Emerging Role of Nutri-Epigenetics in Inflammation and Cancer
Nutrition is a factor involved in inflammation and a modulator of risk toward some cancers, and the complexity of linkages between dietary components and epigenetics mechanisms (e.g., DNA methylation, histone modification, chromatin remodeling) may affect the inflammation phenotype and the development of cancer. An increasing number of studies support the role of diet in cancer development, prevention, and treatment. Although current knowledge regarding nutri-epigenetics is expanding, more work is needed, and nurse scientists have the potential to significantly contribute to the expansion of this knowledge.
Jump to a section
Disease risk and development are influenced by many factors (e.g., lifestyle, environment, nutrition) and genetics. Evidence shows that these factors interact with one another in ways that are unique to each person, making quantification of the risks of developing diseases such as cancer challenging. Cancer is a metabolically driven process with dynamic nutrient-responsive alterations within the human genome (Vander Heiden, Cantley, & Thompson, 2009). The epigenetic machinery acting on the human genome is heavily susceptible to alterations in metabolism and nutrition, particularly during periods of inflammation (Keating & El-Osta, 2015). This emerging knowledge leads to current interest in nutri-epigenetics or nutri-epigenomics. Epigenetics focuses on processes that regulate how and when certain genes are turned on and off, whereas epigenomics refers to analysis of global epigenetic changes across many genes, made possible by high-throughput methods. Therefore, nutri-epigenetics focuses on the process by which nutrition regulates how one specific gene is turned on or off, whereas nutri-epigenomics refers to the analysis of the interaction among multitudes of genes and nutrition, as well as the effects on global gene expression, which may vary among different tissues. This article aims to provide a brief overview of epigenetics and how it can be affected by metabolism and nutrition, discuss nutri-epigenetics and cancer, and consider the implications of nutri-epigenetics knowledge and evidence for nursing practice.
Background
The fields of both epigenetics and epigenomics have been empowered by publicly available databases (e.g., Human Epigenome Browser from Washington University School of Medicine in St. Louis; epigenomegateway.wustl.edu/info) that have focused on gathering epigenetics sequences for epigenome-wide association studies. These studies have demonstrated that epigenetic alterations related to food components and environmental (nutrition) factors, along with other genetic mutations, play a role in the development of inflammatory diseases, such as cancer (van Veldhoven et al., 2015). The field of nutri-epigenomics provides new insights into diet–genome interactions, and these insights have permitted explorations of the use of metabolically based drugs, such as metformin (Glucophage®), as part of cancer therapy (Kasznicki, Sliwinska, & Drzewoski, 2014) and the design of nutrition- and lifestyle-based cancer management strategies (Richman et al., 2013).
The term epigenetics was introduced to describe the interactions between genes and the environment that gave rise to phenotypes during development; it has been expanded to include environmentally responsive cellular processes that can be heritable and have long-term effects on gene expression without alterations in the DNA sequence (change in phenotype without a change in genotype) (Dupont, Armant, & Brenner, 2009). Through epigenetics, sets of genes may be expressed or silent, and this determines which proteins are transcribed in response to an environmental cue.
Two major epigenetic mechanisms that have been found to influence cellular metabolism and nutrient components are DNA methylation and histone modification. Each leads to either silencing or activation of gene expression (see Table 1). DNA methylation is the incorporation of a methyl group on the 5-carbon position of the cytosine ring of DNA by DNA methyltransferases (DNMTs), resulting in 5-methylcytosine. Methylation of cytosine often occurs adjacent to a guanine nucleotide within a gene; consequently, the site is called a CpG site (cytosine [C] lies next to guanine [G] in the DNA sequence, and a phosphodiester [p] bond joins the two nucleotides) (Kulis & Esteller, 2010). The insertion of methyl groups changes the structure of the DNA strands, modifying the condition in which a gene may be accessible to transcription machinery. DNA methylation functions to regulate gene expression, and this regulation has been found to be responsive to environmental cues, such as the presence of interleukins or other cytokines during inflammation (Suzuki, Toyota, Kondo, & Shinomura, 2009).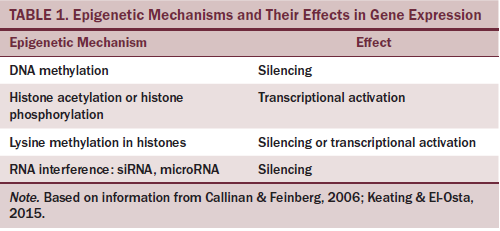
Dietary methyl group consumption, primarily involving methionine-containing protein products, provides an important source of methylation components for the enzyme DNMT through cellular conversion of methionine to S-adenosylmethionine (SAM) (Li & Tollefsbol, 2010). To balance the activity of DNMT, SAM can be converted in the cells to S-adenosylhomocysteine, which is a potent inhibitor of DNMT (Stempak, Sohn, Chiang, Shane, & Kim, 2005). In normal dietary metabolism, when choline and folate (vitamin B12) are present, S-adenosylhomocysteine is recycled back to methionine, which can then be converted back to SAM. The DNA methylation process is sensitive to cellular metabolic reactions, called the methionine cycle, which ultimately relies on dietary methionine, folate, and choline. In contrast to DNA methylation, which modifies the bases within the DNA sequence, histone modifications are changes on the DNA-associated histone proteins. Histones are proteins that chemically bond with DNA molecules to form the chromatin structures that are condensed into chromosomes within the nucleus of the cell (Mariño-Ramírez, Kann, Shoemaker, & Landsman, 2005). Modification of the histone proteins occurs primarily through methylation or acetylation of the amino acids arginine or lysine within the histone proteins. The acetylation of histone occurs by the enzymatic addition of an acetyl group from acetyl coenzyme A, a molecule that is generated by the metabolism of glucose and lipid-derived fatty acids (Verdin & Ott, 2015). The activity of histone acetylation enzymes (histone acetyltransferases) is balanced by histone deacetylases, which determine the transcriptional competency of a gene (Talbert & Henikoff, 2010). An imbalance in the equilibrium of histone acetylation/deacetylation is associated with tumorigenesis and cancer progression (Sobolewski, Sanduja, Blanco, Hu, & Dixon, 2015).
Although different epigenetic mechanisms follow distinctive pathways to regulate gene expression, they tend to be interrelated as part of a harmonized regulation of chromatin/chromosome structural dynamics. Altering structures that are known to affect epigenetic alterations may cause unusual gene activation or silencing of genes. Epigenetic changes have been related to cancer (breast, colon, lung, and leukemia) and to other diseases (asthma, autism, Fragile X syndrome, multiple sclerosis, obesity, rhematoid arthritis, type 2 diabetes mellitus). Cellular processes, such as aging, host defense system, and imprinting, also play a role. These epigenetic processes are key determinants of gene expression, providing new dimensions of interest in epigenetic mechanisms in health outcomes.
Nutri-Epigenetics, Inflammation, and Cancer
Nutrients, such as glucose, lipid-derived fatty acids, and methionine or other amino acids, can affect epigenetics by promoting or inhibiting enzymes involved in DNA methylation and histone modifications (see Figure 1). In normal cells, energy in the form of adenosine triphosphate is produced from glucose and fatty acids through a mitochondrial respiratory chain reaction that requires oxygen. When oxygen is absent, lactate is produced from anaerobic metabolism of glucose. In cancer cells, whether oxygen is absent or present, lactate is continually produced from glucose metabolism, and this overrides the mitochondrial respiratory chain reaction of energy production. This unique preference of cancer cells to metabolize glucose independently of mitochondria, termed the Warburg effect of aerobic glycolysis, has a profound impact on epigenetics because it produces a shift in the availability of acetyl coenzyme A and other molecules needed for epigenetic enzymes (Gogvadze, Zhivotovsky, & Orrenius, 2010). The continual cellular uptake of glucose by cancer cells is indirectly associated with the expression of proinflammatory genes, such as interleukin-8 (Rofstad, Mathiesen, Kindem, & Galappathi, 2006). Evidence of epigenetic dysregulation in cancer is accumulating. The decrease in methylated cytosine results in global hypomethylation. Reduced cytosine methylation characteristically affects DNA sequences and CpG sites. Some cancers also show focal hypermethylation in CpG islands. Genes that are affected become permanently silenced. Methylation affects each patient with cancer differently. Although some patients have negligible variations, others display concordant hypermethylation of several genes, which is seen in colorectal cancer and other cancers (Stirzaker, Zotenko, & Clark, 2016). A buildup of genetic and epigenetic inaccuracies alters normal cells into aberrant tumor cells. In addition, DNA methylation patterns may produce atypical expression of cancer-related genes. Remembering that these DNA methylation arrangements are tissue- and cell-specific is important. 
Review of the Literature
Chronic inflammation is a major risk factor for tumor formation, and studies have noted inflammatory signals as a new epigenetic mechanism that silences specific genes that cause inflammation-induced cellular changes (Abu-Remaileh et al., 2015). Nutrition is thought to be a factor involved in inflammation (Galland, 2010) and a modulator of risk toward some cancers (Day et al., 2013). The complexity of linkages between dietary components and epigenetics mechanisms, such as DNA methylation, histone modification, and chromatin remodeling, including how these may affect the inflammation phenotype and the development of cancer, has been revealed (Keating & El-Osta, 2015). Diets rich in polyunsaturated fatty acids, plant-derived dietary phytochemicals, and macro- and micronutrients may produce mutagenic free radicals, oxidative stress, and inflammatory signaling, which have been implicated in epigenetic alterations (Alegría-Torres, Baccarelli, & Bollati, 2011). DNA modulation of endothelial cells with arachidonic acid promotes upregulation of tumorigenesis (Rodríguez-Blanco et al., 2014). However, diet high in polyunsaturated fatty acids may play a role through reduction of inflammation to reduce tumor formation (Liang et al., 2016).
Fruits and vegetables, which include polyphenolic compounds, such as resveratrol, tea catechins, and flavonoids, have shown chemoprotective properties (He et al., 2011); however, the nutritional consequences may be organ-specific. Studies of tea catechins demonstrate inhibitory activity against cancer development and cancer cell growth (Rahmani, Al shabrmi, Allemailem, Aly, & Khan, 2015; Xiang et al., 2016). In addition, sulforaphane, mostly found in cruciferous vegetables (broccoli and broccoli sprouts), inhibits enzymes involved in cancer initiation (Gills et al., 2006), is chemoprotective, and, in animal studies, has been shown to reduce tumor growth (Myzak, Tong, Dashwood, Dashwood, & Ho, 2007). In the same study, healthy volunteers who consumed a single portion of broccoli were found to suppress histone deacetylase a few hours following consumption, with simultaneous activation of histone acetylation (Myzak et al., 2007). Likewise, another study in human tumor colon cell lines found that elevated doses of diallyl disulfide (found in garlic) increased histone acetylation (Robert, Mouillé, Mayeur, Michaud, & Blachier, 2001). Yi and Su (2013) discussed the molecular mechanisms by which diallyl disulfide aids in reduction of cancer proliferation. Studies focused on folate and vitamin B12 intake, which is important for DNA methylation, have shown that diets low in folate are associated with risk for colon cancer (Kim, 2003), whereas diets high in folate are associated with a decrease in breast cancer (Chen et al., 2014). The effects of folate on epigenetic mechanisms and potential relationship to cancer remain to be seen.
Selenium is another dietary compound that epigenetically modulates DNA and histones by activating methylation-silenced genes (Speckmann & Grune, 2015). Biobehavioral interventions, including nutritional bioactives, are under investigation. Although findings in the area of nutri-epigenetics are exciting and compelling, additional clinical trials and epidemiologic research are needed.
Implications for Nursing
The opportunity exists to optimize patient outcomes through an interplay of host and genomic interactions. Nurses are at the junction to transform health care using genetics and genomics (Tully & Grady, 2015). Knowledge of epigenetic changes in disease processes sheds light into the complex mechanisms underlying tumorigenesis and has unlocked novel therapeutic options and targets for treating malignancies and their associated symptoms. Nurses possess the skills and are strategically positioned to lead research designed to better understand how the environment (i.e., nutrition) and epigenomics may interact mechanistically to add or reduce risks of certain cancers. The knowledge and awareness of epigenetics’ influence on inflammation and cancer and the effects of nutrients and metabolism on this process is critical for nurses who are at the forefront of patient care and education, particularly in the era of personalized medicine. That nutrition influences the epigenome is indisputable. This dynamic link between nutrition and genes provides great insight into how to target health promotion and disease prevention from a nutritional perspective. Nutrient and metabolic needs among individuals may vary greatly, depending on their specific disease process; therefore, the potential role of precision nutrition is key for modulating cancer risk and development.
Conclusion
Epigenetic modifications are key regulators of developmental processes involving differentiation and growth. Differences in epigenetic modifications may contribute to genetic diversity; however, aberrant epigenetic changes often lead to developmental abnormalities and diseases, such as cancer. Knowledge of epigenetic modulations in the development and progression of cancers will not merely allow nurses to recognize different high-risk profiles and to monitor response to drug therapy; it may also provide a stage from which they can design individual lifestyle interventions. Epigenetic changes are easier to modify than modifications that affect the genome. Therefore, nutri-epigenetics is an attractive biological target for modifiable behavioral nutritional interventions. Nurse-led clinical trials with diet modifications will enrich scientific understanding of how nutritional modulation concurrent with pharmacotherapies may enhance health outcomes in cancer.
The authors gratefully acknowledge Alan Hoofring, MS, MA, from the Department of Medical Arts at the National Institutes of Health for his medical illustration assistance.
References
Abu-Remaileh, M., Bender, S., Raddatz, G., Ansari, I., Cohen, D., Gutekunst, J., . . . Lyko, F. (2015). Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer. Cancer Research, 75, 2120–2130. doi:10.1158/0008-5472.CAN-14-3295
Alegría-Torres, J.A., Baccarelli, A., & Bollati, V. (2011). Epigenetics and lifestyle. Epigenomics, 3, 267–277. doi:10.2217.epi.11.22
Callinan, P.A., & Feinberg, A.P. (2006). The emerging science of epigenomics. Human Molecular Genetics, 15(Suppl. 1), R95–R101. doi:10.1093/hmg/ddl095
Chen, P., Li, C., Li, X., Li, J., Chu, R., & Wang, H. (2014). Higher dietary folate intake reduces the breast cancer risk. British Journal of Cancer, 110, 2327–2338. doi:10.1038/bjc.2014.155
Day, S.D., Enos, R.T., McClellan, J.L., Steiner, J.L., Velázquez, K.T., & Murphy, E.A. (2013). Linking inflammation to tumorigenesis in a mouse model of high-fat-diet-enhanced colon cancer. Cytokine, 64, 454–462. doi:10.1016/j.cyto.2013.04.031
Dupont, C., Armant, D.R., & Brenner, C.A. (2009). Epigenetics: Definition, mechanisms and clinical perspective. Seminars in Reproductive Medicine, 27, 351–357. doi:10.1055/s-0029-1237423
Galland, L. (2010). Diet and inflammation. Nutrition in Clinical Practice, 25, 634–640. doi:10.1177/0884533610385703
Gills, J.J., Jeffery, E.H., Matusheski, N.V., Moon, R.C., Lantvit, D.D., & Pezzuto, J.M. (2006). Sulforaphane prevents mouse skin tumorigenesis during the stage of promotion. Cancer Letters, 236, 72–79. doi:10.1016/j.canlet.2005.05.007
Gogvadze, V., Zhivotovsky, B., & Orrenius, S. (2010). The Warburg effect and mitochondrial stability in cancer cells. Molecular Aspects of Medicine, 31, 60–74. doi:10.1016/j.mam.2009.12.004
He, L., Wu, Y., Lin, L., Wang, J., Wu, Y., Chen, Y., . . . Pang, X. (2011). Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Science, 102, 219–225. doi:10.1111/j.1349-7006.2010.01778.x
Kasznicki, J., Sliwinska, A., & Drzewoski, J. (2014). Metformin in cancer prevention and therapy. Annals of Translational Medicine, 2, 57. doi:10.3978/j.issn.2305-5839.2014.06.01
Keating, S.T., & El-Osta, A. (2015). Epigenetics and metabolism. Circulation Research, 116, 715–736. doi:10.1161/CIRCRESAHA.116.303936
Kim, Y.I. (2003). Role of folate in colon cancer development and progression. Journal of Nutrition, 133(Suppl. 1.), 3731S–3739S.
Kulis, M., & Esteller, M. (2010). DNA methylation and cancer. Advances in Genetics, 70, 27–56. doi:10.1016/B978-0-12-380866-0.60002-2
Li, Y., & Tollefsbol, T.O. (2010). Impact on DNA methylation in cancer prevention and therapy by bioactive dietary components. Current Medicinal Chemistry, 17, 2141–2151. doi:10.2174/092986710791299966
Liang, P., Henning, S.M., Schokrpur, S., Wu, L., Doan, N., Said, J., . . . Aronson, W.J. (2016). Effect of dietary omega-3 fatty acids on tumor-associated macrophages and prostate cancer progression. Prostate, 76, 1293–1302. doi:10.1002/pros.23218
Mariño-Ramírez, L., Kann, M.G., Shoemaker, B.A., & Landsman, D. (2005). Histone structure and nucleosome stability. Expert Review of Proteomics, 2, 719–729. doi:10.1586/14789450.2.5.719
Myzak, M.C., Tong, P., Dashwood, W.M., Dashwood, R.H., & Ho, E. (2007). Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Experimental Biology and Medicine, 232, 227–234.
Rahmani, A.H., Al shabrmi, F.M., Allemailem, K.S., Aly, S.M., & Khan, M.A. (2015). Implications of green tea and its consitutients in the prevention of cancer via the modulation of cell signalling pathway. Biomed Research International, 2015, 925640. doi:10.1155/2015/925640
Richman, E.L., Kenfield, S.A., Chavarro, J.E., Stampfer, M.J., Giovannucci, E.L., Willett, W.C., & Chan, J.M. (2013). Fat intake after diagnosis and risk of lethal prostate cancer and all-cause mortality. JAMA, 173, 1318–1326. doi:10.1001/jamainternmed.2013.6536
Robert, V., Mouillé, B., Mayeur, C., Michaud, M., & Blachier, F. (2001). Effects of the garlic compound diallyl disulfide on the metabolism, adherence and cell cycle of HT-29 colon carcinoma cells: Evidence of sensitive and resistant sub-populations. Carcinogenesis, 22, 1155–1161. doi:10.1093/carcin/22.8.1155
Rodríguez-Blanco, G., Burgers, P.C., Dekker, L.J., Ijzermans, J.J., Wildhagen, M.F., Schenk-Braat, E.A., . . . Luider, T.M. (2014). Serum levels of arachidonic acid metabolites change during prostate cancer progression. Prostate, 74, 618–627. doi:10.1002/pros.22779
Rofstad, E.K., Mathiesen, B., Kindem, K., & Galappathi, K. (2006). Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Research, 66, 6699–6707. doi:10.1158/0008-5472.CAN-06-0983
Sobolewski, C., Sanduja, S., Blanco, F.F., Hu, L., & Dixon, D.A. (2015). Histone deacetylase inhibitors activate tristetraprolin expression through induction of early growth response protein 1 (EGR1) in colorectal cancer cells. Biomolecules, 5, 2035–2055.
Speckmann, B., & Grune, T. (2015). Epigenetic effects of selenium and their implications for health. Epigenetics, 10, 179–190. doi:10.1080/15592294.2015.1013792
Stempak, J.M., Sohn, K.-J., Chiang, E.-P., Shane, B., & Kim, Y.-I. (2005). Cell and stage of transformation-specific effects of folate deficiency on methionine cycle intermediates and DNA methylation in an in vitro model. Carcinogenesis, 26, 981–990. doi:10.1093/carcin/bgi037
Stirzaker, C., Zotenko, E., & Clark, S.J. (2016). Genome-wide DNA methylation profiling in triple-negative breast cancer reveals epigenetic signatures with important clinical value. Molecular and Cellular Oncology, 3, e1038424. doi:10.1080/23723556.2015.1038424
Suzuki, H., Toyota, M., Kondo, Y., & Shinomura, Y. (2009). Inflammation-related aberrant patterns of DNA methylation: Detection and role in epigenetic deregulation of cancer cell transcriptome. Methods in Molecular Biology, 512, 55–69. doi:10.1007/978-1-60327-530-9_5
Talbert, P.B., & Henikoff, S. (2010). Histone variants—Ancient wrap artists of the epigenome. Nature Reviews. Molecular Cell Biology, 11, 264–275. doi:10.1038/nrm2861
Tully, L.A., & Grady, P.A. (2015). A path forward for genomic nursing research. Research in Nursing and Health, 38, 177–179. doi:10.1002/nur.21659
Vander Heiden, M.G., Cantley, L.C., & Thompson, C.B. (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science, 324, 1029–1033. doi:10.1126/science.1160809
van Veldhoven, K., Polidoro, S., Baglietto, L., Severi, G., Sacerdote, C., Panico, S., . . . Vineis, P. (2015). Epigenome-wide association study reveals decreased average methylation levels years before breast cancer diagnosis. Clinical Epigenetics, 7, 67. doi:10.1186/s13148-015-0104-2
Verdin, E., & Ott, M. (2015). 50 years of protein acetylation: From gene regulation to epigenetics, metabolism and beyond. Nature Reviews. Molecular Cell Biology, 16, 258–264. doi:10.1038/nrm3931
Xiang, L.P., Wang, A., Ye, J.H., Zheng, X.Q., Polito, C.A., Lu, J.L., . . . Liang, Y.R. (2016). Suppressive effects of tea catechins on breast cancer. Nutrients, 8, E458. doi:10.3390/nu8080458
Yi, L., & Su, Q. (2013). Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food and Chemical Toxicology, 57, 362–370.
About the Author(s)
Joseph is a clinical and translational postdoctoral fellow, Abey is a nurse specialist, and Henderson is investigator and chief of the Digestive Disorder Unit, all in the Biobehavioral Branch of the Division of Intramural Research in the National Institute of Nursing Research at the National Institutes of Health (NIH) in Bethesda, MD. This research was funded by the Division of Intramural Research, National Institute of Nursing Research (1ZIANR000018-01-07), and the NIH Office of the Director, Office of Workforce Diversity, Postdoctoral Intramural Research Training Award, and Summer Internship Award. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society. Henderson can be reached at hendersw@mail.nih.gov, with copy to editor at ONFEditor@ons.org.

