Biomarkers and Cognitive Function in Children and Adolescents During Maintenance Therapy for Leukemia
Objectives: To explore the relationship between biomarkers of oxidative stress and inflmmation in cerebrospinal fluid (CSF) and cognitive function in children receiving maintenance therapy for acute lymphocytic leukemia (ALL).
Sample & Setting: 30 participants aged 4–17 years receiving ALL maintenance therapy at two pediatric cancer centers in the United States.
Methods & Variables: F2-isoprostane (F2-ISoP) and interleukin-8 (IL-8) were evaluated in CSF samples, and cognitive function measures were completed during the first and last cycles of ALL maintenance. The Flanker Inhibitory Control and Attention Test (Flanker) and Dimensional Change Card Sort were completed during the last cycle.
Results: During maintenance therapy, IL-8 decreased, and parent reports of children’s cognitive function improved. Higher IL-8 was associated with better parent reports of children’s cognitive function at each timepoint. Higher F2-ISoP levels were associated with lower Flanker scores.
Implications for Nursing: F2-ISoP may be a useful biomarker in evaluating cognitive dysfunction in children with ALL and merits further investigation.
Jump to a section
Childhood acute lymphoblastic leukemia (ALL) is the most frequently occurring cancer in children younger than age 15 years and makes up 25% of all childhood cancers (National Cancer Institute [NCI], 2021). Childhood ALL survival rates continue to improve because of advances in treatment, with a survival rate of 91% for children younger than age 15 years and 75% for adolescents aged 15–19 years (Siegel et al., 2020). Treatment of childhood ALL is administered in multiple phases. The more intensive phases, remission-induction and consolidation-intensification therapy, are delivered during the first 12–16 months of treatment (NCI, 2021), which is then followed by repeating cycles of less intensive, but lengthier, maintenance chemotherapy. The total length of childhood ALL treatment is two to three years (NCI, 2021). ALL risk groups are determined by multiple patient and clinical disease characteristics at diagnosis, including age (aged younger than 1 year and aged 10 years or older), sex (males), presenting white blood cell count (50,000/mcl or higher), central nervous system (CNS) involvement, and cytogenetics/genomics (NCI, 2021). High- or very high–risk groups require more intensive therapy to achieve and maintain a remission.
Children and adolescents with ALL receive CNS-directed prophylactic chemotherapy as part of their treatment because the CNS is a sanctuary site where leukemia cells can evade the cytotoxic effects of systemic therapy. CNS-directed therapies include intrathecal therapy, higher-dose systemic therapy that crosses the blood–brain barrier, and cranial radiation therapy for those who have leukemia cells in their CNS at diagnosis (NCI, 2021). Methotrexate is one of the chemotherapy agents that is given as part of CNS prophylaxis throughout all phases of ALL treatment; it is administered intrathecally into the cerebrospinal fluid (CSF), via IV in high doses, and orally. Researchers have found that methotrexate is associated with cognitive dysfunction and neuroanatomical white matter magnetic resonance imaging changes in survivors of childhood ALL (Moore et al., 2013). Evidence of cognitive dysfunction caused by CNS-directed chemotherapy has been shown in measurements of impaired attention/concentration, visual/motor integration, and visuospatial awareness, and in decreased information processing speed and working memory (Lofstad et al., 2009; Moore et al., 2013, 2016). Cognitive dysfunction is less severe in ALL survivors because protocols have eliminated cranial–spinal radiation therapy as CNS prophylaxis (Cheung & Krull, 2015). However, the percentage of five-year or greater survivors of childhood ALL who fall two standard deviations below age-based norms in measures of executive function, attention, and memory (13.1%–15.9%) continues to be much higher than the 2% found in healthy controls (Cheung & Krull, 2015). Researchers have tested interventions to remediate cognitive dysfunction in school-aged children and adolescents during and after treatment for ALL. Strategies have included education interventions, such as cognitive remediation programs, school-based math interventions, and computerized training programs, with outcomes showing improvement in working memory, attention, visual working memory, and academic achievements (Castellino et al., 2014). Computerized cognitive training has shown that sustained improvements in working memory and processing speed continued to be evident six months after completing the intervention (Conklin et al., 2017).
At a state-of-the-science symposium convened by the Children’s Oncology Group Nursing Discipline, nurse scientists recognized the need for the science to advance in the identification and confirmation of biomarkers related to specific symptoms in childhood cancers (Mandrell & Withycombe, 2019). Biomarkers are currently used determine evidence-based treatment and evaluate response to treatment, and research is emerging that identifies markers of side effects and symptoms (Mandrell & Withycombe, 2019). Biomarkers of oxidative stress have been associated with distressing symptoms in children with cancer (Mandrell & Withycombe, 2019). Oxidative stress occurs when the body’s natural antioxidant defenses are unable to detoxify and protect against pro-oxidant species; the imbalance results in a disruption to the redox signaling process and molecular damage (Milne et al., 2015; Sies et al., 2017). This increase in reactive oxygen species, typically free radicals, is an indication of oxidative stress (Milne et al., 2015). Cell structures are then damaged by these compounds. Organs with high lipid content, such as the brain, are particularly sensitive to damage through lipid perioxidation (Hockenberry et al., 2019). Methotrexate administered as part of ALL treatment decreases antioxidant defenses, which results in increased oxidative stress. Researchers have reported increases in biomarkers of oxidative stress in CSF of children receiving methotrexate during treatment for ALL (Hockenberry et al., 2014), and these biomarkers have been associated with decreased cognitive function and neurotoxicities (Caron et al., 2009; Cheung et al., 2018; Hooke et al., 2020; Taylor et al., 2015).
One biomarker that measures oxidative stress is F2-isoprostane (F2-IsoP). F2-IsoPs are prostaglandin-like compounds that are created through the free radical–induced peroxidation of arachidonic acid (Milne et al., 2015), which is a polyunsaturated, free fatty acid and has high concentrations in membrane lipids (Hockenberry et al., 2014). A meta-analysis by van’t Erve et al. (2017) classified oxidative stress by F2-IsoP levels measured across 50 human diseases in a variety of body fluid types. Included in their analysis were 10 studies of adults with cancer that focused on F2-IsoP levels as a disease biologic marker but not as a marker of toxicity or symptoms. Overall, these studies showed a small increase in F2-IsoP levels when compared to healthy controls (van’t Erve et al., 2017). Groups that had the largest increases included those with renal diseases, respiratory tract diseases, and preeclampsia (van’t Erve et al., 2017).
F2-IsoPs levels in CSF increase throughout the lifespan (Montine et al., 2011). Although the relationship between F2-IsoPs levels in CSF and cognitive function has been studied in other adult populations, research in children is in its early stages. Increased F2-IsoPs levels have been found to be associated with a biomarker signature for Alzheimer disease (Duits et al., 2013; Montine et al., 2011) and as a biomarker of damage from oxidative stress in adults and children with a traumatic brain injury (Varma et al., 2004; Wagner et al., 2004). In a study of children treated for ALL (n = 47), those who received six or more doses of intrathecal methotrexate during postinduction treatment had higher levels of F2-IsoP than children who received fewer than six doses (Hockenberry et al., 2014). In a case series of three children with ALL, F2-IsoP levels increased before the presentation of acute methotrexate neurotoxicity during the postinduction period (Taylor et al., 2015). F2-IsoP levels were measured four times in relation to a symptom cluster of fatigue, pain, and nausea in a sample of 218 children during their first year of ALL treatment (Hockenberry et al., 2019). Those participants who had an increase in F2-IsoP levels experienced a higher severity of the symptom cluster in the four measurements (Hockenberry et al., 2019). Further research is needed to understand whether there is a relationship between F2-IsoP levels in CSF and cognitive function in children treated for ALL.
Cytokines are proteins that are secreted by cells and affect communication between cells (Becher et al., 2017; Zhang & An, 2007). Cytokines, which can serve as a biomarker that is associated with immune response to disease and inflammation, have been the focus on study of people with cancer (Mandrell & Withycombe, 2019). Interleukin-8 (IL-8) is a proinflammatory cytokine that belongs to the chemokine group; IL-8 stimulates neutrophils and monocytes to migrate to sites of inflammation in the body (Meniailo et al., 2018). Research on IL-8’s relationship to cognitive function has focused on diseases in adult populations, with studies in children lacking. Elevated levels of IL-8 are a measure of the underlying inflammatory mechanism that contributes to cancer-related cognitive impairment. Elevated blood levels of IL-8 in women treated for breast cancer are associated with cognitive deficits (Henneghan et al., 2020; Toh et al., 2020). Hesse et al. (2016) measured IL-8 levels in the CSF of adults diagnosed with Alzheimer disease and compared them to age-matched controls. Individuals with Alzheimer disease had significantly lower levels of IL-8 in their CSF, which was consistent with previous findings (Hesse et al., 2016). The researchers questioned whether the decreased IL-8 levels were because of the loss of reparative mechanisms within the CNS. IL-8 levels may first increase as the body initiates a response to the disease, but levels drop with disease progression (Hesse et al., 2016).
In children undergoing the first year of treatment for ALL, those with higher levels of IL-8 in CSF during induction had higher severity of a symptom cluster of fatigue, pain, and nausea (Hockenberry et al., 2019). In addition, when IL-8 levels in CSF increased during consolidation/intensification therapy, symptom severity also increased (Hockenberry et al., 2019). IL-8 levels in the CSF of children have not been previously associated with performance on measures of cognitive function.
This exploratory study is a companion to a previous multisite study in which symptoms clusters and functional outcomes were investigated in children during the early intensive phases of treatment for newly diagnosed leukemia (Hockenberry et al., 2017; Hooke et al., 2018). The purpose of the current study was to explore the relationship between biomarkers of oxidative stress (F2-IsoPs) and inflammation (IL-8) in CSF and cognitive function during maintenance therapy for childhood ALL.
Methods
Using a single-group, repeated-measures design, this study used a set of CSF biomarkers and cognitive measurements from a small cohort of children and adolescents who participated in the larger primary study. Data on these measures were collected at the beginning of maintenance (BOM) therapy, and these measurements were repeated at the end of maintenance (EOM) therapy as part of this companion study. Maintenance therapy is administered in repeating 12-week cycles; the final cycle of maintenance starts about 1.75 years after the first cycle of maintenance.
Children, adolescents, and families were eligible for the companion study if they had completed the primary study, were between the ages of 3 and 18 years when starting the primary study, were fluent English or Spanish, had not received craniospinal radiation therapy, and were entering the last chemotherapy cycle of maintenance therapy. Two major pediatric cancer treatment programs at Children’s Minnesota in Minneapolis and the Texas Children’s Hospital in Houston served as the study sites. Approval for the study was obtained from the institutional review board at each site. Assent was obtained from child participants who were aged 7–17 years, and their parents provided written consent. Participants who were aged 18 years provided consent.
Cognitive Self-Report Measurements
Study participants were instructed on how to complete the cognitive measurements on a data-secure Apple iPad by the research coordinator. Measurements occurred during an outpatient clinic visit.
Pediatric Quality of Life InventoryTM Cancer Module Cognitive Functioning Subscale: The cancer module of the Pediatric Quality of Life Inventory Cognitive Functioning Scale (PedsQL™) measures pediatric cancer–specific, health-related quality of life. The cancer module has established reliability (Cronbach’s alpha = 0.72 for child reports and 0.87 for parent reports) and is widely used in pediatric cancer research (Varni et al., 2002). The items are consistent in addressing universal concerns of pediatric cancer treatment while using appropriate language across the developmental continuum (Varni et al., 2002). One of the eight domains within the PedsQL is the five-item, Likert-type cognitive functioning subscale. Total scores range from 0 to 100, with higher scores indicating better cognitive quality of life (Varni et al., 2002). In the primary study, parents completed this measure as a proxy if their child was aged 6 years or younger. For consistency in measurement, if parents reported as a proxy at BOM therapy in the primary study, they repeated their proxy role in the companion study.
Parent-Perceived Child Cognitive Function: The Parent-Perceived Child Cognitive Function (PedsPCF) is a 32-item scale that is completed by the child’s parent, in which the parent rates their concerns about their child’s memory and thought processes (Lai et al., 2011). Each question asks the parent to rate the frequency and intensity on a six-point scale, with a higher score indicating better cognitive function. The scale has been validated across the developmental continuum in children and adolescents with cancer for identifying those most at risk for attention, social, and thought problems (Lai et al., 2014). Parents completed the measure for their children at BOM therapy and EOM therapy.
Cognitive Measurements From the National Institutes of Health Toolbox
Two subtests from the National Institute of Health (NIH) Toolbox were used to sample selective attention and executive functioning (cognitive flexibility/inhibitory control), which are areas commonly affected by cancer treatment. These brief, electronically delivered tasks were administered according to standardized instructions on iPads by research coordinators at each of the sites who had completed training in administration (including practice administrations and observation via videotape review) under the supervision of the study’s neuropsychologist. Testing was completed for all participants before they received any sedation for the lumbar puncture procedure. The tests were added to the cognitive measures at the end of leukemia treatment and were not part of the measures performed at BOM therapy in the primary study.
Flanker Inhibitory Control and Attention Test: The Flanker Inhibitory Control and Attention Test (Flanker) is a measure of inhibitory control and attention and is appropriate across the developmental continuum from young children to adults. For each trial that appears on the screen, there is a central directional target (a fish for children aged younger than 8 years and arrows for those aged 8 years and older) (Weintraub et al., 2013). The central target is flanked by similar stimuli to the left and right, and the participant must choose the direction of the central stimulus for inhibiting attention to the irrelevant stimuli (Weintraub et al., 2013). The test includes 20 trials, and children and adolescents are scored using a computer-based algorithm. Scores in this study are reported as age-corrected standard scores, with a normative average of 100 and a standard deviation of 15 (NIH, 2021).
Dimensional Change Card Sort: The Dimensional Change Card Sort (DCCS) measure evaluates executive function and cognitive flexibility in people aged 3–85 years. On the iPad screen, the participant must match one of the two stimuli according to shape or color (Weintraub et al., 2013). If the child is aged younger than 8 years, only one dimension is relevant. In the next block, there is a switch, and the other dimension becomes critical (Weintraub et al., 2013). Children who successfully follow the switch also receive a mixed block, which means that color is the relevant dimension on the majority of tests combined with intermittent, unpredictable shifts to shape (Weintraub et al., 2013). Children who are aged 8 years or older receive only the mixed block, and the relevant criterion word (“color” or “shape”) appears on the screen (Weintraub et al., 2013). Younger children receive the word visually on the screen and orally on the iPad. There are 40 trials in the test. Scores in the current study are reported as age-corrected standard scores, with a normative average of 100 and a standard deviation of 15 (NIH, 2021).
Cerebrospinal Fluid Biomarkers
Two milliliters of CSF were collected during a lumbar puncture procedure, which was part of standard care for children and adolescents with ALL. CSF was withdrawn before the administration of intrathecal chemotherapy. Intrathecal chemotherapy was scheduled at the beginning of each 12-week cycle of maintenance chemotherapy to prevent the growth (relapse) of leukemia cells in the CSF. The detailed methodologies for the processing and testing of the CSF samples for cytokine IL-8 and F2-IsoPs levels are described in the primary study.
Data Analysis
Descriptive statistics were used to summarize participant characteristics. Changes in study variables during maintenance therapy were evaluated using a paired-samples t test. The relationship of the biomarker measurements, cognitive self-report measures, and NIH Toolbox® measures were evaluated using Pearson’s correlations. To interpret the strength of the relationship, a p value of 0.1–0.29 signified a small relationship, 0.3–0.49 represented a medium relationship, and 0.5–1 indicated large relationship (Cohen, 1988).
To further explore significant findings from the correlation analyses, age-corrected Flanker scores were categorized into two groups: participants whose cognitive function scores were normal or higher (scores of 85 or higher) one standard deviation (SD = 15) below the mean (mean = 100) and participants with scores less than 85. Measurements for these two groups were compared using a t test with continuous variables and chi-square tests with categorical variables. SAS®, version 9.4, was used for all analyses.
Results
Sample Characteristics
A summary of the characteristics of the study participants is shown in Table 1. There were slightly more male than female participants. Most participants were aged 3–12 years when they enrolled in the primary study. Non-Hispanic White participants were the largest racial/ethnic group, whereas Hispanic participants consisted of almost one-third of the study sample. The ALL average/standard risk and high-risk categories were each comprised of about 40% of the study sample. None of the study participants had a neurotoxic event requiring a change in their treatment during maintenance therapy. During the final cycle of maintenance therapy when study measures were completed, the mean age of participants was 10.3 years (SD = 4.1), and they had a mean of 85 weeks between the beginning of the first and last cycles of maintenance therapy. 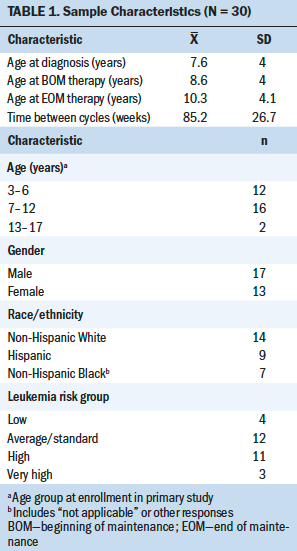
Changes in Biomarkers and Cognitive Function Measures
Levels of F2-IsoP did not change significantly during maintenance therapy in the study sample, whereas IL-8 levels significantly decreased (see Table 2). Scores on the PedsPCF increased significantly, indicating improved cognitive function. Scores on the cognitive subscale of the PedsQL remained stable during maintenance therapy. 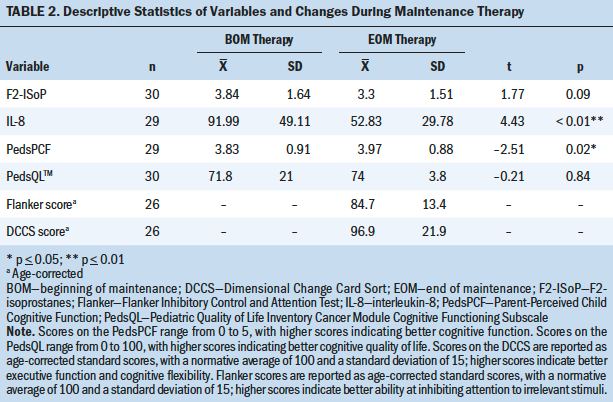
The NIH Toolbox measures were performed at EOM therapy only. These measures were added to the primary study measures as an additional cognitive measure of interest. Twenty-six participants completed these measures, with four refusing to perform the measures. On the Flanker, the age-corrected mean score was 84.7, and the age-corrected score on the DCCS was 96.9.
Biomarker Correlations
F2-IsoP levels at BOM therapy did not correlate with other cognitive measures (see Table 3). F2-IsoP levels at EOM therapy had a moderate negative relationship with scores on the Flanker, with higher F2-IsoP levels significantly associated with lower Flanker scores (p < 0.05). F2-IsoP levels at the two measurement timepoints (BOM therapy and EOM therapy) were significantly correlated. 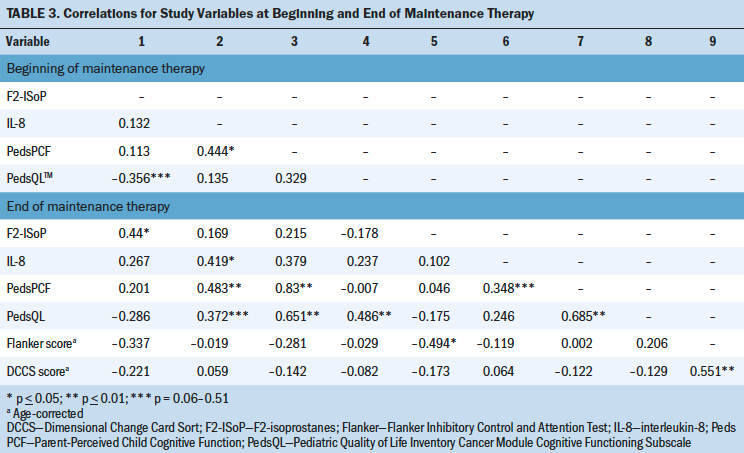
IL-8 levels at BOM therapy had a moderately strong, significant, positive correlation with PedsPCF scores measured at both BOM therapy (p < 0.05) and EOM therapy (p < 0.01), with higher levels of IL-8 associated with higher scores on parent’s perception of their child’s cognitive function. IL-8 levels measured at EOM therapy had a moderate, positive relationship with PedsPCF scores, which trended toward significance (p = 0.06–0.051). IL-8 levels at BOM therapy and EOM therapy were also significantly correlated.
Correlations Among Cognitive Measurements
Parents’ PedsPCF scores at BOM therapy were not significantly associated with scores on the cognitive subscale of the PedsQL at BOM therapy; however there was a significant, strong, positive relationship with PedsQL scores at EOM therapy (p < 0.01), with higher scores on the PedsPCF associated with higher cognitive PedsQL scores. Parents’ PedsPCF scores at EOM therapy also had a significant, strong, positive relationship with PedsQL scores at EOM therapy (p < 0.01). PedsPCF scores at BOM therapy and EOM therapy had a significant, strong, positive relationship (p < 0.01), whereas the relationship between PedsQL scores at both timepoints was significant, moderate, and positive (p < 0.01). Neither PedsPCF nor PedsQL scores at BOM therapy or EOM therapy had significant relationships with age-corrected scores on the Flanker or the DCCS at EOM therapy. At EOM therapy, scores on the Flanker and DCCS had a significant, strong, positive correlation (r = 0.551, p < 0.01).
Differences Between the Flanker Score Groups
After finding the significant relationship between F2-IsoP levels and Flanker scores at EOM, child participants were divided into two groups based on their age-corrected Flanker scores. One group (n = 12) included children whose age-corrected scores were 85 or greater (normal/higher cognitive function), and the other group (n = 14) consisted of children with age-corrected scores of less than 85 (see Table 4). As expected, Flanker scores were significantly different between the groups, and the differences between the Flanker score groups on age-corrected scores on the DCCS trended toward significance (p = 0.06). There was a significant difference in F2-IsoP levels at both BOM therapy and EOM therapy between the two Flanker score groups (p < 0.01 and p < 0.05, respectively). Analysis of demographic characteristics showed that the lower Flanker score group was significantly older (p = 0.03) and had more participants in the high- and very high–risk group (p = 0.02). Flanker score groups did not differ significantly in gender or race/ethnicity. There was not a significant difference in the length of time between the measurements performed during the first and last cycles of maintenance therapy or in length of time of overall ALL treatment. 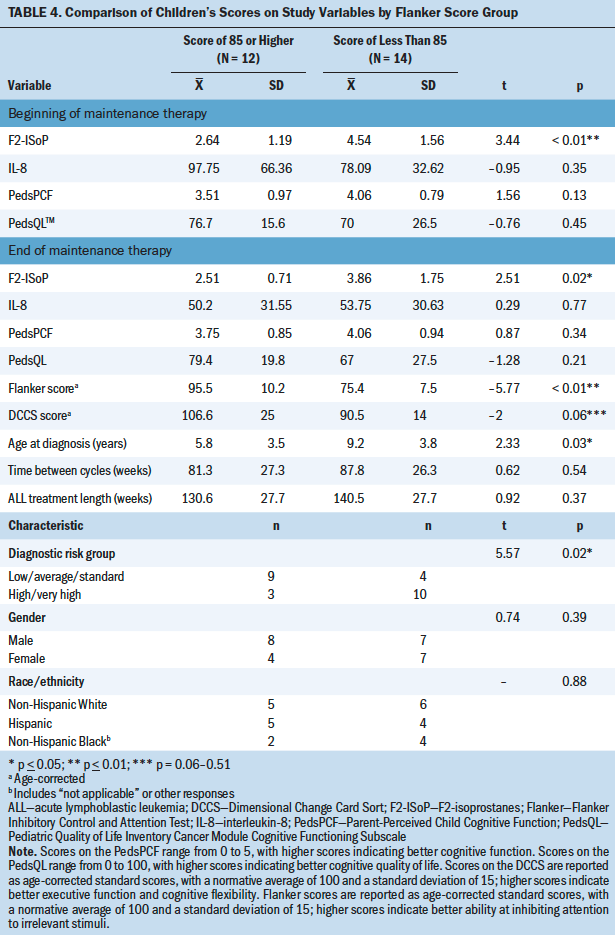
Discussion
The study sample consisted of more children (aged 3–12 years) than adolescents (aged 13–17 years). Although adolescents were invited to participate, they often verbalized that they were ready to be “done” as the end of treatment approached and did not want to volunteer for any further research. The large number of participants of Hispanic ethnicity (n = 9) was reflective of enrollment from the large pediatric cancer program at Texas Children’s Hospital.
Levels of F2-IsoP in CSF remained stable during maintenance therapy, with a slight, nonsignificant decrease. During this time, children continued to receive oral methotrexate weekly and intrathecal methotrexate every 12 weeks, whereas children with high- and very high–risk ALL received an additional dose of intrathecal methotrexate in each of the first four 12-week cycles. In the primary study, the mean level of F2-IsoP at BOM therapy was 6.66 (SD = 8.42) (Hockenberry et al., 2019), and the mean at BOM therapy in the current study was 3.84 (SD= 1.64), showing that this subset of participants started at a lower level. There was a significant, negative correlation between F2-IsoP levels at EOM therapy and Flanker scores, with higher F2-IsoP levels associated with lower Flanker scores. The Flanker is a measure of executive function focusing on inhibitory control and attention. F2-IsoP in CFS has previously been identified as a potential biomarker for symptom severity in children with ALL (Hockenberry et al., 2019); it may also be a biomarker for cognitive function in children with ALL receiving CNS-directed therapies, which merits further investigation.
When the study sample was categorized into two groups based on age-corrected scores of 85 or higher and less than 85 on the Flanker, the group with higher Flanker scores had significantly lower F2-IsoP levels at both BOM therapy and EOM therapy. In addition, the group with the lower scores was significantly older, with more children in the high- and very high–risk treatment groups. One of the factors that determines a high-risk group assignment is being aged 10 years or older at diagnosis, which may have contributed to why these characteristics were significant in the lower-scoring Flanker score group. As previously noted, treatment in these higher-risk groups was more intensive and included additional doses of intrathecal methotrexate. This is consistent with previous research that found that children who received a greater number of intrathecal methotrexate doses had higher levels of F2-IsoP in their CSF (Hockenberry et al., 2014). However, there may have been other characteristics that were not identified that contributed to the lower Flanker scores.
Levels of IL-8 in CSF decreased significantly from BOM therapy to EOM therapy as treatment became less intensive. In this small cohort, levels at BOM therapy were similar (mean = 91.99, SD = 49.11) to those in the larger group of the primary study (mean = 87.22, SD = 90.37) (Hockenberry et al., 2019). IL-8 at BOM therapy was correlated with parents’ perceptions of their child’s cognitive function as measured on the PedsPCF at BOM therapy and EOM therapy. The direction of the relationship was unexpected and surprising, however, with higher levels of IL-8 at BOM therapy associated with better parent reports of cognitive function, which has not been reported previously. Researchers studying adults with Alzheimer disease have hypothesized that higher IL-8 levels in the CSF may indicate an active, inflammatory reparative process that is lost as the disease advances (Hesse et al., 2016). Further research is needed to understand the relationship between changes in levels of IL-8 in the CSF and cognitive function in children with ALL during the treatment trajectory. Intrathecal chemotherapy is administered at regular intervals throughout ALL treatment. CSF samples collected during these standard procedure times could provide more insight into the pattern of inflammatory response mechanism measured by IL-8 levels.
Cognitive function, as measured by parent report, showed a significant increase from BOM therapy to EOM therapy. During maintenance therapy, healthcare providers inform parents that children and adolescents can return to school and activities that are normal for their developmental stage (Kaplan, 2019), so parents’ perceptions of their children’s cognitive function may reflect these efforts to return to normalcy. Of note, the scores on the cognitive subscale of the PedsQL did not improve during maintenance therapy. This measurement was completed by 60% of study participants who self-reported on themselves and by 40% of parents who reported on their child. Scores on the cognitive subscale of the PedsQL were consistent with scores reported previously by children receiving cancer treatment and their parents (Varni et al., 2002). However, there was a strong, positive relationship between scores on the PedsPCF at BOM therapy and EOM therapy and the PedsQL at EOM therapy, indicating consistency in rating the items measuring perceptions about attention, memory, and academics. When examining relationships between self-report/parent report measures of cognitive function and the cognitive tests from the NIH Toolbox, no correlations were found. In a previous study by Lai et al. (2014), the PedsPCF demonstrated a significant relationship to CogState™, a computer-based neuropsychological testing battery, and significant correlations were identified in processing speed, attention, learning, and working memory. Additional research is needed to provide insight into the relationships between measures of functional capacity, such as standardized cognitive tests, and functional status measured by self-report of cognitive function.
Limitations
The study was limited by the small sample size, with only two participants in the oldest age group. Study recruitment began after the conclusion of the larger, primary study. All eligible participants who were receiving maintenance therapy were invited to participate, but many children and adolescents expressed disinterest because they were looking ahead to therapy completion.
In addition, four participants did not complete the measures from the NIH Toolbox that had been added at the end of treatment. This small sample size limited the simultaneous analysis of multiple variables, which may have contributed to the study findings. To the authors’ knowledge, outcomes of cognitive measures from the NIH Toolbox have not been previously reported in children during cancer treatment. Future studies using these measures can provide further insight into their use in this population.
Implications for Nursing
The results of this study create an initial foundation on the relationship between two biomarkers in CSF and cognitive function, which can be built upon in future studies. This study highlights the need for future research in a larger sample to confirm the assessment of the F2-IsoP biomarker in CSF as a valid measure to identify children at risk for poorer cognitive outcomes. Lower levels of inflammation, as measured by IL-8 in the CSF, were not correlated with better cognitive function, and additional research is needed to explore whether IL-8 levels and patterns of change in IL-8 across the trajectory of ALL treatment relate to cognitive function.
As this research progresses, it may guide the management of cognitive dysfunction. Earlier identification using biomarkers can potentially be partnered with evidence-based interventions that remediate cognitive dysfunction (Castellino et al., 2014). ALL treatment is delivered over years, which is a long time in a young life, and survivorship lasts for many decades. Nurses have an important role as researchers who advance symptom science and as advocates for early interventions in children with cognitive dysfunction. Advancing the understanding of adverse side effects of pediatric cancer treatment is essential for improving the lives of childhood cancer survivors and helping them meet their maximum potential. 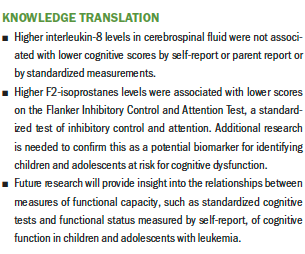
Conclusion
This is the first known study to explore CSF biomarkers of oxidative stress and inflammation, F2-IsoP and IL-8, in relation to cognitive function in children with ALL. As children and adolescents completed their treatment for ALL, those with higher levels of F2-IsoP in CSF had significantly lower scores on the Flanker. In addition, participants whose Flanker scores were one standard deviation below the mean had significantly higher F2-IsoP levels. IL-8 levels did not correlate with cognitive tests from the NIH Toolbox. The results did not support the hypothesis that higher levels of IL-8 in CSF would be associated with worse cognitive function; in fact, higher levels of IL-8 were shown to correlate with better cognitive function as reported by parents. This study provides a foundation for future research on biomarkers and their role in pediatric cancer symptom science research.
About the Author(s)
Mary C. Hooke, PhD, APRN, PCNS, CPON®, FAAN, is an associate professor, and Michelle A. Mathiason, MS, is a statistician, both in the School of Nursing, and Alicia S. Kunin-Batson, PhD, is an assistant professor in the School of Medicine, all at the University of Minnesota in Minneapolis; Audrey Blommer, RN, BSN, PHN, is a research nurse, Jessica Hutter, BS, CCRP, is a clinical research coordinator, and Pauline A. Mitby, MPH, is the research manager of the cancer and blood disorders program, all at Children’s Minnesota in Minneapolis; Ida M. Moore, PhD, RN, FAAN, is a professor and dean of the College of Nursing, and Susan Whitman, MS, is a scientist III in the College of Medicine, both at the University of Arizona in Tucson; and Olga Taylor, MPH, is a clinical research manager, Michael E. Scheurer, PhD, MPH, is a professor of pediatrics and hematology-oncology, and Marilyn J. Hockenberry, PhD, RN, PPCNP, FAAN, is a professor of pediatrics and the director of Global HOPE Nursing, all at the Baylor College of Medicine in Houston, TX. This work was supported, in part, by a grant from the Arthur Olofson Medical Research Fund at the University of Minnesota Foundation and the National Institutes of Health (R01CA1693398). Hooke, Kunin-Batson, Mitby, Moore, Taylor, Scheurer, and Hockenberry contributed to the conceptualization and design. Hooke, Kunin-Batson, Blommer, Hutter, Moore, Whitman, Taylor, and Scheurer completed the data collection. Hooke and Mathiason provided statistical support. Hooke, Mathiason, Moore, and Hockenberry provided the analysis. Hooke, Mathiason, Kunin-Batson, Mitby, Moore, Scheurer, and Hockenberry contributed to the manuscript preparation. Hooke can be reached at hook0035@umn.edu, with copy to ONFEditor@ons.org. (Submitted January 2021. Accepted May 19, 2021.)

