Stigma and Quality of Life in Patients With Advanced Lung Cancer
Objectives: To identify groups of participants with high and low levels of stigma and to examine the influence of stigma on social support, social constraints, symptom severity, symptom interference, and quality of life (QOL).
Sample & Setting: 62 individuals with lung cancer were identified and recruited from a comprehensive cancer center in the southeastern United States.
Methods & Variables: Participants completed a questionnaire that included demographic information and measures of stigma, symptom severity and interference, social support, social constraints, and QOL. IBM SPSS Statistics TwoStep Cluster Analysis was used to identify high- and low-stigma groups. Independent sample t tests were used to compare differences between the groups.
Results: 22 participants had a high level of stigma; they had significantly higher symptom severity on feeling distressed, problems remembering things, and feeling sad, and greater symptom interference related to mood, relations with others, and enjoyment of life. Participants also had significantly higher levels of social support and lower social constraints. Stigma was significantly related to lower levels of QOL.
Implications for Nursing: Nurses should be aware that stigma may influence various factors throughout the disease trajectory; they can privately assess individuals with lung cancer for access to social supports, feelings of stigma, and QOL, and make appropriate referrals as needed.
Jump to a section
Lung cancer, most often diagnosed at an advanced stage, is the leading cause of cancer-related death in the United States (American Cancer Society [ACS], 2018). The five-year relative survival rate for an individual diagnosed with lung cancer is between 4.5% and 8% (Dillman & McClure, 2014), and the median survival time for those with advanced disease is eight months (Dillman & McClure, 2014; Schiller et al., 2002). Advanced lung cancer is a terminal disease with a relatively short survival time after diagnosis; therefore, it is important to examine factors that may influence quality of life (QOL) in individuals diagnosed with this disease.
Cigarette use contributes to 80% of all deaths attributed to lung cancer (ACS, 2018). Regardless of smoking status, studies have reported that between 30% and 95% of those with lung cancer feel stigmatized by their disease (Cataldo, Slaughter, Jahan, Pongquan, & Hwang, 2011; Chambers et al., 2012; Chapple, Ziebland, & McPherson, 2004; Else-Quest, LoConte, Schiller, & Hyde, 2009; Hamann et al., 2014; Lebel et al., 2013; LoConte, Else-Quest, Eickhoff, Hyde, & Schiller, 2008; Weiss et al., 2017). Compared to individuals with cancers in which smoking is not a primary risk factor, those with lung cancer report more stigma (LoConte et al., 2008). Individuals with lung cancer report experiencing the same levels of stigma as those diagnosed with head and neck cancer, in which smoking is also a primary risk factor (Lebel et al., 2013). Stigma has been associated with negative psychological outcomes in individuals with lung cancer (Brown Johnson, Brodsky, & Cataldo, 2014; Chambers et al., 2015; Else-Quest et al., 2009).
Background
Multiple symptoms (e.g., pain, fatigue, dyspnea, distress) and high symptom burden are associated with poor QOL in individuals with lung cancer (Cooley, Short, & Moriarty, 2003; Hopwood & Stephens, 1995; Iyer, Roughley, Ridner, & Taylor-Stokes, 2014; Ma et al., 2014; Polanski, Jankowska-Polańska, Rosinczuk, Chabowski, & Szymanska-Chabowska, 2016). To the current authors’ knowledge, only one study has investigated the relationship between stigma and physical symptoms in lung cancer, finding that stigma was significantly associated with physical symptom severity in individuals with all stages of lung cancer (Cataldo & Brodsky, 2013). It is not known if stigma influences symptom interference or symptom severity in those with advanced lung cancer.
Because social support can be derived from a variety of sources, the social support needs of individuals with lung cancer, including general social support and support from a primary caregiver, should be investigated in multiple ways. Three main types of social support are emotional, informational, and instrumental (House, 1981; House & Kahn, 1985; Kahn & Antonucci, 1980). Among individuals with advanced cancer, those who report higher levels of social support tend to have higher QOL (Applebaum et al., 2014). A qualitative study by Chapple, Ziebland, and McPherson (2004) has suggested that stigma influences the way an individual is able to interact with his or her family and friends, as well as the medical community, after a diagnosis of lung cancer.
The perception of social support from the family is associated with better emotional adjustment (Zemore & Shepel, 1989). In addition, the ability of an individual with cancer to disclose his or her diagnosis affects that individual’s psychological processing of the disease (Lepore & Revenson, 2007). The primary lay caregiver is likely to provide this type of support. If this caregiver is unwilling to offer support to the individual with cancer, that relationship is socially constraining. When social constraints exist within the relationship, the ability of the individual with cancer to discuss, cope, and adjust to the diagnosis is negatively affected (Lepore & Revenson, 2007). A study by Chambers et al. (2015) investigated social constraints among those with all stages of lung cancer, determining that social constraints were found to be a mediating factor between distress and stigma.
Research suggests that QOL, in conjunction with physical condition, is a prognostic indicator in those with lung cancer (Braun, Gupta, & Staren, 2011; Efficace et al., 2006). Consequently, it is important to identify factors that influence QOL, particularly in those with a limited life expectancy. Multiple factors are associated with a decrease in QOL in individuals with lung cancer and include smoking, severe physical symptoms, depression, and anxiety (Polanski et al., 2016), whereas milder physical symptoms, not smoking, and early palliative care are associated with an increase in QOL (Polanski et al., 2016; Temel et al., 2010). Regardless of survival time after diagnosis, individuals with lung cancer should receive holistic care that maximizes QOL.
Brown Johnson et al. (2014) reported that stigma provided a significant explanation of the variance in QOL beyond depression and anxiety in a sample of patients with all stages of lung cancer. Chambers et al. (2015) also found that stigma was associated with QOL in patients newly diagnosed with lung cancer. Distress, social constraints, and illness appraisals were identified as factors that may contribute to QOL in individuals with lung cancer (Chambers et al., 2015). Stigma may influence QOL in individuals with advanced lung cancer, but its unique contribution to QOL in those with advanced lung cancer has not been explored. The objectives of the current study were to (a) identify subgroups of participants who could be identified as having high and low levels of stigma and (b) determine how the stigma groups differed in their levels of social support, social constraints, symptom severity, symptom interference, and QOL.
Methods
Sample and Setting
A cross-sectional, descriptive, exploratory design was used. Appropriate institutional review board approvals were obtained. A convenience sample was identified and recruited from the Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. To be eligible for the study, participants had to be aged 18 years or older, have received chemotherapy for lung cancer at least once, and be able to speak English. All participants had to pass the Short Portable Mental Status Questionnaire prior to enrollment. Those with a known documentation of cognitive impairment, another active cancer, and hospice enrollment were not eligible. A medical oncologist introduced the study to potential participants. Interested participants provided informed consent and completed self-report measures. Participants were recruited from November 2012 to August 2014.
Instruments
Participants had the option to independently complete a questionnaire online in REDCap (Research Electronic Data Capture), which is a secure web-based application to support data capture for research studies, or face-to-face in the clinic with a hard-copy questionnaire. One follow-up email was sent to participants who did not complete the online questionnaire within two weeks. All data were stored in REDCap (Harris et al., 2009). No compensation was provided for this study. Sociodemographic data were collected from participants and included age, gender, race, level of education, marital status, employment status, residence, and smoking history.
Stigma was measured using a six-item scale (Phelan et al., 2013). Each item is a statement or belief about cancer-related stigma and self-blame that is rated on a four-point scale ranging from 1 (not at all) to 4 (very much). The scale was validated in a sample of veterans who had been diagnosed with colorectal cancer; the Cronbach alpha in the validation study was 0.86 (Phelan et al., 2013). This scale, which had just a few items, was chosen to minimize participant burden.
Physical symptoms were measured by the MD Anderson Symptom Inventory–Lung Cancer (MDASI-LC), which has been validated (Mendoza et al., 2011). The MDASI-LC symptom severity subscale consists of 13 core cancer symptoms and three lung cancer–specific symptoms, with possible responses ranging from 0 (not present) to 10 (as bad as you can imagine). The Cronbach alpha coefficient for symptom severity in the current study was 0.92. The MDASI-LC symptom interference subscale contains an additional six items related to activity interference and affective interference and how these symptoms interfere with daily life, with possible responses ranging from 0 (did not interfere) to 10 (interfered completely). The Cronbach alpha coefficient for symptom interference in the current study was 0.93.
The Medical Outcomes Survey, Social Support Survey (MOS-SSS) was initially developed and validated in a sample of individuals with chronic illness (Sherbourne & Stewart, 1991) and has been used in subsequent studies investigating social support in individuals with lung cancer (Poghosyan et al., 2015; Sullivan et al., 2016). The MOS-SSS is a 19-item scale that inquires about how often various types of social support are available to an individual. Possible responses rage from 1 (none of the time) to 5 (all of the time). In addition to measuring overall available social support, several subscales measure specific types of social support, including emotional/informational support, tangible support, affectionate support, and positive social interaction. An additional item—“Someone to do things to help you get your mind off things”—is not part of any subscale and is analyzed as an individual item. Scores are summed and converted to a scale ranging from 0–100, with higher scores indicating more social support (Sherbourne & Stewart, 1991). The Cronbach alpha coefficient in the current study was 0.95.
Social constraints, based on social cognitive processing theory, measure a partner’s socially constraining behaviors toward a loved one (Lepore, 2001). These behaviors may include minimizing emotions and problems or changing the topic when the loved one wants to discuss the illness. Social constraints were measured using the 15-item Social Constraints Scale. It has been used in individuals with cancer (Lepore & Ituarte, 1999; Mosher et al., 2012) and with lung cancer (Chronbach alpha = 0.92) (Chambers et al., 2015). Possible scores range from 0 (never) to 10 (always). This scale is inversely scored, with a higher score indicating more perceived social constraints and an increased perception of constraining behavior from the participant’s primary caregiver. The Cronbach alpha coefficient for the sample in the current study was 0.94.
QOL was measured by a linear analog scale assessment of QOL (Locke et al., 2007) examining five domains of QOL: physical, emotional, spiritual, intellectual, and overall. Each domain is measured with one item rated on a 10-point scale ranging from 0 (as bad as it can be) to 10 (as good as it can be). This tool was validated in individuals with high-grade gliomas and is appropriate for use when short scales are desired to minimize participant burden (Locke et al., 2007). In addition, it has previously been used in those with lung cancer (Clark et al., 2008; Morrison et al., 2017; Solberg Nes et al., 2012). Scores of 6–10 are considered to represent adequate QOL, whereas scores of 0–5 are considered to represent low QOL.
Data Analysis
Descriptive statistics were used to describe the study sample. To determine whether the six-item stigma scale could identify separate groupings of stigma among study participants, the six stigma items were entered into IBM SPSS Statistics TwoStep Cluster Analysis, a tool designed to reveal natural groupings, or clusters, of cases within a given dataset. The resulting analysis included whether clusters could be identified, the number of clusters identified, the number of individuals in each cluster, a qualitative description (from poor to good) of how well the clusters are separated from one another, and the importance of each item on each scale in forming the clusters.
Results of the TwoStep Cluster Analysis determined that participants could be divided into two groups: low stigma (n = 40) and high stigma (n = 22). Independent samples t tests and the eta-squared effect size measure were used to compare scale score means between the two stigma groups. There was good separation between groups.
Results
All participants (N = 62) had been diagnosed with advanced-stage lung cancer (stage III or IV) and had received chemotherapy at least once. The mean age of the sample was 64.45 years (SD = 8.69). The majority of the participants were women (n = 37), married or living with a partner (n = 49), and highly educated (n = 27 with a college degree or higher) (see Table 1). Most participants reported a history of smoking (n = 41), which was defined as an individual smoking more than 100 cigarettes in his or her lifetime. 
Table 2 shows the mean stigma item scores and associated statistics between the high- and low-stigma groups. The only stigma item that was not significantly different between the two groups was the following: “I feel I am to blame for my disease.” The stigma items, in rank order, that were most important in forming the two groups were items 4, 2, 3, and 1. 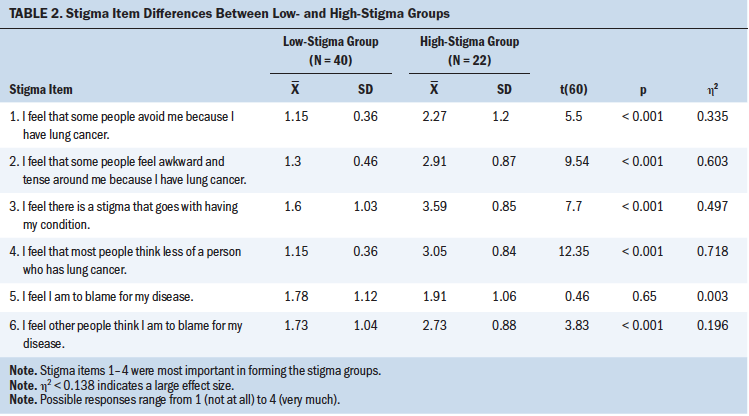
Mean scores on the MDASI-LC between the high- and low-stigma groups were compared (see Table 3). No differences between the groups were found for the core symptoms, the lung cancer–specific symptoms, activity interference, or total symptom severity. There was a significant difference between the high- and low-stigma groups in the affective interference scores (p = 0.005) and the total interference scores (p = 0.025). Individuals in the high-stigma group had higher scores for affective symptom interference and total symptom interference than those in the low-stigma group. 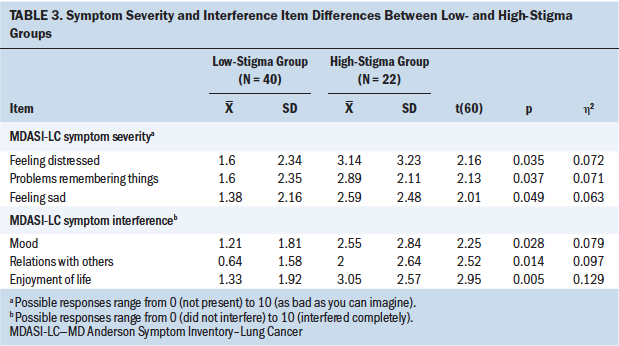
In the low-stigma group, the following symptoms had the highest severity scores: fatigue (mean = 2.95, SD = 2.77), feeling drowsy (mean = 2.03, SD = 2.75), and dyspnea (mean = 1.98, SD = 2.94). In the high-stigma group, the following symptoms had the highest severity scores: fatigue (mean = 4.23, SD = 2.67), dyspnea (mean = 3.50, SD = 2.94), and feeling distressed (mean = 3.14, SD = 3.23). Individual items on the symptom severity subscale were analyzed for group differences. Individuals in the high-stigma group were found to have significantly higher item means, indicating increased symptom severity, for feeling distressed (p = 0.035), problems remembering things (p = 0.037), and feeling sad (p = 0.049). Individual items on the symptom interference subscale were analyzed for group differences. The high-stigma group had significantly higher interference scores for mood (p = 0.28), relations with others (p = 0.014), and enjoyment of life (p = 0.005).
The high- and low-stigma groups’ mean scores on the overall MOSS-SSS scale and subscales were compared (see Table 4). The high-stigma group reported significantly lower mean scores for emotional and informational support (p = 0.001), tangible support (p = 0.005), affectionate support (p = 0.005), positive social interaction (p = 0.001), and the MOS-SSS overall (p < 0.001). The mean score for the additional item on the scale—“Someone to do things to help you get your mind off things”—was also significantly lower for those in the high-stigma group (p = 0.014). 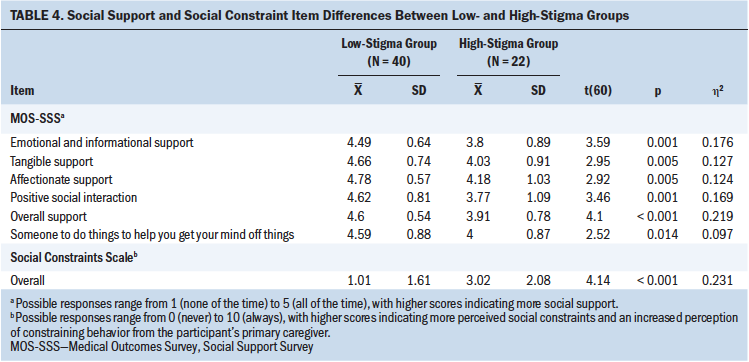
Mean scores on the Social Constraints Scale were compared between groups. A significant difference between the high- and low-stigma groups was found (p < 0.001). Those in the high-stigma group reported more constraining behaviors from their primary caregivers.
Differences in QOL between the two stigma groups were examined, with significant differences identified in physical (p = 0.008), emotional (p = 0.002), spiritual (p = 0.006), intellectual (p = 0.001), and overall QOL (p = 0.001) between the high- and low-stigma groups (see Table 5). Large effect sizes were found for emotional, intellectual, and overall QOL, and medium effect sizes were found for physical and spiritual QOL 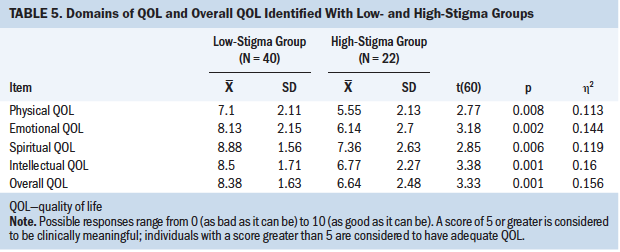 .
.
Discussion
QOL, influenced by a multitude of factors, is a clinical priority for those with a life-limiting illness. Factors such as physical symptom burden and interference and social support have been identified as contributing to the experience of QOL. A higher incidence of physical symptoms decreases QOL in individuals with advanced cancer (Iyer et al., 2014; Ma et al, 2014; Polanski et al., 2016). Decreased social support also negatively influences QOL in those with advanced cancer (Applebaum et al., 2014).
The evidence for stigma as an influencing factor in QOL among those with lung cancer is emerging (Brown Johnson et al., 2014; Chambers et al., 2015), but it has not been fully investigated, particularly in those with advanced lung cancer, for which there is no cure. As an independent factor, stigma may influence physical symptoms, and social support in individuals with advanced lung cancer may play an important role in overall QOL in this population.
The link between stigma and physical symptoms remains unclear, particularly in advanced lung cancer. Only qualitative evidence has suggested that stigma is involved in social support in those with lung cancer (Chapple et al., 2004). Limited evidence exists regarding social constraints in individuals with lung cancer, but socially constraining behavior may be connected to the experience of stigma and QOL in this population (Chambers et al., 2015).
The incidence of stigma in lung cancer ranges from 30% (LoConte et al., 2008) to 95% (Hamann et al., 2014). In the current study’s sample of patients with advanced lung cancer (N = 62), 35% were categorized into the high-stigma group. This finding does not indicate that those categorized into the low-stigma group do not have any feelings of stigma associated with their disease; rather, it indicates that the feelings of stigma among those in the low-stigma group may not rise to the level of clinical significance.
Although physical symptoms and QOL in individuals with lung cancer have been linked (Iyer et al., 2014; Ma et al., 2014; Polanski et al., 2016), the influence of stigma on the experience of physical symptom severity and symptom interference is not well understood. A study by Cataldo and Brodsky (2013) investigated this relationship among individuals with lung cancer, finding that stigma was significantly associated with symptom severity and that stigma made a small but significant contribution to the variance in symptom severity beyond age, anxiety, and depression.
Findings from the current study do not support the association between stigma and physical symptom severity in individuals with lung cancer. The current authors examined stigma in relation to multiple dimensions of physical symptoms in individuals with lung cancer, including severity and interference, finding that stigma was significantly associated with total symptom interference and affective interference but not physical symptom severity. The high- and low-stigma groups reported fatigue as the most troubling symptom and also reported dyspnea as a troubling symptom. Feeling distressed may be more common in those who experience more stigma.
Social support plays a significant role in how patients adjust to their disease after diagnosis (Lepore & Revenson, 2007; Zemore & Shepel, 1989). Social support may be particularly important for individuals diagnosed with advanced disease, who often have high care needs for physical symptoms throughout the disease trajectory. Those who require end-of-life care will eventually be completely reliant on others. Qualitative evidence has suggested that stigma in individuals diagnosed with lung cancer affects how these patients interact with family, friends, and providers; in addition, some patients reported concealing their diagnosis from others, which made obtaining appropriate social support difficult (Chapple et al., 2004). The current study supports earlier qualitative findings. Individuals who were clustered in the high-stigma group reported less social support of all types than those in the low-stigma group.
In addition to social support, the current study examined social constraints. Individuals with high social constraints have less supportive caregivers. Social constraints are important to investigate because most individuals with lung cancer are treated in outpatient facilities and rely on the primary caregiver for day-to-day care in the home. Individuals with advanced lung cancer require more intense home care from their primary caregiver as the disease progresses toward death. Social constraints are understudied in relation to stigma in lung cancer. In a study by Chambers et al. (2015), social constraints were found to have a significant mediating effect on the association between distress and stigma in individuals with all stages of lung cancer. In the current study, similar to the findings regarding social support, those in the high-stigma group reported more socially constraining behaviors from their primary caregiver.
Social support and social constraints may affect the overall experience of lung cancer for patients in the final months of life. Both factors have implications for the quality of care provided in the outpatient and home hospice setting. As symptom burden increases and death approaches, individuals require more care.
The primary finding from this study—that feelings of stigma may influence QOL—is supported by previous studies. A study by Brown Johnson et al. (2014) involving patients with all stages of lung cancer found that stigma provided a significant explanation of variance in QOL beyond depression and anxiety. A study by Chambers et al. (2015) that investigated depression, anxiety, and stigma found that stigma was related to QOL in patients with all stages of cancer. An important difference between these earlier studies and the current study is that the current study’s sample consisted of only those with advanced cancer; in addition, focus was placed on the amount of stigma (high or low) perceived by an individual.
It is noteworthy that individuals in the high-stigma group were more likely to have increased symptom interference, decreased social support, and increased social constraints from a primary caregiver. These findings suggest that individuals who feel the most stigmatized may have fewer available supports and poorer physical health outcomes. It is possible that those who feel stigmatized may be less likely to reach out because of fear of rejection, or they may feel that they caused their disease and, therefore, deserve to suffer.
QOL becomes a goal of care during the final months of life, and individuals facing terminal illness may have a very different end-of-life experience because of stigma. Individuals with advanced lung cancer may be psychologically preparing for the end of life and facing their own mortality. They may be processing life choices that have contributed to their diagnosis and, ultimately, their death while also navigating relationships with those who provide the physical care they need. In addition, individuals with lung cancer may process factors that have contributed to their disease, like smoking, differently than those who have the potential to be cured.
Limitations
Several limitations of this study must be considered. This was a homogeneous sample of primarily White, affluent, and highly educated individuals with lung cancer; this limits the generalizability to other populations. In addition, this was a cross-sectional study, and it is not possible to establish causality between the study variables. Also, individuals in hospice were excluded; those who opt for hospice care have additional supports intended to maximize supportive care and QOL that are not available in the community setting. Self-report measures were employed to gather information; participants may have been unwilling to accurately report social support and social constraints if they felt that this information would reflect negatively on those who cared for them.
Implications for Nursing
Multiple factors influence QOL in individuals with advanced lung cancer. Nurses must be aware that stigma can affect factors associated with QOL and assess for stigma throughout the lung cancer trajectory. In addition, nurses should privately assess available social supports and support from the primary caregiver. Referrals to appropriate interventions, such as psychotherapy, should be made to help resolve feelings and improve available supports. Physical symptoms continue to negatively affect QOL and provide the opportunity for nurses to offer symptom management.
Lung cancer–related stigma is often not part of routine nursing assessment, but emerging evidence suggests it may be beneficial for nurses to screen for stigma in some individuals (Cataldo et al., 2011; Chambers et al., 2012; Chapple et al., 2004; Hamann et al., 2014). One reason that the stigma measure used in the current study was selected is because it consists of just six items; a short instrument is appropriate for use in a population of individuals with advanced cancer because it can be administered quickly and limits patient burden. This stigma measure was able to be completed in only a few minutes; as such, it can easily be incorporated by nurses into the clinical setting to assess for stigma. When nurses identify an individual with feelings of stigma, appropriate referrals to mental health professionals can be made.
Future research should investigate the link between the increase in stigma and the decrease in available social supports and poorer physical health outcomes. Qualitative studies should examine whether individuals with advanced lung cancer process feelings of stigma differently than those with early-stage disease. Lung cancer–related stigma has not been well investigated in ethnic minorities, low-income individuals, or rural dwellers; replicating this study in a more diverse population may yield different results. Further research is needed to explore the concept of social constraints and how this may influence the care received at home and during the end of life. In particular, the link between social constraints and spiritual QOL should be examined. 
Conclusion
Advanced lung cancer has been associated with an extremely high physical symptom burden, but addressing physical symptoms alone is not sufficient to improve QOL. Those facing the end of life should identify QOL goals and be provided with the opportunity to achieve goal resolution. During all phases of care, multiple factors can influence QOL, such as feelings of stigma. Stigma may also affect other factors that contribute to the overall QOL experience, such as general social support, support from a primary caregiver at home, and physical symptoms. 
About the Author(s)
Lee Ann Johnson, PhD, RN, is an assistant professor, Ann M. Schreier, PhD, RN, is a professor, and Melvin Swanson, BS, PhD, is a professor and statistician, all in the Department of Nursing Science in the College of Nursing at East Carolina University in Greenville, NC; Janet P. Moye, PhD, MS, BSN, RN, is the director of palliative care services at Vidant Medical Center in Greenville, NC; and Sheila Ridner, PhD, RN, FAAN, is a Martha Rivers Ingram Professor of Nursing in the School of Nursing at Vanderbilt University in Nashville, TN. This research was funded by a grant from the American Cancer Society. During the writing of this article, Ridner received funding from ImpediMed Tactile Systems. Johnson contributed to the conceptualization and design and completed the data collection. Johnson and Swanson provided statistical support. All authors provided the analysis. Johnson, Schreier, Moye, and Ridner contributed to the manuscript preparation. Johnson can be reached at jarrettl16@ecu.edu, with copy to ONFEditor@ons.org. (Submitted May 2018. Accepted November 8, 2018.)
References
American Cancer Society. (2018). Cancer facts and figures, 2018. Retrieved from https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and…
Applebaum, A.J., Stein, E.M., Lord-Bessen, J., Pessin, H., Rosenfeld, B., & Breitbart, W. (2014). Optimism, social support, and mental health outcomes in patients with advanced cancer. Psycho-Oncology, 23, 299–306. https://doi.org/10.1002/pon.3418
Braun, D.P., Gupta, D., & Staren, E.D. (2011). Quality of life assessment as a predictor of survival in non-small cell lung cancer. BMC Cancer, 11, 353. https://doi.org/10.1186/1471-2407-11-353
Brown Johnson, C.G., Brodsky, J.L., & Cataldo, J.K. (2014). Lung cancer stigma, anxiety, depression, and quality of life. Journal of Psychosocial Oncology, 32, 59–73. https://doi.org/10.1080/07347332.2013.855963
Cataldo, J.K., & Brodsky, J.L. (2013). Lung cancer stigma, anxiety, depression and symptom severity. Oncology, 85, 33–40. https://doi.org/10.1159/000350834
Cataldo, J.K., Slaughter, R., Jahan, T.M., Pongquan, V.L., & Hwang, W.J. (2011). Measuring stigma in people with lung cancer: Psychometric testing of the Cataldo Lung Cancer Stigma Scale [Online exclusive]. Oncology Nursing Forum, 38, E46–E54. https://doi.org/10.1188/11.ONF.E46-E54
Chambers, S.K., Baade, P., Youl, P., Aitken, J., Occhipinti, S., Vinod, S., . . . O’Connell, D.L. (2015). Psychological distress and quality of life in lung cancer: The role of health-related stigma, illness appraisals and social constraints. Psycho-Oncology, 24, 1569–1577. https://doi.org/10.1002/pon.3829
Chambers, S.K., Dunn, J., Occhipinti, S., Hughes, S., Baade, P., Sinclair, S., . . . O’Connell, D.L. (2012). A systematic review of the impact of stigma and nihilism on lung cancer outcomes. BMC Cancer, 12, 184. https://doi.org/10.1186/1471-2407-12-184
Chapple, A., Ziebland, S., & McPherson, A. (2004). Stigma, shame, and blame experienced by patients with lung cancer: Qualitative study. BMJ, 328, 1470–1473. https://doi.org/10.1136/bmj.38111.639734.7C
Clark, M.M., Novotny, P.J., Patten, C.A., Rausch, S.M., Garces, Y.I., Jatoi, A., . . . Yang, P. (2008). Motivational readiness for physical activity and quality of life in long-term lung cancer survivors. Lung Cancer, 61, 117–122. https://doi.org/10.1016/j.lungcan.2007.12.012
Cooley, M.E., Short, T.H., & Moriarty, H.J. (2003). Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psycho-Oncology, 12, 694–708. https://doi.org/10.1002/pon.694
Dillman, R.O., & McClure, S.E. (2014). Steadily improving survival in lung cancer. Clinical Lung Cancer, 15, 331–337. https://doi.org/10.1016/j.cllc.2014.05.006
Efficace, F., Bottomley, A., Smit, E.F., Lianes, P., Legrand, C., Debruyne, C., . . . Van Meerbeeck, J. (2006). Is a patient’s self-reported health-related quality of life a prognostic factor for survival in non-small-cell lung cancer patients? A multivariate analysis of prognostic factors of EORTC study 08975. Annals of Oncology, 17, 1698–1704. https://doi.org/10.1093/annonc/mdl183
Else-Quest, N.M., LoConte, N.K., Schiller, J.H., & Hyde, J.S. (2009). Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychology and Health, 24, 949–964. https://doi.org/10.1080/08870440802074664
Hamann, H.A., Ostroff, J.S., Marks, E.G., Gerber, D.E., Schiller, J.H., & Lee, S.J. (2014). Stigma among patients with lung cancer: A patient-reported measurement model. Psycho-Oncology, 23, 81–92. https://doi.org/10.1002/pon.3371
Harris, P.A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., & Conde, J.G. (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Hopwood, P., & Stephens, R.J. (1995). Symptoms at presentation for treatment in patients with lung cancer: Implications for evaluation of palliative care. The Medical Research Council (MRC) Lung Cancer Working Party. British Journal of Cancer, 7, 633–636.
House, J.S. (1981). Work stress and social support. Reading, MA: Addison-Wesley.
House, J.S. & Kahn, R.L. (Eds.). (1985). Measures and concepts of social support. Orlando, FL: Academic Press.
Iyer, S., Roughley, A., Ridner, A., & Taylor-Stokes, G. (2014). The symptom burden of non-small cell lung cancer in the USA: A real-world cross-sectional study. Supportive Care in Cancer, 22, 181–187. https://doi.org/10.1007/s00520-013-1959-4
Kahn, R.L., & Antonucci, T.C. (1980). Convoys over the life course: Attachment, roles, and social support. In P.B. Baltes & O. Brim (Eds.), Life-span development and behavior (Vol. 3, pp. 253–286). New York, NY: Academic Press.
Lebel, S., Castonguay, M., Mackness, G., Irish, J., Bezjak, A., & Devins, G.M. (2013). The psychosocial impact of stigma in people with head and neck or lung cancer. Psycho-Oncology, 22, 140–152. https://doi.org/10.1002/pon.2063
Lepore, S.J. (2001). A social-cognitive processing model of emotional adjustment to cancer. In A. Baum & B.L. Andersen (Eds.), Psychosocial interventions for cancer (pp. 99–118). Washington, DC: American Psychological Association.
Lepore, S.J., & Ituarte, P.H.G. (1999). Optimism about cancer enhances mood by reducing negative social interactions. Cancer Research, Therapy and Control, 8, 165–174.
Lepore, S.J., & Revenson, T.A. (2007). Social constraints on disclosure and adjustment to cancer. Social and Personality Psychology Compass, 1, 313–333. https://doi.org/10.1111/j.1751-9004.2007.00013.x
Locke, D.E., Decker, P.A., Sloan, J.A., Brown, P.D., Malec, J.F., Clark, M.M., . . . Buckner, J.C. (2007). Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients. Journal of Pain and Symptom Management, 34, 628–638. https://doi.org/10.1016/j.jpainsymman.2007.01.016
LoConte, N.K., Else-Quest, N.M., Eickhoff, J., Hyde, J., & Schiller, J.H. (2008). Assessment of guilt and shame in patients with non–small-cell lung cancer compared with patients with breast and prostate cancer. Clinical Lung Cancer, 9, 171–178. https://doi.org/10.3816/CLC.2008.n.026
Ma, Y., Yang, Y., Huang, Y., Zhao, H., Hou, X. Tian, Y., . . . Zhang, L. (2014). An investigation of symptom burden and quality of life in Chinese chemo-naïve advanced lung cancer patients by using the Instrument-Cloud QOL System. Lung Cancer, 84, 301–306. https://doi.org/10.1016/j.lungcan.2014.01.027
Mendoza, T.R., Wang, X.S., Lu, C., Palos, G.R., Liao, Z., Mobley, G.M., . . . Cleeland, C.S. (2011). Measuring the symptom burden of lung cancer: The validity and utility of the lung cancer module of the M.D. Anderson Symptom Inventory. Oncologist, 16, 217–227. https://doi.org/10.1634/theoncologist.2010-0193
Morrison, E.J., Novotny, P.J., Sloan, J.A., Yang, P., Patten, C.A., Ruddy, K.J., & Clark, M.M. (2017). Emotional problems, quality of life, and symptom burden in patients with lung cancer. Clinical Lung Cancer, 18, 497–503. https://doi.org/10.1016/j.cllc.2017.02.008
Mosher, C.E., Lepore, S.J., Wu, L., Austin, J., Valdimarsdottir, H., Rowley, S., . . . Rini, C. (2012). Social correlates of distress following hematopoietic stem cell transplantation: Exploring the role of loneliness and cognitive processing. Journal of Health Psychology, 17, 1022–1032. https://doi.org/10.1177/1359105311432490
Phelan, S.M., Griffin, J.M., Jackson, G.L., Zafar, S.Y., Hellerstedt, W., Stahre, M., . . . van Ryn, M. (2013). Stigma, perceived blame, self-blame, and depressive symptoms in men with colorectal cancer. Psycho-Oncology, 22, 65–73. https://doi.org/10.1002/pon.2048
Poghosyan, H., Stock, S., Kennedy Sheldon, L., Cromwell, J., Cooley, M.E., & Nerenz, D.R. (2015). Racial disparities in health-related quality of life after lung cancer surgery: Findings from the Cancer Care Outcomes Research and Surveillance Consortium. Journal of Thoracic Oncology, 10, 1404–1412. https://doi.org/10.1097/JTO.0000000000000629
Polanski, J., Jankowska-Polańska, B., Rosinczuk, J., Chabowski, M., & Szymanska-Chabowska, A. (2016). Quality of life of patients with lung cancer. OncoTargets and Therapy, 9, 1023–1028. https://doi.org/10.2147/OTT.S100685
Schiller, J.H., Harrington, D., Belani, C.P., Langer, C., Sandler, A., Krook, J., . . . Johnson, D.H. (2002). Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. New England Journal of Medicine, 346, 92–98. https://doi.org/10.1056/NEJMoa011954
Sherbourne, C.D., & Stewart, A.L. (1991). The MOS Social Support Survey. Social Science and Medicine, 32, 705–714.
Solberg Nes, L., Liu, H., Patten, C.A., Rausch, S.M., Sloan, J.A., Garces, Y.I., . . . Clark, M.M. (2012). Physical activity level and quality of life in long term lung cancer survivors. Lung Cancer, 77, 611–616. https://doi.org/10.1016/j.lungcan.2012.05.096
Sullivan, D.R., Forsberg, C.W., Ganzini, L., Au, D.H., Gould, M.K., Provenzale, D., . . . Slatore, C.G. (2016). Depression symptom trends and health domains among lung cancer patients in the CanCORS study. Lung Cancer, 100, 102–109. https://doi.org/10.1016/j.lungcan.2016.08.008
Temel, J.S., Greer, J.A., Muzikansky, A., Gallagher, E.R., Admane, S., Jackson, V.A., . . . Lynch, T.J. (2010). Early palliative care for patients with metastatic non–small-cell lung cancer. New England Journal of Medicine, 363, 733–742. https://doi.org/10.1056/NEJMoa1000678
Weiss, J., Yang, H., Weiss, S., Rigney, M., Copeland, A., King, J.C., & Deal, A.M. (2017). Stigma, self-blame, and satisfaction with care among patients with lung cancer. Journal of Psychosocial Oncology, 35, 166–179. https://doi.org/10.1080/07347332.2016.1228095
Zemore, R., & Shepel, L.F. (1989). Effects of breast cancer and mastectomy on emotional support and adjustment. Social Science and Medicine, 28, 19–27.




