Using Survivorship Care Plans to Enhance Communication and Cancer Care Coordination: Results of a Pilot Study
Purpose/Objectives: To compare a structured cancer survivorship care plan (SCP) transition visit versus an SCP transition visit coupled with a coordinated follow-up visit from the primary care provider (PCP).
Design: Pilot randomized, controlled study.
Setting: REX Cancer Hospital, a community cancer center in Raleigh, North Carolina.
Sample: 34 adults completing treatment with curative intent.
Methods: Patients and PCPs completed measures at baseline and at six weeks. Wilcoxon signed rank and rank sum tests were used for comparisons of SCP only versus SCP with PCP follow-up visit, as well as between high- and low-activated patients.
Main Research Variables: Confidence in survivorship information and survivor concerns.
Findings: The intervention was feasible and acceptable to patients and their PCPs. All patients (N = 34) had less contradictory information about care after SCP receipt. PCPs reported improved confidence in patients, regardless of intervention arm. Highly activated or empowered patients benefited more and had increased confidence and fewer concerns about cancer care.
Conclusions: The SCP interventions led to increased confidence in survivorship information, but some benefits were greater for highly activated patients. PCPs also had improved confidence in survivorship care after SCP receipt, whether or not they saw the patient in follow-up. A larger study is needed to further explore these findings and the changes over time.
Implications for Nursing: Nurses can be instrumental in facilitating the development and delivery of SCP to survivors and PCPs.
Jump to a section
The report, From Cancer Patient to Cancer Survivor: Lost in Transition (Hewitt, Greenfield, & Stovall, 2005) provides direction for delivering care to survivors by defining four essential components of survivorship care: (a) prevention of recurrence, new primary cancers, or late effects of treatment; (b) surveillance for recurrence, metastases, new primary cancers, and physical or psychosocial late effects of treatment; (c) interventions for physical, psychosocial, and practical consequences of cancer and its treatment; and (d) coordination of care to execute prevention, surveillance, and interventions, specifically between survivors’ primary care providers (PCPs) and oncologists. Hewitt et al. (2005) highlighted the important role of care coordination for the cancer survivor, which should include the patient, the interdisciplinary oncology team, and the patient’s PCP. A survivorship care plan (SCP) was recommended as a tool to facilitate this coordination. Cancer survivorship care includes components of oncology follow-up, as well as primary and preventive care. However, patients and PCPs often feel unprepared to manage these needs when treatment ends. The SCP is currently designed for patients ending treatment with curative intent with no evidence of disease and is a requirement for accreditation by the American College of Surgeons Commission on Cancer (2012). An SCP is meant to be a communication tool for the survivor, as well as for the PCP, to provide a diagnosis and treatment summary, a cancer surveillance plan, and health promotion strategies. Parry, Kent, Forsyth, Alfano, and Rowland (2013) created a framework to evaluate SCPs that includes proximal and distal outcomes. The authors of the current article focused solely on proximal outcomes, given the short duration of the intervention.
For many cancer survivors, the focus of follow-up care is overwhelmingly on cancer surveillance, leaving other important health concerns, such as screening for other cancers, health promotion, and cancer concerns, relatively unaddressed. Survivors need closer collaboration between their oncology specialists and PCPs in caring for all of their health needs; this coordination should include survivors because of the role they have in their own health behavior self-management based on the Wagner Chronic Care Model (Wagner, 1998; Wagner et al., 2010). The model assumes that patients are informed and activated and that clinicians are prepared to take care of them; this includes self-management support and having productive interactions. An SCP is meant to facilitate these interactions.
The authors’ research regarding SCPs has described the preferred format, content, and timing of delivery of these tools and processes for delivery (Birken, Mayer, & Weiner, 2013; Mayer, Gerstel, et al., 2014; Mayer, Gerstel, Leak, & Smith, 2012). Survivors and PCPs preferred a simple and clear SCP that included a treatment summary and a cancer surveillance plan, including who was responsible for the different components (PCP or oncologist), and include information about health promotion and potential long-term and late effects. Preference for delivery was soon after treatment ended but varied in how helpful or useful it might be because of questions about survivors’ readiness to receive and use this information. Others have similar findings (Keesing, McNamara, & Rosenwax, 2015; Klemanski, Browning, & Kue, 2016).
Patient activation, or empowerment, refers to the degree to which the individual understands he or she must play an active role in managing health and health care, and the extent to which he or she feels able to fulfill that role (Hibbard, Mahoney, Stock, & Tusler, 2007; Hibbard & Tusler, 2007). Specifically, it refers to the individual’s knowledge, skill, and confidence (Hibbard & Mahoney, 2010). A number of studies have shown an association between levels of activation (as measured by the Patient Activation Measure) (Hibbard, Stockard, Mahoney, & Tusler, 2004) and survivor outcomes; however, this variable is not typically controlled for when evaluating cancer interventions and outcomes (Greene & Hibbard, 2012; Hibbard, Greene, & Tusler, 2009). This may be important in understanding who may be more or less likely to benefit from an SCP and in identifying different approaches to address these needs.
The Commission on Cancer’s (2012) standard requires that all survivors undergoing treatment with curative intent receive an SCP. Survivors and providers also have expressed an opinion that receiving an SCP is necessary, but not sufficient, to facilitate this transition in care (Mayer et al., 2012). Current research highlights the barriers to the effective development and systematic implementation of personalized SCPs but provides little evidence supporting its use (Birken, Deal, Mayer, & Weiner, 2014; Birken et al., 2013).
Consequently, the authors of the current article compared a survivor visit with the PCP shortly after receiving an SCP compared to the delivery of an SCP alone. The purpose of the PCP visit was to provide an opportunity to discuss care needs and coordination of survivorship care. The SCP was delivered to all participants (N = 37) during a post-treatment transition visit by a nurse practitioner (NP) for patients who were completing treatment with curative intent. Nineteen patients received an additional semistructured planned follow-up visit with their PCP within four weeks after receiving the SCP. The primary aim of this pilot study was to determine feasibility of the intervention and study measures, and to compare the change in confidence in knowledge of future survivorship care needs between survivors and their PCP from baseline to the six-week follow-up. Secondary aims included comparing changes from baseline to the six-week follow-up in measures of PCP visit completion; cancer care coordination; and survivor concerns, expectations, and satisfaction with care. Measures were controlled for levels of patient activation, reflecting the knowledge, skills, and confidence to manage one’s health associated with health-related outcomes (Greene & Hibbard, 2012).
Methods
The authors conducted a single-center, randomized, controlled pilot intervention study at the REX Cancer Hospital, a community cancer center in Raleigh, North Carolina. The study was approved by the institutional review boards at the University of North Carolina–Chapel Hill and at the REX Cancer Hospital.
Participants
Survivor eligibility included being aged 21 years or older, able to read and speak English, diagnosed with an adult onset cancer, having completed treatment with curative intent within four to six weeks, and having a designated PCP. Exclusion criteria included having metastatic cancer. All survivor participants received $50 gift cards at the completion of baseline and six-week follow-up surveys.
PCP eligibility included having a survivor enrolled in this study, a willingness to complete the study measures, and being able to have a visit with the survivor within six weeks of SCP receipt if the patient was randomized to the SCP plus PCP arm. All provider participants received $50 gift cards at the completion of baseline and six-week follow-up surveys.
Measures
After patient consent was obtained, all patient baseline measures were administered in person on the day of the SCP transition visit but prior to SCP receipt and again six weeks later by phone. The PCPs were contacted via their email address or a mailed letter after their patient was enrolled and completed secure online surveys at baseline and again six weeks after the patient had received his or her SCP.
Study measures for survivors included the primary outcome of confidence in knowledge measured with the Confidence in Survivorship Information (CSI), patient version (Palmer, Mao, Jacobs, & Stricker, 2012). Secondary outcomes of survivorship concerns were measured by the Assessment of Survivor Concerns (ASC) (Gotay & Pagano, 2007), and satisfaction with and expectations of care were measured with the Patient Satisfaction With Cancer Care (Jean-Pierre et al., 2011), the adapted Picker Institute Cancer Survey (PICS) (Ayanian & Jacobsen, 2006), and the Patient Expectations for Cancer Survivorship Care (PECSC) (Cheung & Earle, 2009; Cheung, Neville, Cameron, Cook, & Earle, 2009; Cheung, Neville, & Earle, 2010). The level of patient activation, a potential modifier of SCP effect, was measured by the Patient Activation Measure (Green et al., 2010; Hibbard et al., 2004; Hibbard & Tusler, 2007). Demographic and disease- and treatment-related information were collected from the electronic health record.
Study measures for the PCPs included the primary outcome with the CSI, provider version. Secondary measures included PECSC, provider version, and demographic data (see Figure 1).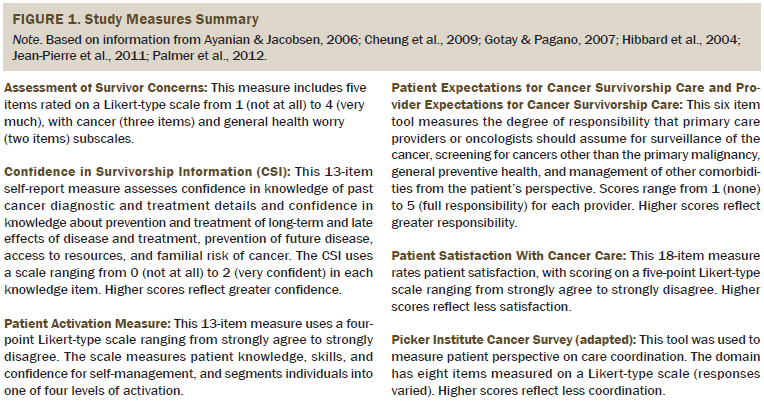
Procedures
Eligible survivor participants were identified each week by the study coordinator, who then contacted the survivor to describe the study, provide the written consent form, and schedule an SCP transition visit within six weeks of ending treatment. Two weeks prior to the scheduled SCP transition visit, the survivor was contacted, given additional details about the study, and consented. The baseline data collection measures were then mailed to the survivor to complete and bring to the visit. Based on information on the survivor’s medical and cancer records, a draft of the SCP was created by the research nurse prior to the visit using an SCP generic template from the American Society of Clinical Oncology (Mayer, Necklyudov, et al., 2014). During the approximately 40-minute transition visit with the oncology NP, the SCP was reviewed with the survivor, revised as needed, and then finalized. A print copy was given to the survivor. All PCPs were sent the final SCP electronically (if accessible within the electronic health record) or by mail.
Survivors’ PCPs were then contacted by email or letter, informing them of the survivor’s participation in the study and inviting them to participate as well. PCPs were given a fact sheet about the study and a confidential email link to complete the secure baseline survey. Completion of the survey implied consent.
Randomization Procedure
Once the consent and baseline measures were collected and the SCP transition visit occurred, participants were then randomized to the SCP only or the SCP plus PCP visit arm. The nurse preparing the SCP and the oncology NP delivering the SCP were blinded to the randomization assignment. The program manager randomized survivors based on a randomized block design, stratifying women with breast cancer—given the anticipated larger enrollment of survivors of breast cancer—to achieve an equal proportion by gender and cancer type.
Similar to the SCP-only arm, survivors in the SCP plus PCP visit arm received a printed copy of their SCP during the SCP transition visit and a clinic staff member then scheduled a PCP visit for the survivor about six weeks after their post-treatment transition visit. Talking points developed for this visit were developed and sent to the PCP along with the SCP to encourage reviewing the SCP, the surveillance schedule, what type of care the PCP would provide, and when and for what type of concerns the survivor should contact the PCP or the oncologist. Talking points also suggested discussing any plans for non–cancer-related health care and health promotion, discussing any residual side effects from treatment that may still be present, and visiting two websites (www.cancersurvivorshipprimarycare.org and www.cancersurvivorshipcentereducation.org) about cancer survivorship care for PCPs.
Analysis
The primary objective in this pilot study was to demonstrate feasibility of the intervention and the measures used. Exploratory analyses were conducted to compare changes in mean CSI scores from baseline to six-week follow-up between survivors receiving the standard SCP delivery and survivors receiving the SCP plus a coordinated PCP visit. For both survivors and PCPs, descriptive statistics are provided for demographics and outcome measures at baseline (overall and separately for the SCP and SCP plus PCP groups) and follow-up at six weeks. Score changes were calculated for each group and compared using Wilcoxon rank sum tests, with test statistics reported as W. In addition, the authors tested whether the changes in scores were significantly different from 0 using Wilcoxon signed rank tests, with test statistics reported as S. Similar tests were conducted for the PCP measures. The authors also explored the effect of patient activation levels on outcomes.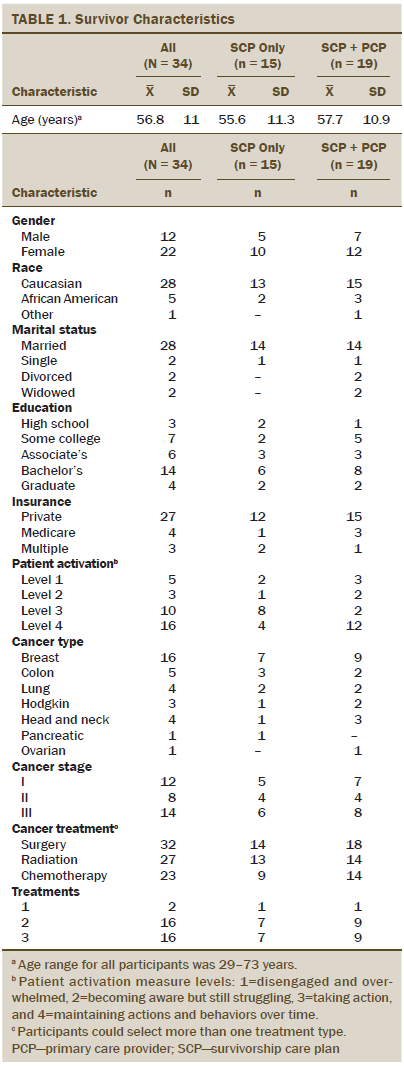
Results
Survivor Participants
Of the 55 eligible survivors approached for this study, 37 consented to participate, received an SCP, and were then randomized. Thirty-four (92%) survivors completed baseline and follow-up measures (19 in the SCP plus PCP group and 15 in the SCP-only group) (see Figure 2), with less than a 10% dropout rate from attrition or disease progression. Demographic and cancer treatment information can be found in Table 1. No significant differences were noted between the SCP group and the SCP plus PCP visit group. Of the 19 survivors who were randomized to the SCP plus PCP arm, 17 successfully attended their PCP visit (two canceled their appointments, and one moved away).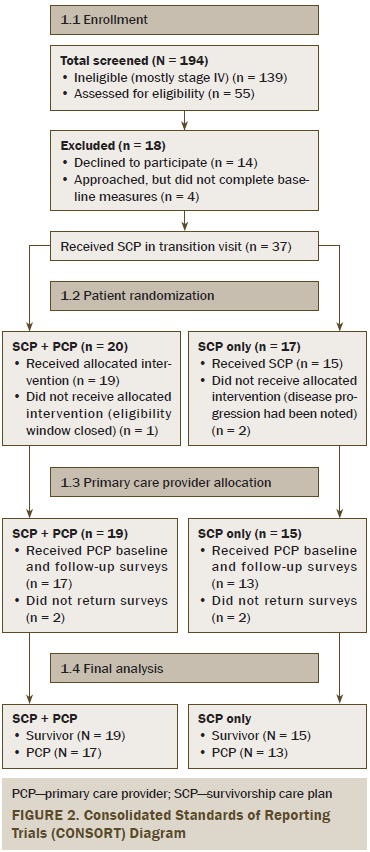
Primary survivor outcome: Confidence in survivorship knowledge improved from baseline to follow-up in all survivors but was not different by group (see Table 2). Mean CSI–past scores (range = 0–2, with higher score indicating more knowledge of diagnosis and treatment) increased from baseline but did not reach significance at six-week follow-up (mean = 1.68, SD = 0.51 versus mean = 1.82, SD = 0.35; S = 21; p = 0.1) for all patients. No difference was noted by intervention group, but there was a difference at baseline between survivors with lower versus higher activation (lower = 1.31 versus higher = 1.79, p = 0.05).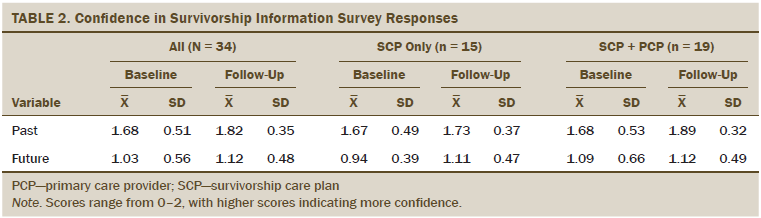
Mean CSI–future scores were not significantly different from baseline to the six-week mark (mean = 1.03, SD = 0.56 versus mean = 1.12, SD = 0.48; S = 39.5; p = 0.38) and were not significantly different between the SCP-only and SCP plus PCP groups (W = 124, p = 0.34) or by level of patient activation (W = 291, p = 0.53).
Secondary survivor outcomes: Cancer worries, measured by the ASC total, general, and cancer specific, were low and did not change before and after SCP delivery (all survivors, p > 0.2) (see Table 3). No significant differences were noted before and after SCP delivery, by level of patient activation, for general worry. There was a borderline difference between SCP and SCP plus PCP groups; the SCP plus PCP group decreased worry by 0.16, whereas the SCP group increased worry by 0.8 (W = 319.5, p = 0.05).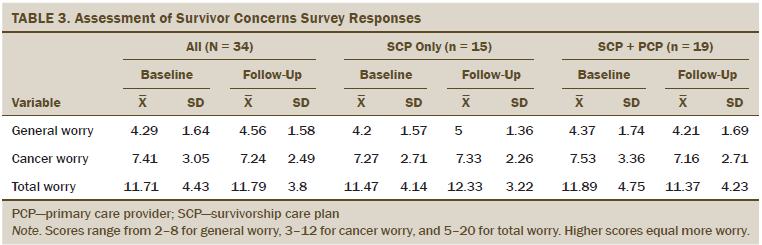
The PICS coordination of care domain scores can be found in Table 4. Although there were no significant differences between baseline and six weeks or by assigned group, differences were seen based on levels of patient activation. At baseline, those with higher levels of activation had better scores (mean = 1.04, SD = 0.2) compared to lower-activated survivors (mean = 1.38, SD = 0.52; W = 100; p = 0.02), reflecting that higher levels of activation were associated with greater coordination of care in follow-up plans. Low-activated survivors improved more from baseline to follow-up (mean = 1.38, SD = 0.52 at baseline to mean = 1, SD = 0 at follow-up; W = 100; p = 0.02). Knowledge about recent medical history, changes in treatment, test results, and who to ask regarding questions did not seem to be an issue and did not change from baseline to follow-up. All survivors reported receiving less confusing information at follow-up (p < 0.0001), and there was a slight, but not significant, improvement in knowing what the next step in care would be after SCP receipt (mean = 1.71, SD = 0.76 to mean = 1.5, SD = 0.56; S = –14; p = 0.06).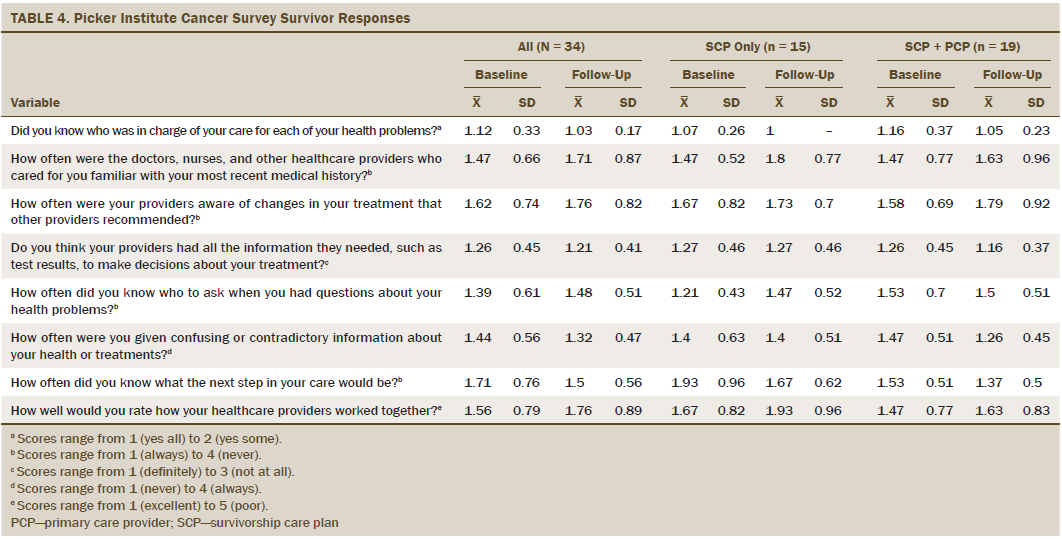
Survivors’ expectations for cancer survivorship care were not significantly different by group, but the authors observed differences between scores at baseline and six weeks in several items (range 1–5, with 5 indicating full responsibility). Survivors reported less responsibility of the oncologist to follow them for cancer at follow-up (baseline mean = 4.65, SD = 0.54; follow-up mean = 4.32, SD = 0.84; S = –40; p = 0.03) and increased discussions about who would provide follow-up for medical problems other than cancer (oncologist, n = 28, 82%; PCP, n = 11, 32%). Survivors had greater expectations that the PCP would provide general preventive health care compared to the oncologist (mean = 4.79, SD = 0.42 versus mean = 2.12, SD = 1.32) and treat other medical problems in addition to cancer (mean = 4.68, SD = 0.53 versus mean = 1.97, SD = 1.16) than the oncologist. Survivors placed similar responsibility on both the PCP (mean = 3.88, SD = 1.15) and oncologist (mean = 3.44, SD = 1.42) in screening for other cancers. All PECSC scores can be found in Table 5.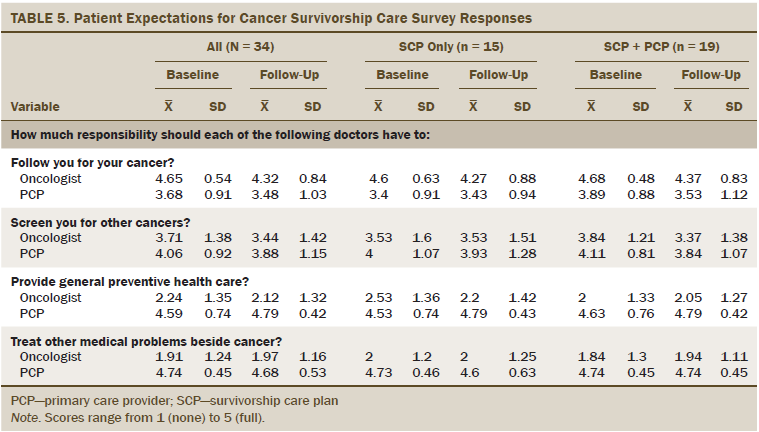
Survivor satisfaction with cancer care scores (range = 18–90, with lower score indicating higher satisfaction) reflected high levels of satisfaction and did not have any significant differences between baseline and six-week follow-up overall, by treatment group, or by level of patient activation.
Primary Care Provider Participants
Of the 34 eligible PCPs, 30 provided baseline and follow-up measures (see Table 6). No significant differences by PCP characteristics were noted between treatment groups, and no PCP saw more than one survivor from the current study’s participant list.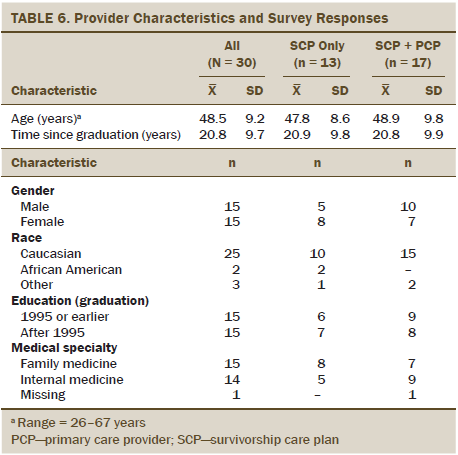
Primary outcome: PCP confidence in survivorship information (range = 0–2, with higher scores indicating more confidence) was assessed using the CSI, with a minor word change of “your patient” in place of “you” (Chronbach alpha = 0.72). CSI scores significantly improved (p = 0.001) from baseline (mean = 1.32, SD = 0.26) to six weeks after SCP delivery (mean = 1.42, SD = 0.29). Improvement in CSI scores did not differ by assigned group, whether they saw a patient in a follow-up visit or not (W = 234, p = 0.19).
Secondary outcomes: PECSC, provider version, scores were not significantly different between baseline and six-week follow-up overall, or between SCP or SCP plus PCP groups in the PCP roles. PCPs clearly identified their role in providing general preventive health care and in treating medical problems other than cancer. Scores were low for discussing with other doctors who would handle medical problems other than cancer, and were also low for discussing this topic with survivors (see Table 7).
[[{"type":"media","view_mode":"media_original","fid":"25656","attributes":{"alt":"","class":"media-image","height":"347","typeof":"foaf:Image","width":"1062"}}]]
Discussion
This randomized, controlled pilot study of the feasibility and impact of a SCP delivered by an oncology NP alone versus with a six-week follow-up visit with the PCP demonstrated improved confidence in survivorship information in both groups. It demonstrated the feasibility of using appropriate measures to evaluate the short-term impact of an SCP as a communication tool for survivors and PCPs (Parry et al., 2013). The findings do raise the question about whether all survivors would benefit equally from the SCP, because there were some differences by level of activation. Patient activation may be a mediator for confidence in understanding and adherence to survivorship care recommendations, but the sample size was too small to test this. Of note, levels of patient activation may influence baseline knowledge.
This study is the third that has identified increased worry after receipt of the SCP, which may be an unintended consequence of SCP delivery, but those who had a PCP visit after SCP delivery had a decreased level in worry. Hershman et al. (2013) found decreased worry over time in women with breast cancer who received the SCP but increases in worry among Latinas in the study (Hershman et al., 2013). Nicolaije et al. (2015) also found increased worry in women with endometrial cancer who received an SCP, which was associated with greater healthcare use. Increased worry may be an unintended consequence in a subset of survivors.
This pilot study successfully demonstrated feasibility in offering an SCP with or without a PCP visit and in measuring short-term outcomes. This will need to be replicated in a larger, more diverse population with longer follow-up to measure additional outcomes, such as adherence to cancer surveillance or adoption of health behaviors, and the impact that patient activation has on them. In the CanCORS study of colorectal and lung cancer survivors, Chrischilles et al. (2015) reported that 25% of participants (n = 205) received the equivalent of an SCP and reported greater certainty in which doctor was in charge, were more likely to have follow-up visits and surveillance, were more likely to report meeting exercise guidelines, and reported better health.
Limitations
A number of limitations exist in the current study that will be addressed in a future larger randomized, controlled trial. These include a small sample size with participants who had high socioeconomic status from a single institution with only six weeks of follow-up after SCP delivery. The clinical significance of the findings are unclear at this point, warranting additional investigation. The strength of this study was the demonstration of the feasibility of the intervention and of the measures used to evaluate the more immediate impact of the interventions in a variety of cancer survivors.
Implications for Nursing Practice
SCPs are beginning to be disseminated as a result of the Commission on Cancer (2012) requirements; however, many questions remain about processes of delivery and the short- and long-term effects of receiving an SCP. “One size may not fit all” (Klemp, 2015, p. 67), and some survivors may benefit from a more tailored approach of SCP delivery considering level of patient activation and worry. Although this study was conducted with NPs to facilitate reimbursement, the authors would expect that nurse navigators would also be able to develop and deliver the SCP, but without reimbursement (Corcoran, Dunne, & McCabe, 2015).
Conclusion
Although the authors were able to demonstrate the feasibility of the intervention and its measurement, the study design and findings will need to be tested in a larger, more diverse population followed during a longer period of time. Booster doses (updating and re-reviewing the SCP) may need to be considered as the SCP outcome data matures. Studying the role of patient activation and worry in SCP outcomes also will be important. Linking the delivery of the SCP to improved patient outcomes documented in other studies will need to be verified in prospective randomized, controlled trials (Chrischilles et al., 2015; Jabson, 2015; Palmer et al., 2015).
References
Ayanian, J.Z., & Jacobsen, P.B. (2006). Enhancing research on cancer survivors. Journal of Clinical Oncology, 24, 5149–5153.
Birken, S.A., Deal, A.M., Mayer, D.K., & Weiner, B.J. (2014). Determinants of survivorship care plan use in United States cancer programs. Journal of Cancer Education, 29, 720–727.
Birken, S.A., Mayer, D.K., & Weiner, B.J. (2013). Survivorship care plans: Prevalence and barriers to use. Journal of Cancer Education, 28, 290–296. doi:10.1007/s13187-013-0469-x
Cheung, W., & Earle, C.C. (2009). Lack of clarity around physician roles during cancer survivorship care planning. American Journal of Hematology/Oncology, 8, 472.
Cheung, W., Neville, B., Cameron, D., Cook, F., & Earle, C. (2009). Comparisons of patient and physician expectations for cancer survivorship care. Journal of Clinical Oncology, 27, 2489–2495.
Cheung, W.Y., Neville, B.A., & Earle, C.C. (2010). Associations among cancer survivorship discussions, patient and physician expectations, and receipt of follow-up care. Journal of Clinical Oncology, 28, 2577–2583. doi:10.1200/JCO.2009.26.4549
Chrischilles, E.A., McDowell, B.D., Rubenstein, L., Charlton, M., Pendergast, J., Juarez, G.Y., & Arora, N.K. (2015). Survivorship care planning and its influence on long-term patient-reported outcomes among colorectal and lung cancer survivors: The CanCORS disease-free survivor follow-up study. Journal of Cancer Survivorship, 9, 269–278. doi:10.1007/s11764-014-0406-y
Commission on Cancer. (2012). Cancer program standards 2012: Ensuring patient-centered care [v1.2.1]. Retrieved from http://www.facs.org/cancer/coc/programstandards2012.pdf
Corcoran, S., Dunne, M., & McCabe, M.S. (2015). The role of advanced practice nurses in cancer survivorship care. Seminars in Oncology Nursing, 31, 338–347. doi:10.1016/j.soncn.2015.08.009
Gotay, D., & Pagano, I. (2007). Assessment of Survivor Concerns (ASC): A newly proposed brief questionnaire. Health and Quality of Life Outcomes, 5, 15.
Green, C.A., Perrin, N.A., Polen, M.R., Leo, M.C., Hibbard, J.H., & Tusler, M. (2010). Development of the patient activation measure for mental health. Administration and Policy in Mental Health, 37, 327–333. doi:10.1007/s10488-009-0239-6
Greene, J., & Hibbard, J.H. (2012). Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. Journal of General Internal Medicine, 27, 520–526. doi:10.1007/s11606-011-1931-2
Hershman, D.L., Greenlee, H., Awad, D., Kalinsky, K., Maurer, M., Kranwinkel, G., . . . Crew, K.D. (2013). Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Research and Treatment, 138, 795–806. doi:10.1007/s10549-013-2486-1
Hewitt, M., Greenfield, S., & Stovall, E. (Eds.). (2005). From cancer patient to cancer survivor: Lost in transition. Washington, DC: Health and Medicine Division of the National Acadamies of Sciences, Engineering, and Medicine.
Hibbard, J.H., Greene, J., & Tusler, M. (2009). Improving the outcomes of disease management by tailoring care to the patient’s level of activation. American Journal of Managed Care, 15, 353–360.
Hibbard, J.H., & Mahoney, E. (2010). Toward a theory of patient and consumer activation. Patient Education and Counseling, 78, 377–381. doi:10.1016/j.pec.2009.12.015
Hibbard, J.H., Mahoney, E.R., Stock, R., & Tusler, M. (2007). Do increases in patient activation result in improved self-management behaviors? Health Services Research, 42, 1443–1463.
Hibbard, J.H., Stockard, J., Mahoney, E.R., & Tusler, M. (2004). Development of the Patient Activation Measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Services Research, 39(4, Pt. 1), 1005–1026.
Hibbard, J.H., & Tusler, M. (2007). Assessing activation stage and employing a “next steps” approach to supporting patient self-management. Journal of Ambulatory Care Management, 30, 2–8.
Jabson, J.M. (2015). Follow-up care instructions, treatment summaries, and cancer survivors’ receipt of follow-up health care and late/long term effects. Supportive Care in Cancer, 23, 1851–1856.
Jean-Pierre, P., Fiscella, K., Freund, K.M., Clark, J., Darnell, J., Holden, A., . . . Winters, P.C. (2011). Structural and reliability analysis of a patient satisfaction with cancer-related care measure: A multisite patient navigation research program study. Cancer, 117, 854–861. doi:10.1002/cncr.25501
Keesing, S., McNamara, B., & Rosenwax, L. (2015). Cancer survivors’ experiences of using survivorship care plans: A systematic review of qualitative studies. Journal of Cancer Survivorship, 9, 260–268. doi:10.1007/s11764-014-0407-x
Klemanski, D.L., Browning, K.K., & Kue, J. (2016). Survivorship care plan preferences of cancer survivors and health care providers: A systematic review and quality appraisal of the evidence. Journal of Cancer Survivorship: Research and Practice, 10, 71–86.
Klemp, J.R. (2015). Survivorship care planning: One size does not fit all. Seminars in Oncology Nursing, 31, 67–72.
Mayer, D., Gerstel, A., Leak, A., & Smith, S. (2012). Patient and provider preferences for survivorship care plans. Journal of Oncology Practice, 8, e80–e86.
Mayer, D.K., Gerstel, A., Walton, A.L., Triglianos, T., Sadiq, T.E., Hawkings, N.A., & Davies, J.M. (2014). Implementing survivorship care plans (SCP) for colon cancer survivors in a comprehensive cancer center. Oncology Nursing Forum, 41, 266–273.
Mayer, D.K., Necklyudov, L., Snyder, C., Merrill, J., Wollins, D., & Shulman, L. (2014). ASCO clinical expert statement on cancer survivorship care planning. Journal of Oncology Practice, 10, 345–351.
Nicolaije, K., Ezendam, N., Vos, M., Pijnenborg, J., Boll, D., Boss, E.A., . . . van de Poll-Franse, L.V. (2015). Impact of an automatically generated cancer survivorship care plan on patient-reported outcomes in routine clinical practice. Journal of Clinical Oncology, 33, 3550–3559.
Palmer, S., Mao, J., Jacobs, L., & Stricker, C. (2012). Are cancer survivors confident in their knowledge about their disease and care? Retrieved from http://www.cancer.org/acs/groups/content/@researchadministration/docume…
Palmer, S.C., Stricker, C.T., Panzer, S.L., Arvey, S.A., Baker, S.K., Casillas, J., . . . Ganz, P.A. (2015). Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. Journal of Oncology Practice, 11, e222–e229.
Parry, C., Kent, E.E., Forsythe, L.P., Alfano, C.M., & Rowland, J.H. (2013). Can’t see the forest for the care plan: A call to revisit the context of care planning. Journal of Clinical Oncology, 31, 2651–2653. doi:10.1200/JCO.2012.48.4618
Wagner, E.H. (1998). Chronic disease management: What will it take to improve care for chronic illness? Effective Clinical Practice, 1, 2–4.
Wagner, E.H., Aiello Bowles, E.J., Greene, S.M., Tuzzio, L., Wiese, C.J., Kirlin, B., & Clauser, S.B. (2010). The quality of cancer patient experience: Perspectives of patients, family members, providers and experts. Quality and Safety in Health Care, 19, 484–489.
About the Author(s)
Mayer is a professor in the School of Nursing at the University of North Carolina (UNC)–Chapel Hill and director of Cancer Survivorship at the UNC Lineberger Comprehensive Cancer Center in Chapel Hill; Deal is a biostatistician at the UNC Lineberger Comprehensive Cancer Center; Crane is a staff physician in Hematology/Medical Oncology at the REX Cancer Center in Raleigh, NC; and Chen is an associate professor in the Department of Radiation Oncology, Asher is an associate professor in the Department of Family Medicine, Hanson is a professor in the School of Medicine, Wheeler is an associate professor in the Gillings School of Global Public Health, Gerstel is a project coordinator in the School of Nursing, Green is the deputy director for communication and recruitment for the Clinical Scholars Program, and Birken is an assistant professor in the Gillings School of Global Public Health, all at UNC–Chapel Hill; and Rosenstein is a professor in the School of Medicine at UNC–Chapel Hill and director of the cancer support program at the UNC Lineberger Comprehensive Cancer Center. This research was funded by a grant from the Health-e-NC signature initiative of the University Cancer Research Fund. Mayer, Deal, Chen, Asher, Hanson, Wheeler, Green, and Rosenstein contributed to the conceptualization and design. Mayer, Deal, Crane, Chen, Gerstel, and Green completed the data collection. Mayer, Deal, and Green provided statistical support. Mayer, Deal, Asher, Hanson, Green, Birken, and Rosenstein provided the analysis. Mayer, Deal, Chen, Asher, Hanson, Wheeler, Green, Birken, and Rosenstein contributed to the manuscript preparation. Mayer can be reached at dmayer@unc.edu, with copy to editor at ONFEditor@ons.org. Submitted November 2015. Accepted for publication January 12, 2016.

