.22777
American Diabetes Association. (2015). Standards of medical care in diabetes—2015. Diabetes Care, 38(Suppl.), S1–S94.
Benfield, T., Jensen, J.S., & Nordestgaard, B.G. (2007). Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia, 50, 549–554.
Biernacka, K.M., Uzoh, C.C., Zeng, L., Persad, R.A., Bahl, A., Gillatt, D., . . . Holly, J.M. (2013). Hyperglycemia-induced chemoresistance of prostate cells due to IGFBP2. Endocrine-Related Cancer, 20, 741–751. doi:10.1530/ERC-13-0077
Carr, M.E. (2001). Diabetes mellitus: A hypercoagulable state. Journal of Diabetes and Its Complications, 15, 44–54.
Centers for Disease Control and Prevention. (2015). Bloodstream infection event (central line–associated bloodstream infection and non–central line–associated bloodstream infection). Retrieved from http://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf
Charlson, M.E., Pompei, P., Ales, K.L., & Mackenzie, C.R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40, 373–383. doi:10.1016/0021-9681(87)90171-8
Collier, B., Dossett, L.A., May, A.K., & Diaz, J.J. (2008). Glucose control and the inflammatory response. Nutrition in Clinical Practice, 23, 3–15. doi:10.1177/011542650802300103
Fuji, S., Kim, S.W., Mori, S., Fukuda, T., Kamiya, S., Yamasaki, S., . . . Takaue, Y. (2007). Hyperglycemia during the neutropenia period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation, 84, 814–820.
Fuji, S., Kim, S.W., Mori, S., Kamiya, S., Yoshimura, K., Yokoyama, H., . . . Fukuda, T. (2009). Intensive glucose control after allogeneic hematopoietic stem cell transplantation: A retrospective matched-cohort study. Bone Marrow Transplantation, 44, 105–111. doi:10.1038/bmt.2008.431
Fulop, T., Kotb, R., Fortin, C.F., Pawelec, G., de Angelis, F., & Larbi, A. (2010). Potential role of immumosenescence in cancer development. Annals of the New York Academy of Sciences, 1197, 158–165.
Garg, R., Bhutani, H., Alyea, E., & Pendergrass, M. (2007). Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation. Diabetes Care, 30, 993–994. doi:10.2337/dc06-2563
Hammer, M.J., Casper, C., Gooley, T.A., O’Donnell, P.V., Boeckh, M., & Hirsch, I.B. (2009). The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biology of Blood and Marrow Transplantation, 15, 344–351.
Hammer, M.J., Motzer, S.A., Voss, J.G., & Berry, D.L. (2010). Glycemic control among older adult hematopoietic cell transplant recipients. Journal of Gerontological Nursing, 36, 40–50.
Hardy, S.J., Nowacki, A.S., Bertin, M., & Weil, R.J. (2010). Absence of an association between glucose levels and surgical site infections in patients undergoing craniotomies for brain tumors. Journal of Neurosurgery, 113, 161–166. doi:10.3171/2010.2.JNS09950
Karnchanasorn, R., Malamug, L.R., Jin, R., Karanes, C., & Chiu, K.C. (2012). Association of hyperglycemia with prolonged hospital stay but no effect on engraftment after autologous hematopoietic stem cell transplantation. Endocrine Practice, 18, 508–518.
Kaukonen, K.M., Bailey, M., Pilcher, D., Cooper, D.J., & Bellomo, R. (2015). Systemic inflammatory response syndrome criteria in defining severe sepsis. New England Journal of Medicine, 372, 1629–1638. doi:10.1056/NEJMoa1415236
Kreutziger, J., Schlaepfer, J., Wenzel, V., & Constantinescu, M.A. (2009). The role of admission blood glucose in outcome prediction of surviving patients with multiple injuries. Journal of Trauma, 67, 704–708. doi:10.1097/TA.0b013e3181b22e37
Krinsley, J.S. (2003). Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clinic Proceedings, 78, 1471–1478.
Ma, Y.S., Yang, I.P., Tsai, H.L., Huang, C.W., Juo, S.H., & Wang, J.Y. (2014). High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells. DNA and Cell Biology, 33, 64–72. doi:10.1089/dna.2013.2161
Martin, G.S. (2012). Sepsis, severe sepsis and septic shock: Changes in incidence, pathogens and outcomes. Expert Review of Anti-Infective Therapy, 10, 701–706. doi:10.1586/eri.12.50
Matias Cdo, N., Lima, V., Teixeira, H.M., Souto, F.R., & Magalhães, V. (2013). Hyperglycemia increases the complicated infection and mortality rates during induction therapy in adult acute leukemia patients. Revista Brasileira de Hematologia e Hemoterapia, 35, 39–43. doi:10.5581/1516-8484.20130013
Mraovic, B., Hipszer, B.R., Epstein, R.H., Pequignot, E.C., Parvizi, J., & Joseph, J.I. (2010). Preadmission hyperglycemia is an independent risk factor for in-hospital symptomatic pulmonary embolism after major orthopedic surgery. Journal of Arthroplasty, 25, 64–70. doi:10.1016/j.arth.2008.10.002
National Cancer Institute. (2014). Office of Cancer Survivorship: Statistics. Retrieved from http://cancercontrol.cancer.gov/ocs/statistics/statistics.html
O’Donnell, M.R., Abboud, C.N., Altman, J., Appelbaum, F.R., Arber, D.A., Attar, E., . . . Gregory, K.M. (2012). Acute myeloid leukemia. Journal of the National Comprehensive Cancer Network, 10, 984–1021.
Onitilo, A.A., Engel, J.M., Glurich, I., Stankowski, R.V., Williams, G.M., & Doi, S.A. (2012). Diabetes and cancer II: Role of diabetes medications and influence of shared risk factors. Cancer Causes and Control, 23, 991–1008. doi:10.1007/s10552-012-9971-4
Payandeh, M., Aeinfar, M., & Aeinfar, V. (2012). Treating the elderly patient with acute myelogenous leukemia. In S. Koschmieder & S. Krug (Eds.), Myeloid leukemia—Clinical diagnosis and treatment (pp. 235–258). Rijeka, Croatia: InTech.
Price, C.L., & Knight, S.C. (2009). Methyglyoxal: Possible link between hyperglycaemia and immune suppression? Trends in Endocrinology and Metabolism, 20, 312–317. doi:10.1016/j.tem.2009.03.010
Richardson, L.C., & Pollack, L.A. (2005). Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nature Clinical Practice. Oncology, 2, 48–53.
Rodak, B.F., Fritsma, G.A., & Keohane, E. (2011). Hematology: Clinical principles and applications (4th ed.). St. Louis, MO: Saunders.
Sheean, P.M., Freels, S.A., Helton, W.S., & Braunshweig, C.A. (2006). Adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation, 12, 656–664. doi:10.1016/j.bbmt.2006.01.010
Soysal, D.E., Karakus, V., Seren, A.R., Tatar, E., Celik, M., & Hizar, S. (2012). Evaluation of transient hyperglycemia in non-diabetic patients with febrile neutropenia. European Journal of Internal Medicine, 23, 342–346. doi:10.1016/j.ejim.2011.12.010
Storey, S., & Von Ah, D. (2012). Impact of malglycemia on clinical outcomes in hospitalized patients with cancer: A review of the literature. Oncology Nursing Forum, 39, 458–465. doi:10.1188/12.ONF.458-465
Storey, S., & Von Ah, D. (2015). Incidence and impact of hyperglycemia on hospitalized leukemia patients. European Journal of Oncology Nursing, 19, 13–17. doi:10.1016/j.ejon.2014.08.005
Swanson, C.M., Potter, D.J., Kongable, G.L., & Cook, C.B. (2011). Update on inpatient glycemic control in hospitals in the United States. Endocrine Practice, 17, 853–861. doi:10.4158/EP11042.OR
Tefferi, A., & Letendre, L. (2012). Going beyond 7 + 3 regimens in the treatment of adult acute myeloid leukemia. Journal of Clinical Oncology, 30, 2425–2428. doi:10.1200/JCO.2011.38.9601
Trédan, O., Galmarini, C.M., Patel, K., & Tannock, I.F. (2007). Drug resistance and the solid tumor microenvironment. Journal of the National Cancer Institute, 99, 1441–1454. doi:10.1093/jnci/djm135
Umpierrez, G.E., Isaacs, S.D., Bazargan, N., You, X., Thaler, L.M., & Kitabchi, A.E. (2002). Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes. Journal of Clinical Endocrinology and Metabolism, 87, 978–982.
Van den Berghe, G., Wouters, P., Weekers, F., Verwaest, C., Bruyninckx, F., Schetz, M., . . . Bouillon, R. (2001). Intensive insulin therapy in critically ill patients. New England Journal of Medicine, 345, 1359–1367. doi:10.1210/jc.2009-0663
Weiser, M.A., Cabanillas, M.E., Konopleva, M., Thomas, D.A., Pierce, S.A., Escalante, C.P., . . . O’Brien, S.M. (2004). Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer, 100, 1179–1185. doi:10.1002/cncr.20071
World Health Organization. (2015). BMI classification. Retrieved from http://apps.who.int/bmi/index.jsp?introPage=intro_3.html
Zeng, L., Biernacka, K.M., Holly, J.M.P., Jarrett, C., Morrison, A.A., Morgan, A., . . . Perks, C.M. (2010). Hyperglycaemia confers resistance to chemotherapy on breast cancer cells: The role of fatty acid synthase. Endocrine-Related Cancer, 17, 539–551. doi:10.1677/ERC-09-0221
About the Author(s)
Storey is a visiting assistant scientist and Von Ah is an associate professor and chair of the Department of Community and Health Systems, both in the School of Nursing at Indiana University in Indianapolis. This research was funded, in part, by Sigma Theta Tau International, Alpha Chapter, and the St. Vincent Foundation. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society. Storey and Von Ah contributed to the conceptualization and design and the manuscript preparation. Storey completed the data collection and provided the statistical support and analysis. Storey can be reached at sustorey@iu.edu, with copy to editor at ONFEditor@ons.org. Submitted September 2015. Accepted for publication October 28, 2015.

