Research Agenda of the Oncology Nursing Society: 2019–2022
Problem Statement: To define the Oncology Nursing Society Research Agenda for 2019–2022.
Design: Multimethod, consensus-building approach by members of the Research Agenda Project Team.
Data Sources: Expert opinion, literature review, surveys, interviews, focus groups, town hall, and review of research priorities from other cancer care organizations and funding agencies.
Analysis: Content analysis and descriptive statistics were used to synthesize research priority themes that emerged.
Findings: Three priority areas for scientific development were identified: symptom science, health disparities, and palliative and psychosocial care in oncology. In addition, cross-cutting themes that provide context and elaboration for these priorities emerged.
Implications for Nursing: The Research Agenda can be used to focus oncology nurses’ research, scholarship, leadership, and health policy efforts to advance quality cancer care, inform research funding priorities, and align initiatives and resources across the ONS enterprise.
Jump to a section
The Oncology Nursing Society (ONS) promotes excellence in oncology nursing and quality cancer care. In keeping with this mission, since 2001, ONS has developed and disseminated a Research Agenda identifying priority areas where new knowledge is urgently needed. The purpose of the Research Agenda is two-fold: it includes both the development and dissemination of contemporary research priorities needed to advance cancer care and delineates critical areas to be considered by research funders.
A multimethod approach was used to develop the Research Agenda. ONS identified content experts to serve on the project team. This team was broken into smaller work groups to address various tasks that needed to be accomplished to update the Research Agenda. The first work group was convened to review the previous process used to formulate the prior ONS Research Agenda and discuss the best way to update the process in a scientifically rigorous yet time- and resource-efficient manner (Knobf et al., 2015). The previous process spanned multiple years and involved surveying ONS members, publishing survey results, and then convening an expert panel to update the Research Agenda based on survey responses. Given the low response rates (11%) and the lack of a clear set of focused research priorities that emerged from analysis of the survey results, an alternative approach was discussed to actively engage ONS members with expertise in research together with other key stakeholders to yield a focused set of research priorities (LoBiondo-Wood et al., 2014).
A condensed process was recommended by the work group to identify a focused set of contemporary research priorities. As Figure 1 illustrates, the first step in the process consisted of conducting a targeted survey and interviews with ONS members with research expertise as well as key stakeholders from funding agencies to identify research priorities needed to transform cancer care. Once this information was gathered and collated, the entire project team convened to discuss the results, review relevant literature, and examine research priorities from other professional organizations and funding agencies. 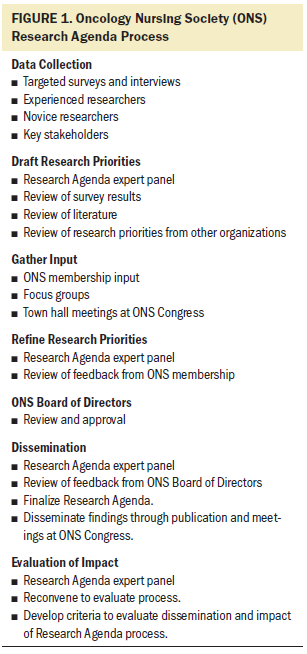
Once content areas where new knowledge is needed had been identified, work groups were convened to draft research priorities within each topical area. A consensus-building process consisting of iterative cycles of peer review and refinement of the research priorities was used to generate and approve the initial draft of the Research Agenda. Once the draft was approved, the Research Agenda was shared with a broader group of ONS members at the annual ONS Congress. The feedback from these sessions was presented to the Research Agenda team for consideration and inclusion in the agenda. The final Research Agenda was approved by the ONS Board of Directors. An evaluation process will be used to refine the process.
The purpose of this article is to disseminate the Research Agenda to the ONS membership and the larger interprofessional oncology community. The 2019–2022 Research Agenda provides a scientific road map that may be used to inform research, scholarship, leadership, and health policy efforts to advance quality cancer care, inform research funding priorities, and align initiatives and resources across the ONS enterprise.
The 2019–2022 Research Agenda project team identified three overarching priority areas where new scientific knowledge is needed: symptom science, health disparities, and palliative and psychosocial care in oncology. In addition, four cross-cutting themes emerged that add context and elaboration to these priorities: aging, survivorship, healthcare delivery models, and advanced research methods (see Figure 2). The following summary presents each research priority separated into three sections, including the context of the problem, research gaps, and the research priorities. 
Symptom Science: Immunotherapy and Emerging Therapies
Immunotherapy (IO) is one of the fastest-emerging areas of cancer treatment, and the availability of this new class of agents has created a paradigm shift in the treatment of many malignancies. The initial research priority related to IO focuses on immune checkpoint inhibitor (ICPI) therapies because so many new agents targeting this mechanism have received recent U.S. Food and Drug Administration (FDA) approval (Hargadon, Johnson, & Williams, 2018). Other emerging IOs, including chimeric antigen receptor (CAR) T cells and combinatorial therapies (Holzinger & Abken, 2020) are also relevant within the context of this priority as the science evolves (Iafolla et al., 2018; Tang, Shalabi, & Hubbard-Lucey, 2018). The immune checkpoint pathway, part of the adaptive immune system, serves to balance the body’s responses to foreign antigens and prevents autoimmune dysfunction. Tumors have the capacity to exploit these pathways, thereby evading immune surveillance. Inhibitory ICPIs targeting cytotoxic T-lymphocyte antigen 4 and programed cell death protein 1 (PD-1) and its ligand (PD-L1) are the inhibitory ICPIs that have received the most study to date. ICPIs that target each of these checkpoints have been approved for use as first- and second-line therapy for metastatic disease in several malignancies, as well as in the adjuvant setting (Hargadon et al., 2018).
Although generally well tolerated, ICPI is associated with a unique profile of adverse effects referred to as immune-related adverse events (irAEs) (Friedman, Proverbs-Singh, & Postow, 2016; Postow, Sidlow, & Hellmann, 2018). irAEs reflect a generalized inflammatory reaction and can lead to significant morbidity. Although any organ system can be affected, the most common irAEs involve the gastrointestinal tract, endocrine glands, skin, and liver. Less often, the central nervous system and cardiovascular, pulmonary, musculoskeletal, and hematologic systems are involved.
There is interest in understanding factors that influence patients’ responses to ICPI and irAE development. Clinical trials have observed variability in the therapeutic response to ICPI based on the tumor microenvironment, tumor burden, PD-L1 expression, age, and gender (Yan et al., 2018). Diversity of the gut microbiome is associated with improved response to ICPI, and antibiotic use may abrogate this response (Chaput et al., 2017; Havel, Chowell, & Chan, 2019; Matson et al., 2018).
Research Gaps
ICPI therapies have been associated with numerous adverse events. Adverse events of cancer therapies are typically graded and reported by clinicians using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (U.S. Department of Health and Human Services, 2017). Systematic reviews of clinical trials describe significant gaps in the grading, reporting, and attribution of irAEs of patients treated with ICPI (Chen, Razak, Bedard, Siu, & Hansen, 2015). Although patients may derive clinical benefit from ICPI, few studies have examined changes in symptoms, quality of life, physical function, and/or cognition using tools that are specific to the unique symptom and irAE profile associated with these therapies (Hall et al., 2019; Mendoza, 2018). To date, the patient experience has been captured using generic instruments developed for clinical trials on cytotoxic agents (Hall et al., 2019). Therefore, current instruments used may not accurately reflect the patient experience, underscoring the need to develop patient-reported outcomes (PROs) that are specific to ICPI.
Because patients are living longer after ICPI, it is unclear what will be the profile of late- and long-term adverse events (Johnson et al., 2015). A gap exists in identifying the impact of factors such as age, gender, diet, weight, exercise, stress, sleep patterns, and the environment, individually and collectively, on the development of irAEs across patient populations and over time (Andrews, Reuben, Gopalakrishnan, & Wargo, 2018; Elias, Hartshorn, Rahma, Lin, & Snyder-Cappione, 2018; Idorn & Thor Straten, 2017; King-Kallimanis, Kanapuru, Blumenthal, Theoret, & Kluetz, 2018; Rassy, Ghosn, Rassy, Assi, & Robert, 2018; Sattar, Kartolo, Hopman, Lakoff, & Baetz, 2019; Soldati et al., 2018; Wallis et al., 2019; Yan et al., 2018).
New knowledge is also required to provide the evidence base for optimal supportive care during and following treatment with IO. Although clinical practice guidelines have been developed to guide clinicians in the management of irAEs, those recommendations are based largely on a consensus of clinical expert opinion, rather than empirical evidence (Brahmer, Lacchetti, & Thompson, 2018; Haanen et al., 2017; Thompson et al., 2019). Oncology nurse scientists have significant expertise in the testing of symptom-focused interventions and, therefore, are well-poised to address this knowledge gap (Miaskowski, Barsevick, et al., 2017).
Research Priorities
Areas for future research were developed based on the current state of the science and gaps identified by the expert panel, and included the following recommendations:
• Develop, test, and refine reliable, valid, and sensitive PRO tools to capture treatment experiences in patients receiving IO, and link those measures to clinical decision support and treatment pathways to improve clinical outcomes.
• Characterize variability in presentation, trajectory, and management of the irAEs across various patient populations.
• Examine factors (age, gender, diet, weight, exercise, stress, and sleep patterns) that may influence patient responses to ICPI therapy and irAE development.
• Conduct randomized trials to test the efficacy of supportive care interventions to alleviate irAEs.
Symptom Science: Precision Health and Biosignatures
In alignment with a number of precision health initiatives (Ashley, 2015; Fiore & Goodman, 2016; Ginsburg & Phillips, 2018; McCarthy, 2016; National Research Council, 2011), oncology nursing research is focused on the identification of phenotypic and molecular characteristics that will identify individuals at highest risk for greater symptom burden, as well as target novel and effective supportive care interventions, thereby improving patient outcomes.
At the current time, oncology nurse scientists have made significant contributions to precision health in the areas of symptom management, quality of life, and functional status during and following cancer treatment (Hickey et al., 2019). Patients undergoing treatment for cancer experience multiple co-occurring symptoms. The pattern of symptoms has been classified based on the severity of specific symptoms or symptom clusters among patients with a variety of cancer diagnoses (Mazor et al., 2018; Russell et al., 2019; Sullivan et al., 2018) and by treatments (Abid et al., 2017; Miaskowski, Conley, et al., 2017; Wright et al., 2019). In addition, research is uncovering underlying genomic variations that increase the risk for adverse symptom experiences (Dhruva et al., 2015; Kober et al., 2016; Miaskowski, Conley, et al., 2017; Page et al., 2018).
Determining optimal approaches to reveal the underlying mechanisms of individual variability in symptom experiences requires a comprehensive understanding of the potential factors that contribute to higher symptom burden. Defining individual biosignatures may aid in this process. Biosignatures reflect both genomic and phenotypic components and may include biomarkers and integrative “omics,” as well as lifestyle, environmental, and psychosocial factors (Institute of Medicine, 2008). A number of approaches are available to obtain this information. The use of valid and reliable self-report measures along with the measurement of various biomarkers has provided some of the most salient information about the mechanisms underlying symptom experiences (Dhruva et al., 2015; Kober et al., 2016; Page et al., 2018).
Identifying biosignatures for common individual symptoms and symptom clusters will require the collection and analysis of multiple factors that accurately capture phenotypic and molecular characteristics. In addition to determining phenotypic characteristics, measuring different types of biomarkers (e.g., genomic, metabolomics, proteomic, epigenomic) is an essential component of this research. By definition, a biomarker is a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention (Califf, 2018; FDA-NIH Biomarker Working Group, 2016). Biomarkers provide an indication of molecular functioning and can be applied to risk assessment, diagnosing or monitoring a state or condition, and/or evaluation of an intervention (Califf, 2018). Nurse scientists have incorporated many types of biomarkers into studies that target symptom experiences as well as functional status and responses to biobehavioral interventions (Ferranti, Grossmann, Starkweather, & Heitkemper, 2017).
Research Gaps
A significant gap in the development of a precision health approach to symptom science is that most of the research on individual and/or multiple symptoms relies on statistical approaches that model means and standard deviations. However, a large amount of the individual variability and the latent patterns in patients’ symptom experiences will not be reflected through such statistical modeling. In contrast, there is increasing interest in using finite mixture modeling approaches that are more flexible about the distributional shape of the data, and can be used both to draw inferences and to explore the data to derive clusters or trajectory groups. In addition, the use of heterogenous measures across studies makes it difficult to aggregate data to support integrative analysis across studies (Hesse, Moser, & Riley, 2015; Yu & Zeng, 2018). Strengthening the use of common data elements in symptom science, including patient demographics, self-reported symptoms, behavioral factors, and biological measures, sets the stage for integrative data analysis, which has been a key priority of the National Institute of Nursing Research (Page et al., 2018; Redeker et al., 2015).
More information is needed about the phenotypes of individuals with stable symptoms as well as individuals whose symptoms are changing over time. A better understanding of those characteristics of patients with fluctuating symptoms will predict patients who, at baseline, are at greatest risk for adverse symptoms, allowing clinicians to target intensive symptom screening and management approaches. Such predictive analytics will also support the delivery of anticipatory patient and family education and other preemptive interventions to improve symptom outcomes. There is also a knowledge gap with respect to the phenotype(s) of symptom burden in the survivor population. Although the number of survivors is increasing, there has been little exploration of symptom clusters in survivors, and most of the work that has been conducted has limited the focus to symptom clusters in breast cancer survivors. A majority of the reports of the symptom experience in cancer survivors emphasize the occurrence, severity, and management of single symptoms (Kim, Kim, Lim, & Kim, 2018; Nho, Kim, Park, & Kweon, 2018). Cross-sectional and longitudinal studies are needed to characterize the symptom experiences of patients with cancer in different age groups, with different types of cancers and treatments, and across the cancer control continuum. Also urgently needed are studies to identify effective interventions to mitigate and manage symptom clusters, both during and following cancer treatment and at the end of life (Miaskowski, Barsevick, et al., 2017). Conducting and analyzing such intervention studies, particularly those testing complex, multicomponent interventions, will require knowledge of advanced methods, including adaptive research designs and personalized dynamic treatment regimens, and the statistical methods needed to conduct finite mixture modeling and to examine mediation and moderation of treatment effects.
Determining optimal methodologic approaches to identify patients at risk for a higher symptom burden is an area that warrants additional research. The use of large data sets that include biological determinants may expedite filling this gap in knowledge. For example, a study by Papachristou et al. (2018) used predictive modeling and a robust dataset to predict which patients would be most likely to have severe symptoms during a cycle of chemotherapy.
Although many biosignature studies do capture data over time, the number of time points may be limited. Newer approaches, such as ecological momentary assessment, that capture the dynamic nature of patients’/survivors’ symptom experiences and their associated biomarker profiles, are warranted. Approaches that incorporate web-based patient and survivor interfaces, such as the Electronic Patient Self-Reporting of Adverse Events: Patient Information and aDvice (eRAPiD) system (Absolom, Gibson, & Velikova, 2019; Cowan et al., 2016) or the Symptom Care at Home System, may be helpful for real-time symptom reporting (Mooney, Whisenant, & Beck, 2019). However, characterizing individual variability in symptom experiences will require a multifaceted approach that also includes wearable devices and genomic/epigenomic evaluations, as well as the collection of patients, treatment, biobehavioral, and psychosocial factors to optimize the identification of symptom biosignatures (Lucas et al., 2018).
Research Priorities
Areas for future research that were developed based on the current state of the science and gaps identified by the expert panel included the following recommendations:
• Harmonize assessment measures and strengthen the use of common data elements.
• Identify the optimal approaches to characterize patients’ and survivors’ symptom profiles and their associated genotypes and phenotypes.
• Comparatively evaluate approaches to examine the mechanisms underlying variation in patients’ and survivors’ symptom experiences.
• Determine optimal methodologic approaches to predict patients and survivors at greatest risk for symptom burden.
• Establish the biosignature (i.e., phenotypic and molecular characteristics) of common individual symptoms and symptom clusters in patients and survivors.
• Develop and test interventions to manage single symptoms and symptom clusters.
Health Disparities
The National Institutes of Health (NIH) has designated several groups, including Blacks/African Americans, Hispanics/Latinos, American Indians/Alaska Natives, Asian Americans, Native Hawaiians and other Pacific Islanders, socioeconomically disadvantaged populations, sexual and gender minorities, and rural communities, as groups who are underserved and who may experience disparities in health outcomes and access to care (NIH, 2019). An analysis of U.S. socioeconomic and racial/ethnic disparities in incidence and mortality from all cancers, combined from 1950 to 2014, demonstrates that those who live in more areas with fewer resources, and those with lower levels of education and income, have higher cancer incidence and mortality rates compared with those with more resources (Singh & Jemal, 2017). In addition, those who identified as lesbian, gay, bisexual, transgender, or queer (LGBTQ) are at higher risk for specific cancers compared to heterosexuals (Hudson et al., 2017). Disparities with respect to incidence and mortality are most prominent among those with lung, colorectal, cervical, stomach, and liver cancers, and these trends in disparities are increasing.
Similarly, there is a higher incidence of cancer-related deaths in rural populations compared to those living in urban communities. Risk factors for adverse outcomes in rural populations include tobacco use, sedentary behavior, and higher rates of obesity (Henley et al., 2017). Many barriers to accessing cancer care and participating in cancer clinical trials that exist for minorities and other vulnerable populations are also experienced by those living in rural communities. These barriers include lower rates of insurance coverage, poverty, transportation problems, and difficulties in accessing cancer screening, diagnosis, and staging services (Charlton, Schlichting, Chioreso, Ward, & Vikas, 2015).
Research Gaps
In 1993, the U.S. government mandated that NIH-funded clinical trials must include women and minorities and must assess outcomes by gender and race or ethnicity. However, roughly 25 years after this mandate, the proportion of racial/ethnic minorities enrolled in cancer clinical trials does not reflect the U.S. general population (Chen, Lara, Dang, Paterniti, & Kelly, 2014). An analysis of 782 published clinical trials showed overall enrollment of women at 46%. However, 15% of trials enrolled less than 30% women, only 26% reported at least one outcome by gender, and 13% reported outcomes by race or ethnicity. Progress has been meager, with no changes or improvements made in inclusion, analysis, or reporting by sex, race, or ethnicity compared to studies conducted prior to this mandate (Geller et al., 2018). Research also continues to identify factors related to health disparities in cancer incidence; for example, approximately 40% of all cancers diagnosed are associated with obesity and women and non-Hispanic Blacks/African Americans are among those at highest risk (Steele et al., 2017).
Very limited research has focused on the care of LGBTQ patients with cancer. Cancer outcomes in this population have been understudied, and the information that is known is less likely to reach oncology care professionals (Margolies & Brown, 2018). An analysis of NIH-funded sexual and gender minority research indicated that 75% of projects focused on HIV/AIDS, whereas only 10% focused on cancer (the second leading cause of death in this population) (NIH, 2015). There is an urgency to better understand the specific needs and experiences of sexual and gender minorities and to improve access to care and care quality for this population, as well as to improve their participation in clinical trials (Quinn et al., 2015).
Similarly, limited research has focused on rural populations, a group that has a higher incidence of cancer-related deaths and prominent risk factors that include tobacco use, sedentary behavior, obesity, and barriers to accessing needed care (Henley et al., 2017). Of note, the availability of cancer care providers in rural areas is lacking; less than 3% of physicians practice in rural areas. To reduce travel time to large metropolitan hospitals, reduce family burden and stress, and reduce healthcare and out-of-pocket costs for the system and patients, alternative models of healthcare delivery need to be evaluated to identify those who provide quality, cost-effective care (Blake, Moss, Gaysynsky, Srinivasan, & Croyle, 2017; Hazin & Qaddoumi, 2010; Sabesan, Simcox, & Marr, 2012).
As a result of the rising cost of cancer treatment, Altice, Banegas, Tucker-Seeley, and Yabroff (2016) observed that 47%–49% of cancer survivors reported experiencing financial distress, and 12%–62% reported being in debt because of their treatment. A population-based study identified several factors associated with cancer-related financial difficulties, including younger age, being a member of a minority group, and recent receipt of chemotherapy and/or radiation therapy. Survivors who had financial problems were more likely to delay or forego medical care, prescription medications, dental care, glasses, and mental health care. To minimize additional health disparities among disadvantaged groups, it is critical to conduct research identifying effective approaches to address the financial burden of cancer care.
Research Priorities
Priorities for continued research include the following:
• Develop and test interventions to increase minority and vulnerable population participation in cancer clinical trials.
• Examine the effects on cancer outcomes of social determinants of health (i.e., physical, social, and economic factors).
• Develop and test interventions to address health disparities related to behavioral factors such as obesity, physical inactivity, diet, tobacco use, and immunizations that can prevent malignancies associated with human papillomavirus and hepatitis B.
• Evaluate interventions to address financial toxicity associated with cancer treatment.
• Examine the role of technology, including telehealth strategies, to improve access to care, particularly among rural populations.
Palliative Care and Psychosocial Oncology
The National Consensus Project (NCP) of Quality Palliative Care developed guidelines for quality care, which were endorsed by 80 organizations, including ONS. Palliative care attends to the physical, functional, psychological, practical, and spiritual consequences of a serious illness through early integration of these needs and concerns into the plan of care. As such, palliative care improves quality of life for the patient and his or her family (Ahluwalia et al., 2018).
Palliative care has been integral to oncology care; cancer is recognized as an illness with a significant quality-of-life burden for patients and their family caregivers. The NCP guidelines recognize eight dimensions of palliative care, including structure and processes of care; physical aspects of care; psychological and psychiatric aspects of care; social aspects of care; spiritual, religious, and existential aspects of care; cultural aspects of care; care of the patient nearing the end of life; and ethical and legal aspects of care. Palliative care is now available in most oncology care settings and has demonstrated significant benefits to patients, families, and health systems. The availability of quality palliative care services is of particular importance given the shift to outpatient care and the aging of the population. There is also a strong recommendation for specialty palliative care to be accompanied by generalist-level palliative care, meaning palliative care provided by oncology nurses, physicians, and other cancer clinicians. Models of palliative care continue to evolve in oncology settings, with increasing attention being given to each of the eight domains across the trajectory of cancer diagnosis, treatment, survivorship, recurrence, and end of life.
Research Gaps
Ahluwalia et al. (2018) examined 139 systematic reviews to synthesize the evidence about the components and effectiveness of palliative care and to identify research gaps. Despite the volume of evidence supporting the benefits of palliative care, much of the evidence is of low quality because of inconsistent findings, the lack of precise effect estimates to support the clinical significance of intervention effects, and a reliance on study designs with a high risk of bias. High-quality evidence does exist that supports the delivery of home-based palliative care; the expansion of these services will be essential as the population ages. Challenges that need to be addressed include the assessment and treatment of symptom burden, particularly given the multimorbidity (defined as two or more chronic conditions) that is experienced by 70% of adults aged 75 years or older (Ritchie & Zulman, 2013). Expansion and testing of new care delivery models, particularly among those in rural areas who may have difficulty accessing palliative care services, are needed (Hui & Bruera, 2015).
A significant gap in knowledge was noted with respect to the provision of culturally sensitive palliative care. Given the importance of acknowledging and incorporating sociocultural norms into care, additional research in this area is needed. Similarly, Ferrell and Wittenberg (2017) found that the majority of research conducted with family caregivers of adults with cancer had been conducted in predominantly White populations.
It is important to focus on both patient and family caregiver needs because family caregivers often experience increasing distress as the patient’s function declines (Northouse, Williams, Given, & McCorkle, 2012; Williams & McCorkle, 2011). Although the testing of caregiver interventions has received greater emphasis recently, the next step in the research is to determine which type or types of interventions work best to improve outcomes, and then to manualize these research-tested interventions for implementation in real-world settings (Ferrell & Wittenberg, 2017; Kent et al., 2016).
Research Priorities
Priorities for continued study include the following:
• Develop and test interventions for culturally sensitive palliative and psychosocial oncology care.
• Examine the effects of telehealth on improving patient and caregiver symptoms and health outcomes.
• Determine the most effective interventions to improve patient and caregiver health-related quality of life (HRQOL), satisfaction with care, and use of healthcare resources.
• Determine the effects of early/integrated palliative care intervention on patient and family caregiver outcomes (e.g., symptoms, HRQOL, psychological health, rehospitalizations).
• Understand the impact of single or multiple symptoms on function, disease outcomes, HRQOL, and treatment decision making in seriously ill older adults with cancer, particularly those with multiple comorbidities.
Cross-Cutting Themes
Four cross-cutting themes emerged as important and timely considerations as clinicians, scientists, and policy makers use this Research Agenda to prioritize their activities. These cross-cutting themes include aging, survivorship, healthcare delivery implications, and advanced research methods. A brief description of each of these themes, highlighting their relevance to contemporary oncology nursing research, is presented. Advanced research methods that have the potential to improve the rigor, depth, and efficiency of research and accelerate the translation of new knowledge into clinical practice are highlighted.
Aging
Cancer incidence is expected to rise 67% in those aged 65 years or older from 2010 to 2030, compared to a rise in incidence of only 11% for those younger than age 65 years. It is also estimated that, by 2040, 73% of cancer survivors in the United States will be 65 years or older (National Cancer Institute, 2019). Older adults typically have other medical conditions that need to be considered when choosing cancer treatment. Research is needed to identify the most effective care delivery models for delivering services to older adults with cancer, and to test, tailor, and target supportive care and rehabilitative interventions that can preserve and enhance HRQOL and function.
Survivorship
The number of cancer survivors is projected to increase from 16.9 million in 2019 to 21.7 million by 2029, and many survivors are living longer (National Cancer Institute, 2019). In addition, many survivors, even among those who are cancer-free, must cope with the long-term effects of treatment, as well as psychosocial concerns such as fear of recurrence and being at high risk for developing other malignancies.
Healthcare Delivery System Implications
Innovative models of care delivery and reimbursement are needed to deliver high-quality, cost-effective care, and to position nurses as leaders in transforming cancer care.
Advanced Research Methods
Biobehavioral, population, and data science are advancing at a rapid rate, and developments in these offer oncology nurse scientists new tools, technologies, and methods related to research design, measurement, and data analysis. At the same time, the receipt of research funding from federal and other sources is highly competitive. For oncology nurse scientists to be successful, innovative advanced research methods that have the potential to offer robust new insights and speed the translation of science into real-world settings need to be incorporated into research training programs and grant applications.
Overview of Advanced Research Methods
To address the challenges of cancer care delivery research, a thorough understanding of contemporary research designs, methods, and data analytics is essential. New methods are continuously emerging, and scientists are required to leverage developments and techniques from a wide range of disciplines, both basic and translational. As such, continuous lifelong learning to extend and develop new skills is essential for all scientists. In addition, interprofessional collaboration is needed to bring expertise to bear on solving problems of interest to oncology nursing. The authors present a brief overview of some salient trends in advanced research methods, which include contemporary research designs, data science, team science, and implementation science. These topics were chosen because they are relevant to the current research environment and have the potential to speed translation of research findings into real-world practice settings.
Contemporary Research Designs
Although randomized controlled trials (RCTs) are often the gold standard for determining intervention effects, alternative approaches to testing interventions are receiving increased attention and may be particularly useful in situations where individual randomization is not possible, contamination threatens internal validity, and investigators wish to test adaptive complex interventions. In these circumstances, several new research designs have emerged, including Multiphase Optimization STrategy (MOST), Sequential Multiple Assignment Randomized Trials (SMART), and stepped wedge or cluster randomized trial designs. MOST and SMART are related approaches and are used to adaptively test an intervention, thereby achieving a more effective, efficient, acceptable, and scalable intervention. They are ideal for optimizing multicomponent complex interventions, including mobile health efforts. For circumstances in which individual-level randomization is not possible, or desirable, a pragmatic trial using a stepped wedge or cluster randomized design allows an investigator to examine an intervention’s real-world effectiveness across diverse patient groups while, at the same time, preserving the experimental control needed to draw conclusions about a cause and effect relationship between the intervention and the outcome patient groups (Song, DeVito Dabbs, & Ward, 2016; Wilbur, Kolanowski, & Collins, 2016).
Optimal management of health conditions often require a sequential, individualized approach where treatment is adjusted over time in response to the specific needs of the individual. Adaptive interventions, also known as stepped care or dynamic treatment algorithms, are one way to provide individualized sequences of treatment. With an adaptive intervention, the dose and even the components of the intervention may be varied based on individual characteristics or tailoring variables (Almirall, Nahum-Shani, Sherwood, & Murphy, 2014). MOST and SMART approaches can be used to develop and test these types of individualized interventions. MOST systematically and efficiently optimizes behavioral interventions and consists of three phases: preparation, optimization, and evaluation (Collins, Nahum-Shani, & Almirall, 2014; Wyrick, Rulison, Fearnow-Kenney, Milroy, & Collins, 2014). In the preparation phase, efficacious components of the intervention are identified and optimization criteria are pre-selected, such as selecting specific outcomes and/or effect size. In the optimization or refinement phase, a series of experiments are conducted to obtain information about the performance of each component. Finally, a confirmatory RCT of the adaptive or optimized intervention versus a comparator is conducted during the evaluation phase.
The SMART trial design is a tool to identify the best time-varying adaptive intervention strategy and can be used within the refinement phase of MOST (Lei, Nahum-Shani, Lynch, Oslin, & Murphy, 2012; Sikorskii et al., 2017). SMART is a special type of factorial design and differs significantly from a standard RCT. The main goal for a SMART design is to construct an adaptive intervention based on data, whereas the main goal of an RCT is to evaluate an already developed intervention versus a comparator condition (Almirall et al., 2014). Another difference between a SMART and RCT design is that randomization takes place more than once in the SMART design. Each randomization corresponds to a decision point and aims to address a scientific question concerning two or more treatment options at that decision point.
The stepped wedge design involves the collection of observations during a baseline period in which no clusters are exposed to the intervention. Following this, at regular intervals or steps, a cluster (or group of clusters) is randomized to receive the intervention and all participants are once again measured. This continues until all eligible participants or clusters have entered the intervention condition (Barker, McElduff, D’Este, & Campbell, 2016; Murray et al., 2018). A cluster randomized trial (CRT) is a trial in which individuals are randomized in groups—the group as a whole is randomized and not the individual. Stepped wedge and cluster randomized designs offer several advantages, although they are complex to design, perform, and interpret (Hemming, Carroll, Thompson, Forbes, & Taljaard, 2019). They may mitigate the concerns that arise when studying an intervention that is expected to produce positive effects, where it would be ethically or logistically problematic to withhold that treatment. In addition, because each cluster in the stepped wedge design receives both the control and the treatment condition by the end of the trial and each cluster switches randomly from control to treatment condition at different times, analyses comparing between- and within-cluster effects and the effects of time can be performed. Therefore, CRT and stepped wedge trial designs may have increased statistical power, compared to a traditional RCT, and may also help to surmount issues of feasibility, referral bias, and intervention contamination.
Traditional clinical trials are costly, may be slow to produce results, and have limited external validity (Ford & Norrie, 2016; Volpp, Terwiesch, Troxel, Mehta, & Asch, 2013). As a result, efforts are being made to strengthen the capacity to implement cost-effective, large-scale, pragmatic trials (Weinfurt et al., 2017). Technological advances have created the opportunity to use real-world data to improve current methods of generating clinical evidence to enhance healthcare interventions. Multiple sources of real-world data are available for use in pragmatic trials, which include electronic health records, insurance claims, patient registries, and digital health solutions (Khozin, Blumenthal, & Pazdur, 2017).
Pragmatic trials measure the effectiveness of interventions in real-world settings. As a result, eligibility criteria are typically broad and protocols are brief and designed so that data can be collected as part of routine care. In contrast, traditional explanatory RCTs measure the efficacy of an intervention and are conducted under ideal conditions using tightly controlled study protocol procedures, comparatively smaller sample sizes, and carefully selected participants for inclusion. The PRagmatic Explanatory Continuum Indicator Summary-2 (PRECIS-2) tool provides guidance to enable researchers to design a trial that matches the intention of the proposed study (Loudon et al., 2015).
Data Science
Data science uses an interprofessional approach to solve complex problems and extract knowledge through methods, tools, and analytics that support the organization, integration, and analysis (including visual analysis) of digital data (Alyass, Turcotte, & Meyre, 2015; Broome, 2016; Founds, 2018). Typically, the data are of a scope and magnitude that can only be analyzed using computers for data storage and processing. Big data may include well-defined and/or structured data elements (e.g., patient-reported measures) as well as loosely defined and/or unstructured elements (e.g., data obtained from wearable devices and social media) (Westra et al., 2017). Big data resources that are useful for oncology nurse scientists include self-report and survey data sources, including those linked to cancer or practice registries. Other sources of big data include instrumented measurements (e.g., actigraphy), social media, electronic devices (e.g., robot/avatar), sensors (e.g., pulse oximeters, glucose monitors), electronic health records, mobile and geospatial applications, and wearable tracking devices (Brennan & Bakken, 2015). Big data management and analysis cannot generally be performed using software such as R or SAS because big data require computing infrastructures that are usually cloud-based and use algorithms to achieve data linkages and synthesis. For example, Apache Spark™ is a leading platform for large-scale structured query language, batch processing, stream processing, and machine learning. Apache Hadoop®, on the other hand, is currently the most widely used open-source distributed processing framework that manages data processing and storage for big data applications running in clustered systems. Hadoop handles various forms of structured and unstructured data, and supports advanced analytics initiatives, including predictive analytics, data mining, and machine learning applications.
Machine learning is often incorporated in data science. Machine learning is an artificial intelligence tool that uses advanced algorithms to structure, analyze, and aggregate large volumes of data. Machine learning (either supervised or unsupervised) is particularly useful in identifying patterns in big data (Bose, Maganti, Bowles, Brueshoff, & Monsen, 2019). Supervised machine learning usually aims to learn a function that best approximates the relationship between a set of predictors and one or more outcomes observed in a dataset (Papachristou et al., 2018). Unsupervised machine learning, in contrast, does not define predictors and outcomes a priori, but rather allows the analytic modeling to define the structure of relationships among variables within a dataset (Bose & Radhakrishnan, 2018). After collecting and processing the structured data from machine learning tools, data scientists interpret, convert, and summarize the data so that they are useful for clinical decision making (Kwon, Karim, Topaz, & Currie, 2019).
A key priority is to strengthen the educational curriculum so that nurse scientists have the needed data science skills and quantitative competencies to lead and contribute to these research teams (Shea et al., 2019). Other challenges associated with the use of big data, including the dentification and analysis of vast amounts of unstructured data, can prove too complex, expensive, and time-consuming to analyze (Westra et al., 2015). In addition, given that machine learning is algorithm-based rather than theory-based, it may be challenging to determine the importance of each variable in contributing to the statistical overall model. Therefore, machine learning is often used for predictive purposes rather than to draw inferences (Brennan & Bakken, 2015). Machine learning can be combined with classic inferential statistics to identify the variables that account for most of the variance in the overall model. Challenges exist in integrating disparate data (particularly real-time information) at the right time to make clinical decisions, and with interpretation and generalization of findings from studies that leverage big data to better understand health outcomes (Founds, 2018; Hesse et al., 2015).
Team Science
Team science is a collaborative effort to address a scientific challenge that leverages the strengths and expertise of professionals trained in different fields (Little et al., 2017). Cross-disciplinary team science brings together investigators with diverse knowledge and skills, and may be particularly helpful for studies of complex problems with multiple causes. Team members with different training work together to integrate their perspectives in a single research endeavor and are able to address a broad array of complex and interacting variables and apply complementary research methods. Team science is seen as a promising approach to accelerate scientific innovation and the translation of scientific findings into effective policies and practices.
The increased complexity of cancer care requires that researchers move beyond the boundaries of their respective disciplines and gain knowledge about the principles of team science so that these insights can be leveraged in conducting observational research, testing new interventions, and evaluating care delivery models (Hall et al., 2018; Osarogiagbon et al., 2016). Research training has typically been discipline-specific, with some overlap in research methods, but with very little emphasis on understanding the science of team science or gaining experience operating in interprofessional research teams. Models of research training for nurse scientists must also evolve and incorporate meaningful interprofessional cross-educational opportunities.
Implementation Science
The translation of evidence-based interventions into healthcare settings has been a slow and arduous process. It takes, on average, 17 years for scientific evidence to be taken up in the practice setting (Balas & Boren, 2000). Implementation science has evolved to facilitate more rapid uptake of evidence-based practice. It is defined as the study of methods to promote the adoption and integration of evidence-based interventions and policies into routine health care.
An essential component of implementation science is evaluating the process of implementation and its impact on the adoption of the evidence-based practice of interest. Implementation science research employs a multilevel (system, provider, and patient), cross-setting, and transdisciplinary approach, and plays an important role in identifying barriers to, and enablers of, effective health care (Mitchell & Chambers, 2017). Through both theory and specific research methods, implementation science leverages that knowledge to anticipate barriers and facilitators, tests the efficacy of implementation strategies, and helps to understand contextual factors that enhance the uptake of evidence-based practice.
Conclusion
The ONS Research Agenda synthesizes the state of the science in key areas of cancer care, identifies gaps, and proposes directions for continued research to address contemporary challenges in oncology nursing and to drive improvements in health outcomes, care quality, and access for patients across the cancer control continuum. The ONS Research Agenda identifies three priority areas where new knowledge is urgently needed: symptom science, health disparities, and palliative and psychosocial care in oncology. Considerations to enhance the significance, rigor, and relevance of oncology nursing science include a focus on priority populations (older adults and cancer survivors), innovative care delivery models, and advanced research methods and data analytics. This focused set of priorities serves to both concentrate the professional efforts of scientists and clinicians and to direct resources for scientific training, career development, and research funding opportunities.
The ONS Research Agenda can be used to align initiatives and resources across the ONS enterprise, and provides direction for knowledge generation, evidence-based practice, and healthcare policy to support the delivery of quality cancer care.
About the Author(s)
Diane Von Ah, PhD, RN, FAAN, is a professor and associate dean of academic operations in the School of Nursing at Indiana University in Indianapolis; Carlton G. Brown, PhD, RN, AOCN®, NEA-BC, FAAN, is the president and nurse research consultant at Zenith Healthcare Solutions in Palm Springs, CA; Susan J. Brown, PhD, MSN, RN, NEA-BC, is the senior vice president of Patient Care Services and chief nursing officer at the City of Hope Comprehensive Cancer Center in Duarte, CA; Ashley Leak Bryant, PhD, RN-BC, OCN®, is an assistant professor in the School of Nursing at the University of North Carolina at Chapel Hill; Marianne Davies, DNP, MSN, RN, APRN, CNS-BC, ACNP-BC, AOCN®, is an associate professor in the Yale School of Nursing and Yale Comprehensive Cancer Center at Yale University in West Haven, and an oncology nurse practitioner–thoracic at the Smilow Cancer Center at Yale New Haven, both in Connecticut; Marylin Dodd, PhD, RN, FAAN, is a professor emeritus in the Department of Physiological Nursing at the University of California, San Francisco; Betty Ferrell, RN, PhD, MA, FAAN, FPCN®, CHPN®, is a professor and director of nursing research in the Division of Nursing Research and Education, Department of Population Sciences, at the City of Hope National Medical Center; Marilyn Hammer, PhD, RN, DC, FAAN, is the director of the Phyllis F. Cantor Center Research in Nursing and Patient Care Services at the Dana-Farber Cancer Institute in Boston, MA; M. Tish Knobf, PhD, RN, FAAN, is a professor and chair of the Acute Care/Health Systems Division and the administrative director of the Graduate Entry Prespecialty in Nursing (GEPN) program, both at Yale University School of Nursing, in Orange, CT; Teresa J. Knoop, MSN, RN, AOCN®, is the assistant director of Clinical Operations Clinical Trials at the Ingram Cancer Center at Vanderbilt University Medical Center in Nashville, TN; Geri LoBiondo-Wood, PhD, RN, FAAN, is the Bette P. Thomas Distinguished Professor for Innovative Healthcare Delivery and director of the PhD program in the Cizik School of Nursing at the University of Texas Health Science Center in Houston; Deborah K. Mayer, PhD, RN, AOCN®, FAAN, is the Frances Hill Fox Distinguished Professor in the School of Nursing and the director of cancer survivorship in the Lineberger Comprehensive Cancer Center at the University of North Carolina at Chapel Hill, and the interim director of the Office of Cancer Survivorship in the Division of Cancer Control and Population Sciences at the National Cancer Institute in Bethesda, MD; Christine Miaskowski, PhD, RN, FAAN, is a professor and the Sharon A. Lamb Endowed Chair in the Department of Physiological Nursing in the School of Nursing at the University of California, San Francisco; Sandra A. Mitchell, PhD, CRNP, AOCN®, FAAN, is a research scientist in the Outcomes Research Branch at the National Cancer Institute; Lixin Song, PhD, RN, FAAN, is an associate professor and the Beerstecher-Blackwell Distinguished Term Scholar at the Lineberger Comprehensive Cancer Center, and an adjunct associate professor in the Department of Urology in the School of Medicine, both at the University of North Carolina at Chapel Hill; Deborah Watkins Bruner, PhD, RN, FAAN, is the senior vice president for research at Emory University in Atlanta, GA; Susan Wesmiller, PhD, RN, is an assistant professor in Health Promotion and Development in the School of Nursing at the University of Pittsburgh in Pennsylvania; and Mary E. Cooley, PhD, RN, FAAN, is a nurse scientist in the Phyllis F. Cantor Center Research in Nursing and Patient Care Services at the Dana-Farber Cancer Institute, and the scholar-in-residence at the Oncology Nursing Society in Pittsburgh, PA. No relevant financial relationships to disclose. Von Ah, S.J. Brown, Leak Bryant, Davies, Dodd, Ferrell, Hammer, Knobf, Knoop, Mayer, Miaskowski, Mitchell, Song, Watkins Bruner, Wesmiller, and Cooley contributed to the conceptualization and design. Von Ah, Davies, Dodd, Ferrell, LoBiondo-Wood, Mitchell, Song, Watkins Bruner, and Cooley completed the data collection. Ferrell provided statistical support. S.J. Brown, Leak Bryant, Davies, Ferrell, Knobf, Mayer, Miaskowski, Mitchell, Song, Watkins Bruner, Wesmiller, and Cooley provided analysis. Von Ah, C.G. Brown, S.J. Brown, Leak Bryant, Davies, Dodd, Ferrell, Hammer, Knobf, Knoop, Mayer, Miaskowski, Mitchell, Song, Watkins Bruner, and Wesmiller contributed to the manuscript preparation. Cooley can be reached at mcooley@ons.org, with copy to ONFEditor@ons.org.
References
Abid, H., Kober, K.M., Smoot, B., Paul, S.M., Hammer, M., Levine, J.D., . . . Miaskowski, C. (2017). Common and distinct characteristics associated with trajectories of morning and evening energy in oncology patients receiving chemotherapy. Journal of Pain and Symptom Management, 53, 887–900. https://doi.org/10.1016/j.jpainsymman.2016.12.339
Absolom, K., Gibson, A., & Velikova, G. (2019). Engaging patients and clinicians in online reporting of adverse effects during chemotherapy for cancer: The eRAPID System (Electronic Patient Self-Reporting of Adverse Events: Patient Information and aDvice). Medical Care, 57(Suppl. 5), S59–S65. https://doi.org/10.1097/mlr.0000000000001085
Ahluwalia, S.C., Chen, C., Raaen, L., Motala, A., Walling, A.M., Chamberlin, M., . . . Hempel, S. (2018). A systematic review in support of the National Consensus Project Clinical Practice Guidelines for Quality Palliative Care, fourth edition. Journal of Pain and Symptom Management, 56, 831–870. https://doi.org/10.1016/j.jpainsymman.2018.09.008
Almirall, D., Nahum-Shani, I., Sherwood, N.E., & Murphy, S.A. (2014). Introduction to SMART designs for the development of adaptive interventions: With application to weight loss research. Translational Behavioral Medicine, 4, 260–274. https://doi.org/10.1007/s13142-014-0265-0
Altice, C.K., Banegas, M.P., Tucker-Seeley, R.D., & Yabroff, K.R. (2016). Financial hardships experienced by cancer survivors: A systematic review. Journal of the National Cancer Institute, 109, djw205. https://doi.org/10.1093/jnci/djw205
Alyass, A., Turcotte, M., & Meyre, D. (2015). From big data analysis to personalized medicine for all: Challenges and opportunities. BMC Medical Genomics, 8, 33.
Andrews, M.C., Reuben, A., Gopalakrishnan, V., & Wargo, J.A. (2018). Concepts collide: Genomic, immune, and microbial influences on the tumor microenvironment and response to cancer therapy. Frontiers in Immunology, 9, 946.
Ashley, E.A. (2015). The precision medicine initiative: A new national effort. JAMA, 313, 2119–2120. https://doi.org/10.1001/jama.2015.3595
Balas, E.A., & Boren, S.A. (2000). Managing clinical knowledge for health care improvement. Yearbook of Medical Informatics, 1, 65–70.
Barker, D., McElduff, P., D’Este, C., & Campbell, M.J. (2016). Stepped wedge cluster randomized trials: A review of the statistical methodology used and available. BMC Medical Research and Methodologies, 16, 69. https://doi.org/10.1186/s12874-016-0176-5
Blake, K.D., Moss, J.L., Gaysynsky, A., Srinivasan, S., & Croyle, R.T. (2017). Making the case for investment in rural cancer control: An analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiology and Biomarkers Prevention, 26, 992–997. https://doi.org/10.1158/1055-9965.Epi-17-0092
Bose, E., Maganti, S., Bowles, K.H., Brueshoff, B.L., & Monsen, K.A. (2019). Machine learning methods for identifying critical data elements in nursing documentation. Nursing Research, 68, 65–72. https://doi.org/10.1097/nnr.0000000000000315
Bose, E., & Radhakrishnan, K. (2018). Using unsupervised machine learning to identify subgroups among home health patients with heart failure using telehealth. Computers and Informatics in Nursing, 36, 242–248. https://doi.org/10.1097/cin.0000000000000423
Brahmer, J.R., Lacchetti, C., & Thompson, J.A. (2018). Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline summary. Journal of Oncology Practice, 14, 247–249. https://doi.org/10.1200/jop.18.00005
Brennan, P.F., & Bakken, S. (2015). Nursing needs big data and big data needs nursing. Journal of Nursing Scholarship, 47, 477–484. https://doi.org/10.1111/jnu.12159
Broome, M.E. (2016). Big data, data science, and big contributions. Nursing Outlook, 64, 113–114. https://doi.org/10.1016/j.outlook.2016.02.001
Califf, R.M. (2018). Biomarker definitions and their applications. Experimental Biology and Medicine, 243, 213–221. https://doi.org/10.1177/1535370217750088
Chaput, N., Lepage, P., Coutzac, C., Soularue, E., Le Roux, K., Monot, C., . . . Carbonnel, F. (2017). Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Annals of Oncology, 28, 1368–1379. https://doi.org/10.1093/annonc/mdx108
Charlton, M., Schlichting, J., Chioreso, C., Ward, M., & Vikas, P. (2015). Challenges of rural cancer care in the United States. Oncology, 29, 633–640.
Chen, M.S., Jr., Lara, P.N., Dang, J.H., Paterniti, D.A., & Kelly, K. (2014). Twenty years post-NIH Revitalization Act: Enhancing minority participation in clinical trials (EMPaCT): Laying the groundwork for improving minority clinical trial accrual: Renewing the case for enhancing minority participation in cancer clinical trials. Cancer, 120(Suppl. 7), 1091–1096. https://doi.org/10.1002/cncr.28575
Chen, T.W., Razak, A.R., Bedard, P.L., Siu, L.L., & Hansen, A.R. (2015). A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Annals of Oncology, 26, 1824–1829. https://doi.org/10.1093/annonc/mdv182
Collins, L.M., Nahum-Shani, I., & Almirall, D. (2014). Optimization of behavioral dynamic treatment regimens based on the sequential, multiple assignment, randomized trial (SMART). Clinical Trials, 11, 426–434. https://doi.org/10.1177/1740774514536795
Cowan, R.A., Suidan, R.S., Andikyan, V., Rezk, Y.A., Einstein, M.H., Chang, K., . . . Chi, D.S. (2016). Electronic patient-reported outcomes from home in patients recovering from major gynecologic cancer surgery: A prospective study measuring symptoms and health-related quality of life. Gynecologic Oncology, 143, 362–366. https://doi.org/10.1016/j.ygyno.2016.08.335
Dhruva, A., Aouizerat, B.E., Cooper, B., Paul, S.M., Dodd, M., West, C., . . . Miaskowski, C. (2015). Cytokine gene associations with self-report ratings of morning and evening fatigue in oncology patients and their family caregivers. Biological Research for Nursing, 17, 175–184. https://doi.org/10.1177/1099800414534313
Elias, R., Hartshorn, K., Rahma, O., Lin, N., & Snyder-Cappione, J.E. (2018). Aging, immune senescence, and immunotherapy: A comprehensive review. Seminars in Oncology, 45, 187–200. https://doi.org/10.1053/j.seminoncol.2018.08.006
FDA-NIH Biomarker Working Group. (2016). BEST (biomarkers, endpoints, and other tools) resource. Silver Spring, MD: U.S. Food and Drug Administration.
Ferranti, E.P., Grossmann, R., Starkweather, A., & Heitkemper, M. (2017). Biological determinants of health: Genes, microbes, and metabolism exemplars of nursing science. Nursing Outlook, 65, 506–514. https://doi.org/10.1016/j.outlook.2017.03.013
Ferrell, B., & Wittenberg, E. (2017). A review of family caregiving intervention trials in oncology. CA: A Cancer Journal for Clinicians, 67, 318–325. https://doi.org/10.3322/caac.21396
Fiore, R.N., & Goodman, K.W. (2016). Precision medicine ethics: Selected issues and developments in next-generation sequencing, clinical oncology, and ethics. Current Opinion in Oncology, 28, 83–87. https://doi.org/10.1097/cco.0000000000000247
Ford, I., & Norrie, J. (2016). Pragmatic trials. New England Journal of Medicine, 375, 454–463. https://doi.org/10.1056/NEJMra1510059
Founds, S. (2018). Systems biology for nursing in the era of big data and precision health. Nursing Outlook, 66, 283–292. https://doi.org/10.1016/j.outlook.2017.11.006
Friedman, C.F., Proverbs-Singh, T.A., & Postow, M.A. (2016). Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncology, 2, 1346–1353. https://doi.org/10.1001/jamaoncol.2016.1051
Geller, S.E., Koch, A.R., Roesch, P., Filut, A., Hallgren, E., & Carnes, M. (2018). The more things change, the more they stay the same: A study to evaluate compliance with inclusion and assessment of women and minorities in randomized controlled trials. Academic Medicine, 93, 630–635. https://doi.org/10.1097/acm.0000000000002027
Ginsburg, G.S., & Phillips, K.A. (2018). Precision medicine: From science to value. Health Affairs, 37, 694–701.
Haanen, J.B.A.G., Carbonnel, F., Robert, C., Kerr, K.M., Peters, S., Larkin, J., & Jordan, K. (2017). Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 28(Suppl. 4), iv119–iv142. https://doi.org/10.1093/annonc/mdx225
Hall, E.T., Singhal, S., Dickerson, J., Gabster, B., Wong, H.N., Aslakson, R.A., & Schapira, L. (2019). Patient-reported outcomes for cancer patients receiving checkpoint inhibitors: Opportunities for palliative care-a systematic review. Journal of Pain and Symptom Management, 58, 137–156. https://doi.org/10.1016/j.jpainsymman.2019.03.015
Hall, K.L., Vogel, A.L., Huang, G.C., Serrano, K.J., Rice, E.L., Tsakraklides, S.P., & Fiore, S.M. (2018). The science of team science: A review of the empirical evidence and research gaps on collaboration in science. American Psychologist, 73, 532–548. https://doi.org/10.1037/amp0000319
Hargadon, K.M., Johnson, C.E., & Williams, C.J. (2018). Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. International Immunopharmacology, 62, 29–39. https://doi.org/10.1016/j.intimp.2018.06.001
Havel, J.J., Chowell, D., & Chan, T.A. (2019). The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nature Reviews Cancer, 19, 133–150.
Hazin, R., & Qaddoumi, I. (2010). Teleoncology: Current and future applications for improving cancer care globally. Lancet Oncology, 11, 204–210. https://doi.org/10.1016/s1470-2045(09)70288-8
Hemming, K., Carroll, K., Thompson, J., Forbes, A., & Taljaard, M. (2019). Quality of stepped-wedge trial reporting can be reliably assessed using an updated CONSORT: Crowd-sourcing systematic review. Journal of Clinical Epidemiology, 107, 77–88. https://doi.org/10.1016/j.jclinepi.2018.11.017
Henley, S.J., Anderson, R.N., Thomas, C.C., Massetti, G.M., Peaker, B., & Richardson, L.C. (2017). Invasive cancer incidence, 2004–2013, and deaths, 2006–2015, in nonmetropolitan and metropolitan counties-United States. Morbidity and Mortality Weekly Report Surveillance Summary, 66(14), 1–13. https://doi.org/10.15585/mmwr.ss6614a1
Hesse, B.W., Moser, R.P., & Riley, W.T. (2015). From big data to knowledge in the social sciences. Annals of the American Academy of Political and Social Science, 659, 16–32. https://doi.org/10.1177/0002716215570007
Hickey, K.T., Bakken, S., Byrne, M.W., Bailey, D.C.E., Demiris, G., Docherty, S.L., . . . Grady, P.A. (2019). Precision health: Advancing symptom and self-management science. Nursing Outlook, 67, 462–475. https://doi.org/10.1016/j.outlook.2019.01.003
Holzinger, A., & Abken, H. (2020). Advances and challenges of CAR T cells in clinical trials. Recent Results in Cancer Research, 214, 93–128. https://doi.org/10.1007/978-3-030-23765-3_3
Hudson, J., Schabath, M.B., Sanchez, J., Sutton, S., Simmons, V.N., Vadaparampil, S.T., . . . Quinn, G.P. (2017). Sexual and gender minority issues across NCCN guidelines: Results from a national survey. Journal of the National Comprehensive Cancer Network, 15, 1379–1382. https://doi.org/10.6004/jnccn.2017.0169
Hui, D., & Bruera, E. (2015). Models of integration of oncology and palliative care. Annals of Palliative Medicine, 4, 89–98. https://doi.org/10.3978/j.issn.2224-5820.2015.04.01
Iafolla, M.A.J., Selby, H., Warner, K., Ohashi, P.S., Haibe-Kains, B., & Siu, L.L. (2018). Rational design and identification of immuno-oncology drug combinations. European Journal of Cancer, 95, 38–51. https://doi.org/10.1016/j.ejca.2018.02.027
Idorn, M., & Thor Straten, P. (2017). Exercise and cancer: From “healthy” to “therapeutic”? Cancer Immunology, Immunotherapy, 66, 667–671. https://doi.org/10.1007/s00262-017-1985-z
Institute of Medicine. (2008). Neuroscience biomarkers and biosignatures: Converging technologies, emerging partnerships, workshop summary. Washington, DC: Author.
Johnson, D.B., Friedman, D.L., Berry, E., Decker, I., Ye, F., Zhao, S., . . . Lovly, C.M. (2015). Survivorship in immune therapy: Assessing chronic immune toxicities, health outcomes, and functional status among long-term ipilimumab survivors at a single referral center. Cancer Immunology Research, 3, 464–469. https://doi.org/10.1158/2326-6066.Cir-14-0217
Kent, E.E., Rowland, J.H., Northouse, L., Litzelman, K., Chou, W.Y., Shelburne, N., . . . Huss, K. (2016). Caring for caregivers and patients: Research and clinical priorities for informal cancer caregiving. Cancer, 122, 1987–1995. https://doi.org/10.1002/cncr.29939
Khozin, S., Blumenthal, G.M., & Pazdur, R. (2017). Real-world data for clinical evidence generation in oncology. Journal of the National Cancer Institute, 109, djx187.
Kim, M., Kim, K., Lim, C., & Kim, J.S. (2018). Symptom clusters and quality of life according to the survivorship stage in ovarian cancer survivors. Western Journal of Nursing Research, 40, 1278–1300. https://doi.org/10.1177/0193945917701688
King-Kallimanis, B.L., Kanapuru, B., Blumenthal, G.M., Theoret, M.R., & Kluetz, P.G. (2018). Age-related differences in patient-reported outcomes in patients with advanced lung cancer receiving anti-PD-1/PD-L1 therapy. Seminars in Oncology, 45, 201–209. https://doi.org/10.1053/j.seminoncol.2018.06.003
Knobf, M.T., Cooley, M.E., Duffy, S., Doorenbos, A., Eaton, L., Given, B., . . . Mallory, G. (2015). The 2014–2018 Oncology Nursing Society Research Agenda. Oncology Nursing Forum, 42, 450–465. https://doi.org/10.1188/15.ONF.450-465
Kober, K.M., Smoot, B., Paul, S.M., Cooper, B.A., Levine, J.D., & Miaskowski, C. (2016). Polymorphisms in cytokine genes are associated with higher levels of fatigue and lower levels of energy in women after breast cancer surgery. Journal of Pain and Symptom Management, 52, 695–708.e4.
Kwon, J.Y., Karim, M.E., Topaz, M., & Currie, L.M. (2019). Nurses “seeing forest for the trees” in the age of machine learning: Using nursing knowledge to improve relevance and performance. Computers, Informatics, Nursing, 37, 203–212.
Lei, H., Nahum-Shani, I., Lynch, K., Oslin, D., & Murphy, S.A. (2012). A “SMART” design for building individualized treatment sequences. Annual Review of Clinical Psychology, 8, 21–48. https://doi.org/10.1146/annurev-clinpsy-032511-143152
Little, M.M., St Hill, C.A., Ware, K.B., Swanoski, M.T., Chapman, S.A., Lutfiyya, M.N., & Cerra, F.B. (2017). Team science as interprofessional collaborative research practice: A systematic review of the science of team science literature. Journal of Investigative Medicine, 65, 15–22. https://doi.org/10.1136/jim-2016-000216
LoBiondo-Wood, G., Brown, C.G., Knobf, M.T., Lyon, D., Mallory, G., Mitchell, S.A., . . . Fellman, B. (2014). Priorities for oncology nursing research: The 2013 national survey. Oncology Nursing Forum, 41, 67–76. https://doi.org/10.1188/14.ONF.67-76
Loudon, K., Treweek, S., Sullivan, F., Donnan, P., Thorpe, K.E., & Zwarenstein, M. (2015). The PRECIS-2 tool: Designing trials that are fit for purpose. BMJ, 350, h2147. https://doi.org/10.1136/bmj.h2147
Lucas, A.R., Bass, M.B., Rothrock, N.E., O’Connor, M.L., Sorkin, M.R., Nawyn, J., . . . Wagner, L.I. (2018). Development of an eHealth system to capture and analyze patient sensor and self-report data: Mixed-methods assessment of potential applications to improve cancer care delivery. JMIR Medical Informatics, 6(4), e46. https://doi.org/10.2196/medinform.9525
Margolies, L., & Brown, C.G. (2018). Current state of knowledge about cancer in lesbians, gay, bisexual, and transgender (LGBT) people. Seminars in Oncology Nursing, 34, 3–11. https://doi.org/10.1016/j.soncn.2017.11.003
Matson, V., Fessler, J., Bao, R., Chongsuwat, T., Zha, Y., Alegre, M.L., . . . Gajewski, T.F. (2018). The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science, 359(6371), 104–108.
Mazor, M., Cataldo, J.K., Lee, K., Dhruva, A., Cooper, B., Paul, S.M., . . . Miaskowski, C. (2018). Differences in symptom clusters before and twelve months after breast cancer surgery. European Journal of Oncology Nursing, 32, 63–72.
McCarthy, M. (2016). US president endorses “moonshot” effort to cure cancer. BMJ, 352, i213. https://doi.org/10.1136/bmj.i213
Mendoza, T.R. (2018). Symptoms as patient-reported outcomes in cancer patients undergoing immunotherapies. Advances in Experimental Medicine and Biology, 995, 165–182. https://doi.org/10.1007/978-3-030-02505-2_9
Miaskowski, C., Barsevick, A., Berger, A., Casagrande, R., Grady, P.A., Jacobsen, P., . . . Marden, S. (2017). Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. Journal of the National Cancer Institute, 109, dhw253. https://doi.org/10.1093/jnci/djw253
Miaskowski, C., Conley, Y.P., Mastick, J., Paul, S.M., Cooper, B.A., Levine, J.D., . . . Kober, K.M. (2017). Cytokine gene polymorphisms associated with symptom clusters in oncology patients undergoing radiation therapy. Journal of Pain and Symptom Management, 54, 305–316.https://doi.org/10.1016/j.jpainsymman.2017.05.007
Mitchell, S.A., & Chambers, D.A. (2017). Leveraging implementation science to improve cancer care delivery and patient outcomes. Journal of Oncology Practice, 13, 523–529. https://doi.org/10.1200/jop.2017.024729
Mooney, K., Whisenant, M.S., & Beck, S.L. (2019). Symptom care at home: A comprehensive and pragmatic PRO system approach to improve cancer symptom care. Medical Care, 57(Suppl. 5), S66–S72. https://doi.org/10.1097/mlr.0000000000001037
Murray, D.M., Pals, S.L., George, S.M., Kuzmichev, A., Lai, G.Y., Lee, J.A., . . . Nelson, S.M. (2018). Design and analysis of group-randomized trials in cancer: A review of current practices. Preventive Medicine, 111, 241–247. https://doi.org/10.1016/j.ypmed.2018.03.010
National Cancer Institute. (2019). Division of Cancer Control and Population Sciences: Statistics. Retrieved from https://cancercontrol.cancer.gov/ocs/statistics/statistics.html
National Institutes of Health. (2015). SGM research portfolio analysis. Retrieved from https://dpcpsi.nih.gov/sites/default/files/SGMRO_Portfolio_Analysis_RF5…
National Institutes of Health. (2019). National Institute on Minority Health and Health Disparities: Overview. Retrieved from https://www.nimhd.nih.gov/about/overview
National Research Council. (2011). Toward precision medicine: Building a knowledge network for biomedical research and a new taxonomy of disease. Washington, DC: National Academies Press.
Nho, J.H., Kim, S.R., Park, M.H., & Kweon, S.S. (2018). Symptom clusters and quality of life in breast cancer survivors after cancer treatment in a tertiary hospital in Korea. European Journal of Cancer Care, 27(6), e12919. https://doi.org/10.1111/ecc.12919
Northouse, L., Williams, A.L., Given, B., & McCorkle, R. (2012). Psychosocial care for family caregivers of patients with cancer. Journal of Clinical Oncology, 30, 1227–1234. https://doi.org/10.1200/jco.2011.39.5798
Osarogiagbon, R.U., Rodriguez, H.P., Hicks, D., Signore, R.S., Roark, K., Kedia, S.K., . . . Krasna, M.J. (2016). Deploying team science principles to optimize interdisciplinary lung cancer care delivery: Avoiding the long and winding road to optimal care. Journal of Oncology Practice, 12, 983–991. https://doi.org/10.1200/jop.2016.013813
Page, G.G., Corwin, E.J., Dorsey, S.G., Redeker, N.S., McCloskey, D.J., Austin, J.K., . . . Grady, P. (2018). Biomarkers as common data elements for symptom and self-management science. Journal of Nursing Scholarship, 50, 276–286.
Papachristou, N., Puschmann, D., Barnaghi, P., Cooper, B., Hu, X., Maguire, R., . . . Miaskowski, C. (2018). Learning from data to predict future symptoms of oncology patients. PLOS ONE, 13(12), e0208808. https://doi.org/10.1371/journal.pone.0208808
Postow, M.A., Sidlow, R., & Hellmann, M.D. (2018). Immune-related adverse events associated with immune checkpoint blockade. New England Journal of Medicine, 378, 158–168. https://doi.org/10.1056/NEJMra1703481
Quinn, G.P., Sanchez, J.A., Sutton, S.K., Vadaparampil, S.T., Nguyen, G.T., Green, B.L., . . . Schabath, M.B. (2015). Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA: A Cancer Journal for Clinicians, 65, 384–400. https://doi.org/10.3322/caac.21288
Rassy, E.E., Ghosn, M., Rassy, N.A., Assi, T., & Robert, C. (2018). Do immune checkpoint inhibitors perform identically in patients with weight extremes? Immunotherapy, 10, 733–736. https://doi.org/10.2217/imt-2018-0053
Redeker, N.S., Anderson, R., Bakken, S., Corwin, E., Docherty, S., Dorsey, S.G., . . . Grady, P. (2015). Advancing symptom science through use of common data elements. Journal of Nursing Scholarship, 47, 379–388. https://doi.org/10.1111/jnu.12155
Ritchie, C.S., & Zulman, D.M. (2013). Research priorities in geriatric palliative care: Multimorbidity. Journal of Palliative Medicine, 16, 843–847. https://doi.org/10.1089/jpm.2013.9491
Russell, J., Wong, M.L., Mackin, L., Paul, S.M., Cooper, B.A., Hammer, M., . . . Miaskowski, C. (2019). Stability of symptom clusters in patients with lung cancer receiving chemotherapy. Journal of Pain and Symptom Management, 57, 909–922. https://doi.org/10.1016/j.jpainsymman.2019.02.002
Sabesan, S., Simcox, K., & Marr, I. (2012). Medical oncology clinics through videoconferencing: An acceptable telehealth model for rural patients and health workers. Internal Medicine Journal, 42, 780–785. https://doi.org/10.1111/j.1445-5994.2011.02537.x
Sattar, J., Kartolo, A., Hopman, W.M., Lakoff, J.M., & Baetz, T. (2019). The efficacy and toxicity of immune checkpoint inhibitors in a real-world older patient population. Journal of Geriatric Oncology, 10, 411–414. https://doi.org/10.1016/j.jgo.2018.07.015
Shea, K.D., Brewer, B.B., Carrington, J.M., Davis, M., Gephart, S., & Rosenfeld, A. (2019). A model to evaluate data science in nursing doctoral curricula. Nursing Outlook, 67, 39–48. https://doi.org/10.1016/j.outlook.2018.10.007
Sikorskii, A., Wyatt, G., Lehto, R., Victorson, D., Badger, T., & Pace, T. (2017). Using SMART design to improve symptom management among cancer patients: A study protocol. Research in Nursing and Health, 40, 501–511. https://doi.org/10.1002/nur.21836
Singh, G.K., & Jemal, A. (2017). Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: Over six decades of changing patterns and widening inequalities. Journal of Environmental and Public Health, 2017, 2819372. https://doi.org/10.1155/2017/2819372
Soldati, L., Di Renzo, L., Jirillo, E., Ascierto, P.A., Marincola, F.M., & De Lorenzo, A. (2018). The influence of diet on anti-cancer immune responsiveness. Journal of Translational Medicine, 16, 75. https://doi.org/10.1186/s12967-018-1448-0
Song, M.K., DeVito Dabbs, A., & Ward, S.E. (2016). A SMART design to optimize treatment strategies for patient and family caregiver outcomes. Nursing Outlook, 64, 299–305. https://doi.org/10.1016/j.outlook.2016.04.008
Steele, C.B., Thomas, C.C., Henley, S.J., Massetti, G.M., Galuska, D.A., Agurs-Collins, T., . . . Richardson, L.C. (2017). Vital signs: Trends in incidence of cancers associated with overweight and obesity—United States, 2005–2014. Morbidity Mortality Weekly Report, 66, 1052–1058. https://doi.org/10.15585/mmwr.mm6639e1
Sullivan, C.W., Leutwyler, H., Dunn, L.B., Cooper, B.A., Paul, S.M., Levine, J.D., . . . Miaskowski, C.A. (2018). Stability of symptom clusters in patients with breast cancer receiving chemotherapy. Journal of Pain and Symptom Management, 55, 39–55. https://doi.org/10.1016/j.jpainsymman.2017.08.008
Tang, J., Shalabi, A., & Hubbard-Lucey, V.M. (2018). Comprehensive analysis of the clinical immuno-oncology landscape. Annals of Oncology, 29, 84–91. https://doi.org/10.1093/annonc/mdx755
Thompson, J.A., Schneider, B.J., Brahmer, J., Andrews, S., Armand, P., Bhatia, S., . . . Scavone, J.L. (2019). Management of immunotherapy-related toxicities, version 1.2019. Journal of the National Comprehensive Cancer Network, 17, 255–289. https://doi.org/10.6004/jnccn.2019.0013
U.S. Department of Health and Human Services. (2017). Common terminology criteria for adverse events (CTCAE), version 5.0. Retrieved from https://ctep.cancer.gov/protocoldevelopment/electronic_applications/doc…
Volpp, K.G., Terwiesch, C., Troxel, A.B., Mehta, S., & Asch, D.A. (2013). Making the RCT more useful for innovation with evidence-based evolutionary testing. Healthcare, 1(1–2), 4–7. https://doi.org/10.1016/j.hjdsi.2013.04.007
Wallis, C.J.D., Butaney, M., Satkunasivam, R., Freedland, S.J., Patel, S.P., Hamid, O., . . . Klaassen, Z. (2019). Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: A systematic review and meta-analysis. JAMA Oncology, 5, 529–536. https://doi.org/10.1001/jamaoncol.2018.5904
Weinfurt, K.P., Hernandez, A.F., Coronado, G.D., DeBar, L.L., Dember, L.M., Green, B.B., . . . Curtis, L.H. (2017). Pragmatic clinical trials embedded in healthcare systems: Generalizable lessons from the NIH Collaboratory. BMC Medical Research Methodologies, 17, 144. https://doi.org/10.1186/s12874-017-0420-7
Westra, B.L., Clancy, T.R., Sensmeier, J., Warren, J.J., Weaver, C., & Delaney, C.W. (2015). Nursing knowledge: Big data science-implications for nurse leaders. Nursing Administration Quarterly, 39, 304–310. https://doi.org/10.1097/naq.0000000000000130
Westra, B.L., Sylvia, M., Weinfurter, E.F., Pruinelli, L., Park, J.I., Dodd, D., . . . Delaney, C.W. (2017). Big data science: A literature review of nursing research exemplars. Nursing Outlook, 65, 549–561. https://doi.org/10.1016/j.outlook.2016.11.021
Wilbur, J., Kolanowski, A.M., & Collins, L.M. (2016). Utilizing MOST frameworks and SMART designs for intervention research. Nursing Outlook, 64, 287–289. https://doi.org/10.1016/j.outlook.2016.04.005
Williams, A.L., & McCorkle, R. (2011). Cancer family caregivers during the palliative, hospice, and bereavement phases: A review of the descriptive psychosocial literature. Palliative and Supportive Care, 9, 315–325. https://doi.org/10.1017/s1478951511000265
Wright, F., Dunn, L.B., Paul, S.M., Conley, Y.P., Levine, J.D., Hammer, M.J., . . . Kober, K.M. (2019). Morning fatigue severity profiles in oncology outpatients receiving chemotherapy. Cancer Nursing, 42, 355–364.
Wyrick, D.L., Rulison, K.L., Fearnow-Kenney, M., Milroy, J.J., & Collins, L.M. (2014). Moving beyond the treatment package approach to developing behavioral interventions addressing questions that arose during an application of the Multiphase Optimization Strategy (MOST). Translational Behavioral Medicine, 4, 252–259. https://doi.org/10.1007/s13142-013-0247-7
Yan, X., Zhang, S., Deng, Y., Wang, P., Hou, Q., & Xu, H. (2018). Prognostic factors for checkpoint inhibitor-based immunotherapy: An update with new evidences. Frontiers in Pharmacology, 9, 1050. https://doi.org/10.3389/fphar.2018.01050
Yu, X.T., & Zeng, T. (2018). Integrative analysis of omics big data. Methods in Molecular Biology, 1754, 109–135. https://doi.org/10.1007/978-1-4939-7717-8_7

