Interventions to Support Adherence to Oral Anticancer Medications: Systematic Review and Meta-Analysis
Problem Identification: This systematic review compared the efficacy of interventions to usual care on adherence to oral anticancer regimens.
Literature Search: Embase®, PubMed®, and CINAHL® were searched for eligible comparative studies published between January 2000 and May 2021. Outcomes of interest included adherence, cancer-related morbidity, quality of life, patient satisfaction, and other patient-specific outcomes.
Data Evaluation: Reviewers assessed risk of bias using the Cochrane Risk of Bias 2 tool and Risk of Bias in Nonrandomized Studies of Interventions. Certainty of evidence was assessed using the GRADE framework.
Synthesis: Risk assessment, ongoing or periodic assessment, proactive follow-up, motivational interviewing, or structured programs may improve adherence. Education or coaching interventions may improve or have little to no effect on adherence. Technological interventions may improve adherence, but interactive compared to noninteractive technology may have little to no effect.
Implications for Research: As more cancer treatments move to oral formulations, work remains to identify the most effective interventions to support people receiving oral anticancer regimens.
Jump to a section
Oral anticancer medications (OAMs) provide patients with recommended treatment in a less invasive, more convenient form than traditional cancer therapies and are transforming how cancer care is delivered. The transition of treatment from IV to oral methods of delivery has been a change for both patients and providers. The National Comprehensive Cancer Network estimates that as many as 25% of all new cancer treatments are being developed in oral formulations (Weingart et al., 2008). The shift from patients receiving care at infusion centers to managing their treatment at home has changed how clinicians educate, monitor, and follow up with patients. Patients and their care partners are being asked to understand and manage complex regimens at home without active assistance from healthcare professionals. Adherence to therapy has emerged as a primary concern with this shift in treatment (Weingart et al., 2008).
Adherence has been defined broadly through a collaborative approach to decision-making as agreement on choice and manner of treatment and, more specifically, as the extent to which patients take their medications as prescribed (Atkinson et al., 2016; Greer et al., 2016). Rates of adherence to OAMs have been reported to vary widely depending on population, cancer type, regimen, and measurement of adherence, with many patients reporting difficulty taking OAMs as prescribed (Greer et al., 2016; Milata et al., 2016; Salgado et al., 2017). The relationship between adherence and patient outcomes is well documented. Patients who report nonadherence have a lower likelihood of response to treatment and higher mortality (Greer et al., 2016). This underscores the importance of assessing patient adherence to OAMs and improving support for patients to receive optimal outcomes from treatment.
Despite this shift in cancer care, professional organizations are only beginning to set standards and procedures to support patients on OAM regimens. Processes for prescribing, educating, documenting, and monitoring patients taking OAMs are still being developed (Rodriguez et al., 2017; Weingart et al., 2012). To address this gap in practice, the Oncology Nursing Society (ONS) and the American Society of Clinical Oncology have updated standards of practice guiding chemotherapy administration to include safe administration and management of oral chemotherapy (Neuss et al., 2013, 2016). The standards include practice guidance for OAMs, including treatment planning, prescribing, drug preparation and administration, toxicity management, and documentation. They also include education related to storage, handling, disposal, drug–drug or drug–food interactions, planning for missed doses, and including care partners when appropriate. The standards recommend that institutions complete an initial assessment of adherence, with a plan for clinical staff to address any issues in a time frame appropriate to the patient and regimen (Neuss et al., 2013, 2016). The Hematology/Oncology Pharmacist Association has published practice standards for OAM management as well (Mackler et al., 2019). These standards cover prescription, education, dispensing, distribution, follow-up, and monitoring of symptoms and adherence, and they highlight the important role of the interprofessional oncology team. Although these national standards and best practices are important for patient management, a gap exists on specific recommendations for interventions that support patients taking OAMs.
Supporting patients taking OAMs depends on patient-, provider-, and system-level interventions. A review of current evidence on supportive care for patients and interventions to improve adherence was conducted to inform the development of an ONS Guideline™ on interventions and processes to support patients receiving OAMs (Belcher et al., 2022). This systematic review and meta-analysis compared the efficacy of nine types of adherence interventions given to patients taking OAMs.
Methods
This systematic review followed guidance from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009). The protocol is registered in PROSPERO (CRD42021250368). Supplemental data on outcomes taxonomy and search strategy are presented in the appendices.
PICO Questions
The PICO framework was used to develop questions with the following elements: a specific patient population (P), intervention (I), comparison (C), and outcome (O). A team of clinical experts was responsible for developing questions and identifying timely outcomes aimed at improving adherence for patients taking OAMs. The PICO questions are listed in Figure 1. After the outcomes and measures were extracted from the literature, a taxonomy was developed by the research team to organize the various ways in which outcomes of interest were reported. Instead of using a strict definition of adherence, this review measured adherence as defined by the authors of each study. Regarding specific PICO questions, coaching was broadly defined to encompass counseling interventions as well. Technology was defined as interventions entailing the use of webpages, mobile applications, text messaging, emails, and automated voicemails; follow-ups conducted via telephone alone were not considered technological interventions. Interactive technology entailed patients needing to respond to the technological intervention, and noninteractive technology required no follow-up (i.e., an automated voice message reminder to take medication). Because no standard care guideline currently exists for the management of adherence to OAMs, the reference for comparison was usual care.

Search Strategy and Inclusion Criteria
A clinical librarian searched Embase®, PubMed®, and CINAHL® databases for articles published between January 2000 and May 2021. Two reviewers screened titles and abstracts independently and in duplicate using Covidence software to find eligible articles that met the inclusion and exclusion criteria. Inclusion criteria were a comparison group, an adult population on an oral anticancer regimen, measurement of a patient-specific outcome measure (e.g., adherence, cancer-related morbidity, quality of life, patient satisfaction), and an intervention relevant to at least one of the PICO questions. Exclusion criteria were non-English studies, systematic reviews, noncomparative studies, and pediatric populations. Full texts for studies that met these criteria were then screened by two reviewers, independently and in duplicate. Conflict resolution was conducted by the team leader.
Data Extraction
Data were extracted independently and in duplicate by two investigators into a standardized and pilot-tested Microsoft Excel spreadsheet. Information was collected on the following variables: study characteristics, patient population, adherence intervention (e.g., type, dosage [if relevant], duration), and reported outcomes. Conflicts in the extraction process were resolved by referring to the source material, and consensus between the two extractors was reached. For cases in which consensus was challenging, the team leader would help with the decision-making process. RevMan, version 5.4, was used to input and analyze the extracted outcome data.
Data Synthesis and Analysis
RevMan was used to analyze and pool data whenever possible in a quantitative synthesis by researchers experienced with meta-analytic methods. Continuous variables were reported as a mean difference (MD) or a standardized MD (SMD) depending on which was more appropriate for the data being compared, and dichotomous variables in the analysis were reported as a risk ratio (RR). Adherence MDs were reported in percentages, and other MDs were reported in points. The software used the DerSimonian and Laird (1986) method for random- and fixed-effect models to estimate the effect size and 95% confidence intervals (CIs). Heterogeneity between pooled studies was assessed using the I2 statistic. Pooled data was presented and analyzed using forest plots. When quantitative synthesis was not possible, the outcomes were reported narratively.
Risk of Bias and Assessing the Certainty of the Evidence
Risk of bias for randomized controlled trials (RCTs) was assessed using the Cochrane Risk of Bias 2 tool, and the Risk of Bias in Nonrandomized Studies of Interventions was used for observational studies (Higgins et al., 2021; Sterne et al., 2016). Assessments were independently conducted by two reviewers for all studies included in this systematic review. When assessments disagreed, consensus was reached through discussion and input from the team leader.
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework was used to evaluate the overall certainty of the evidence (COE) (Guyatt et al., 2011). Risk of bias, inconsistency, indirectness, imprecision, and publication bias were rated to classify the overall COE as very low, low, moderate, or high. The estimate of the effect and COE were presented using the GRADEpro Guideline Development Tool in the form of evidence profiles.
Synthesis
Supplemental data on study inclusion processes, characteristics, risk-of-bias assessments, and evidence profiles—including number of patients, RRs, MDs or SMDs—and COE for each study are presented in the appendices.
Search Results
The search identified 10,265 titles and abstracts. Of those, 354 full-text articles were screened, and 49 full-text studies were included in the quantitative synthesis. Reviewed studies included a total of 50,379 patients, and most studies consisted of 200 patients or fewer (range = 29–42,366). Study characteristics and risk-of-bias assessments for eligible studies reporting on each PICO are presented in the appendices.
Data Synthesis
PICO 1: Should standardized assessment for risk for nonadherence/barriers to adherence be used rather than usual care in patients starting a new oral anticancer medication regimen?
One RCT reported on adherence rates among patients receiving a risk assessment in conjunction with a tailored coaching intervention compared to usual care (Schneider et al., 2014). Participants (n = 25) who received a risk assessment along with a tailored intervention had an adherence rate of 95.1%, and 20 participants in the control arm had an adherence rate of 82.4%. The COE is very low because of imprecision from a small sample size and indirectness from the addition of a coaching intervention.
PICO 2: Should standardized oral anticancer medication educational programs that address adherence be used rather than usual care in patients on an oral anticancer medication regimen?
Sixteen studies that addressed this question were identified in this review (Berry et al., 2015; Byrne et al., 2018; Gönderen Çakmak & Kapucu, 2021; Hendricks, 2015; Krikorian et al., 2019; Krolop et al., 2013; Lin et al., 2020; Morgan et al., 2018; Patel et al., 2016; Ribed et al., 2016; Schneider et al., 2014; Simons et al., 2011; Suttmann et al., 2020; Vacher et al., 2020; Zerbit et al., 2020; Ziller et al., 2013). Some of the studies reported on outcomes of interest that were not presented in the evidence profile and were ineligible for inclusion in analysis. They are described in the appendices (Gönderen Çakmak & Kapucu, 2021; Hendricks, 2015; Morgan et al., 2018; Patel et al., 2016; Ribed et al., 2016; Schneider et al., 2014).
Two RCTs demonstrated that education programs may have little or no effect on adherence rates compared to usual care in patients on an OAM regimen (MD = 0.4%, 95% CI [–1.87, 2.68], very low COE) (Krikorian et al., 2019; Ziller et al., 2013). In contrast, two RCTs demonstrated that education programs may increase the proportion of patients with high adherence in comparison to usual care (RR = 1.16, 95% CI [1.01, 1.33], moderate COE) (Berry et al., 2015; Suttmann et al., 2020). Four cohort studies reported that educational programs may increase adherence rates compared to usual care, but the evidence is very uncertain (MD = 10.61%, 95% CI [7.21, 14.01], very low COE) (Krolop et al., 2013; Simons et al., 2011; Vacher et al., 2020; Zerbit et al., 2020).
Two studies reported on patient satisfaction and knowledge of the regimen. One cohort study demonstrated that patients in an educational program may be less likely to report satisfaction with their care after receiving the educational intervention in comparison to usual care. Satisfaction was assessed using pharmacist check-in (RR = 0.89, 95% CI [0.72, 1.1], very low COE), a medication information sheet (RR = 0.85, 95% CI [0.63, 1.14], very low COE), and check-in with a medication navigator (RR = 0.75, 95% CI [0.6, 0.95], very low COE) (Lin et al., 2020). One cohort study demonstrated that patients in educational programs were more likely to have knowledge of the regimen after the educational intervention compared to before the intervention. Patient knowledge on dosage and frequency (RR = 1.26, 95% CI [1.03, 1.52], very low COE), management of missed doses (RR = 1.51, 95% CI [1.16, 1.98], very low COE), and dosage schedule (RR = 1.31, 95% CI [1.06, 1.62], very low COE) were assessed (Byrne et al., 2018).
PICO 3: Should standardized, periodic/ongoing assessment of adherence instead of usual care be used for patients on an oral anticancer medication regimen?
Twelve studies that addressed this question were identified in this review (Bordonaro et al., 2014; Bouleftour et al., 2021; Dennison et al., 2021; Eldeib et al., 2019; Greer et al., 2020; Lin et al., 2020; Mir et al., 2020; Muluneh et al., 2018; Spoelstra et al., 2015, 2017; Suttmann et al., 2020; Zerbit et al., 2020). Some of the studies reported on outcomes of interest that were not presented in the evidence profile and were ineligible for inclusion in analysis. They are described in the appendices (Bouleftour et al., 2021; Dennison et al., 2021; Eldeib et al., 2019; Greer et al., 2020; Lin et al., 2020; Mir et al., 2020; Muluneh et al., 2018; Spoelstra et al., 2017; Suttmann et al., 2020).
Three studies reported on adherence (Greer et al., 2020; Spoelstra et al., 2015; Zerbit et al., 2020). One RCT showed that patients on OAM regimens receiving ongoing assessment of adherence may have higher adherence rates as compared to patients receiving usual care (MD = 2.34%, 95% CI [–5.58, 10.26], very low COE) (Greer et al., 2020). Similarly, a cohort study reported that adherence rates may increase in patients receiving periodic assessment in comparison to those receiving usual care (MD = 7%, 95% CI [0.66, 13.34], very low COE) (Zerbit et al., 2020). One RCT reported that there may be little to no effect on relative dose intensity for patients receiving ongoing assessment compared to usual care (MD = 0.32%, 95% CI [–0.08, 0.72], very low COE) (Spoelstra et al., 2015).
Three studies assessed cancer-related morbidity by global toxicity score and symptom experience. One RCT showed that ongoing assessment of adherence may have little or no effect on cancer-related morbidity compared to usual care (MD = 1 point, 95% CI [–1.72, 3.72], very low COE) (Bouleftour et al., 2021). Similarly, another RCT showed that periodic assessment may have little or no effect on cancer-related morbidity compared to patients receiving usual care (MD = –1.75 points, 95% CI [–9.48, 5.98], very low COE) (Spoelstra et al., 2013). Likewise, a cohort study reported there may be little or no effect on cancer-related morbidity for patients on an OAM regimen receiving ongoing assessment of adherence in comparison to those receiving usual care (MD = –4.78 points, 95% CI [–7.8, –1.76], very low COE) (Spoelstra et al., 2017).
Four studies reported on the outcomes of quality of life, satisfaction, and self-efficacy. Quality of life was assessed using Functional Assessment of Cancer Therapy–General (FACT-G) and European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 (EORTC QLQ-C30) (Maringwa et al., 2011; Musoro et al., 2019). One RCT showed that patients receiving periodic assessment of adherence may have little to no difference in quality of life compared to patients receiving usual care (MD = 2.28 points; 95% CI [1.93, 2.63], moderate COE) (Greer et al., 2020). Minimally important difference (MID) was defined as 5–7 points on the FACT-G (King et al., 2010; Yost et al., 2013; Yost & Eton, 2005). In contrast, one cohort study reported a meaningful increase in quality of life according to the EORTC QLQ-C30 in patients receiving ongoing assessment in comparison to those receiving usual care (MD = 15.7 points, 95% CI [8.84, 22.56], MID = 4–11, low COE) (Bordonaro et al., 2014). One cohort study showed that patients receiving ongoing assessment of adherence may be more likely to report satisfaction with their care than patients receiving usual care (RR = 1.32, 95% CI [1.02, 1.72], very low COE) (Dennison et al., 2021). Patient self-efficacy was assessed across two studies using the original and revised versions of the Medication Adherence Self-Efficacy Scale (Fernandez et al., 2008). One RCT showed that patients receiving periodic assessment of adherence may have little to no difference in self-efficacy compared to usual care (MD = –0.51 points, 95% CI [–1.3, 0.28], very low COE) (Spoelstra et al., 2015). Similarly, a cohort study showed that patients receiving ongoing assessment of adherence may have little to no difference in self-efficacy compared to patients receiving usual care (MD = –0.01 points, 95% CI [–0.36, 0.34], very low COE) (Spoelstra et al., 2017).
PICO 4: Should proactive follow-up outside of routine medical visits be done rather than usual care for patients on an oral anticancer medication regimen who have additional risk factors?
Three studies that addressed this question were identified in this review (Eldeib et al., 2019; Hendricks, 2015; Vacher et al., 2020). Some of the studies reported on outcomes of interest that were not presented in the evidence profile and were ineligible for inclusion in analysis. They are described in the appendices (Eldeib et al., 2019; Hendricks, 2015). One cohort study demonstrated that proactive follow-up in patients on an OAM regimen with additional risk factors may increase adherence rate compared to usual care (MD = 17.8%, 95% CI [6.43, 29.17], very low COE) (Vacher et al., 2020).
PICO 5: Should a coaching intervention be used instead of usual care for patients on an oral anticancer medication regimen?
Eight studies were identified in this review (Bordonaro et al., 2014; Komatsu et al., 2020; Krikorian et al., 2019; Lam & Cheung, 2016; Middendorff et al., 2018; Muluneh et al., 2018; Patel et al., 2016; Schneider et al., 2014; Vacher et al., 2020). Some of the studies reported on outcomes of interest that were not presented in the evidence profile and were ineligible for inclusion in analysis. They are described in the appendices (Muluneh et al., 2018; Patel et al., 2016; Schneider et al., 2014). Because of the broad definition of coaching, there is heterogeneity in the interventions used amongst studies.
Five studies reported on adherence to OAMs (Komatsu et al., 2020; Krikorian et al., 2019; Lam & Cheung, 2016; Middendorff et al., 2018; Vacher et al., 2020). One RCT showed that coaching may have little or no effect on adherence rates when compared to usual care (MD = 0.8%, 95% CI [–2.24, 3.84], very low COE) (Krikorian et al., 2019). Another RCT reported that patients who received coaching may have little or no difference in adherence compared to those receiving usual care (RR = 1.01, 95% CI [0.91, 1.12], very low COE) (Komatsu et al., 2020). One cohort study showed that adherence rates may improve after receiving the coaching intervention in comparison to baseline adherence rates (MD = 17.8%, 95% CI [6.43, 29.17], very low COE) (Vacher et al., 2020). Similarly, two cohort studies reported there may be an improvement in adherence in patients who received coaching compared to those who received usual care (MD = 2.98%, 95% CI [2.95, 3.01], very low COE) (Lam & Cheung, 2016; Middendorff et al., 2018).
Four studies reported on the outcomes of cancer-related morbidity, quality of life, satisfaction, and self-efficacy. Morbidity was assessed using the MD Anderson Symptom Inventory (Mendoza et al., 2019). One RCT reported that coaching had little or no effect on cancer-related morbidity compared to usual care (MD = 0 points, 95% CI [–0.55, 0.55], MID = 1–10, very low COE) (Komatsu et al., 2020). Quality of life was assessed using the FACT–Breast and EORTC QLQ-C30 in two studies. MID was defined as 7–8 points on the FACT–Breast (Eton et al., 2004). One RCT reported that coaching had little to no effect on quality of life when compared to usual care (MD = 0.2 points, 95% CI [–6.18, 6.58], very low COE) (Komatsu et al., 2020). The same study showed that patients receiving the coaching intervention may have similar satisfaction scores compared to those receiving usual care (MD = 0.1 points, 95% CI [–0.9, 1.1], very low COE) (Komatsu et al., 2020). Self-efficacy was measured using the General Self-Efficacy Scale. Patients receiving the coaching intervention may have improved self-efficacy scores compared to those receiving usual care (MD = 1.8 points, 95% CI [–0.01, 3.61], very low COE) (Komatsu et al., 2020).
PICO 6: Should motivational interviewing be used instead of usual care for patients on an oral anticancer medication regimen?
Four studies that addressed this question were identified in this systematic review (Gönderen Çakmak & Kapucu, 2021; Ribed et al., 2016; Spoelstra et al., 2017; Ziller et al., 2013). A variety of components were used in motivational interviewing interventions. Some of the studies reported on outcomes of interest that were not presented in the evidence profile and were ineligible for inclusion in analysis. They are described in the appendices (Gönderen Çakmak & Kapucu, 2021; Ribed et al., 2016; Spoelstra et al., 2017).
One RCT reported that motivational interviewing may improve adherence rates compared to usual care in patients on an OAM regimen (MD = 3.23%, 95% CI [0.45, 6.02], low COE) (Ziller et al., 2013).
Three studies reported on the outcomes of cancer-related morbidity and self-efficacy. Morbidity was assessed using the Symptom Experience Inventory. One cohort study reported that patients receiving motivational interviewing may experience less cancer-related morbidity compared to those receiving usual care (MD = –4.78 points, 95% CI [–7.8, –1.76], very low COE) (Spoelstra et al., 2017). Self-efficacy was assessed using the Medication Adherence Self-Efficacy Scale (Fernandez et al., 2008). One RCT demonstrated that motivational interviewing may improve self-efficacy in comparison to those receiving usual care (MD = 9.9 points, 95% CI [9.68, 0.12], low COE) (Gönderen Çakmak & Kapucu, 2021). In contrast, a cohort study showed little to no effect of motivational interviewing on self-efficacy compared to usual care (MD = –0.01 points, 95% CI [–0.36, 0.34], very low COE) (Spoelstra et al., 2017).
PICO 7: Should a technological intervention be used rather than usual care for patients on an oral anticancer medication regimen?
Twelve studies that addressed this question were identified in this review (Collado-Borrell et al., 2020; Fischer et al., 2018; Greer et al., 2020; Hershman et al., 2020; Kim et al., 2018; Krok-Schoen et al., 2019; Mauro et al., 2019; McKay et al., 2019; Mir et al., 2020; Sikorskii et al., 2018; Spoelstra et al., 2015, 2016) Some of the studies reported on outcomes of interest that were not presented in the evidence profile and were ineligible for inclusion in analysis. They are described in the appendices (Fischer et al., 2018; Greer et al., 2020; Hershman et al., 2020; Kim et al., 2018; Krok-Schoen et al., 2019; McKay et al., 2019; Mir et al., 2020; Spoelstra et al., 2016).
Five studies reported on adherence in the evidence profile (Collado-Borrell et al., 2020; Greer et al., 2020; Mauro et al., 2019; Sikorskii et al., 2018; Spoelstra et al., 2015). Two RCTs showed that patients receiving a technological intervention may have higher adherence rates compared to those receiving usual care (MD = 8.23%, 95% CI [2.9, 13.55], very low COE) (Greer et al., 2020; Mauro et al., 2019). One cohort study reported that adherence rates may increase in patients on an oral anticancer regimen in comparison to those receiving only usual care (MD = 4.7%, 95% CI [1.19, 8.21], very low COE) (Collado-Borrell et al., 2020). Two RCTs reported little to no effect on relative dose intensity when comparing patients receiving a technology intervention to those receiving usual care (MD = 0.01%, 95% CI [0.04, 0.02], very low COE) (Sikorskii et al., 2018; Spoelstra et al., 2015).
Five studies reported on the outcomes of cancer-related morbidity, quality of life, and satisfaction. Morbidity was assessed using the Symptom Experience Inventory. One RCT showed that patients receiving a technology intervention may have little or no difference in cancer-related morbidity compared to those receiving usual care (MD = –3.5 points, 95% CI [–12.48, 5.48], low COE) (Spoelstra et al., 2015). Quality of life was assessed using the FACT-G and World Health Organization Quality-of-Life Instrument–Short Form. Two RCTs reported there may be little to no effect on quality of life when comparing patients receiving a technology intervention to patients receiving usual care (SMD = 1.44 SD, 95% CI [1.15, 1.74], very low COE) (Greer et al., 2020; Kim et al., 2018). Conversely, a cohort study showed that patients receiving a technology intervention may have higher quality of life when compared to those receiving usual care (MD = 0.13 points, 95% CI [–0.07, 0.2], MID = 0.061, very low COE) (Collado-Borrell et al., 2020). Quality of life in this study was assessed using the EuroQol-5 Dimension Questionnaire (McClure et al., 2017). Satisfaction was assessed using the Functional Assessment of Chronic Illness Therapy–Treatment Satisfaction–Patient Satisfaction. One RCT reported little to no effect on satisfaction when comparing patients receiving a technology intervention versus usual care (MD = 0 points, 95% CI [–1.31, 1.31], very low COE) (McKay et al., 2019).
PICO 8: Should interactive technology rather than noninteractive technology be used for patients on an oral anticancer medication regimen?
One RCT reported that patients receiving interactive technology interventions may be less likely to have an adherence rate of at least 80% compared to those receiving passive technology (RR = 0.86, 95% CI [0.7, 1.05], very low COE) (Spoelstra et al., 2013).
The study also showed that interactive technology may have little or no effect on reducing symptom severity compared to noninteractive technology in patients on OAM regimens (MD = 4.12 points, 95% CI [–0.4, 8.64], very low COE) (Spoelstra et al., 2013).
PICO 9: Should structured oral anticancer medication programs rather than no structured oral anticancer medication programs be used by institutions providing care to patients on an oral anticancer medication regimen?
Fourteen studies that addressed this question were identified in this review (Bordonaro et al., 2012, 2014; Curry et al., 2020; Dennison et al., 2021; Gebbia et al., 2013; Khandelwal et al., 2012; Krolop et al., 2013; Lam & Cheung, 2016; Middendorff et al., 2018; Muluneh et al., 2018; Ribed et al., 2016; Stokes et al., 2017; Tschida et al., 2012; Vacher et al., 2020). All were cohort studies. Some of the studies reported on outcomes of interest that were not presented in the evidence profile and were ineligible for inclusion in analysis. They are described in the appendices (Bordonaro et al., 2012; Curry et al., 2020; Gebbia et al., 2013; Khandelwal et al., 2012; Middendorff et al., 2018; Muluneh et al., 2018; Ribed et al., 2016; Vacher et al., 2020).
Adherence was measured differently across studies, but all found an increase in adherence for patients in an OAM program. Two studies reported that adherence rates, measured using the Medication Event Monitoring System, may increase in patients on an OAM program compared to those receiving usual care (MD = 12.22%, 95% CI [9.19, 15.24], very low COE) (Krolop et al., 2013; Vacher et al., 2020). Four studies reported that adherence rates, measured using medication possession ratio, may increase in patients on an OAM program compared to usual care (MD = 6%, 95% CI [4, 8], very low COE) (Lam & Cheung, 2016; Middendorff et al., 2018; Stokes et al., 2017; Tschida et al., 2012). One study reported that adherence rates, using pill counts, may increase in patients on an OAM program in comparison to usual care (RR = 1.14, 95% CI [0.96, 1.36], very low COE) (Gebbia et al., 2013).
Two studies reported on cancer-related morbidity, quality of life, satisfaction, and financial toxicity. One cohort study reported that cancer-related morbidity, assessed using the EORTC QLQ-C30, may increase in patients on an OAM program compared those receiving usual care (MD = 11.1 points, 95% CI [7.45, 14.75]; MID = 6 points, very low COE) (Bordonaro et al., 2014). This study also reported that quality of life, assessed using the EORTC QLQ-C30, may increase in patients on an OAM program compared to usual care (MD = 15.7 points, 95% CI [12.7, 18.7], MID = 4–11 points, very low COE) (Bordonaro et al., 2014). This cohort study also reported that financial toxicity, assessed using the EORTC QLQ-C30, may not be affected in patients on an OAM program in comparison to those receiving usual care (MD = 0 points, 95% CI [–1.57, 1.57], very low COE) (Bordonaro et al., 2014). Another cohort study reported that patient satisfaction may increase in those on an OAM program compared to those receiving usual care (RR = 1.32, 95% CI [1.02, 1.72], very low COE) (Dennison et al., 2021).
Discussion
Statement of the Principal Findings
This systematic review serves as the evidence base for a clinical practice guideline on interventions to support patients taking OAMs (Belcher et al., 2022). It aims to synthesize and evaluate the quality of evidence available. The following findings suggest that compared to usual care:
- Risk assessment may improve adherence (very low COE).
- Educational programs may improve or have little to no effect on adherence (very low COE).
- Periodic assessment of adherence may improve adherence (very low COE).
- Active oral adherence follow-up may improve adherence (very low COE).
- Coaching interventions may improve or have little to no effect on adherence (very low COE).
- Motivational interviewing may improve adherence (low COE).
- Technological intervention may improve adherence (very low COE).
- There was no difference in adherence between interactive and noninteractive technology (very low COE).
- Structured oral anticancer medication programs may improve adherence (very low COE).
Strengths and Limitations
This systematic review and meta-analysis explored supporting OAM adherence with a variety of interventions, such as education, follow-up, counseling, technology, and structured programs. It was conducted with transparency and appropriate methodology to identify eligible studies, implement statistical analysis whenever possible, and evaluate COE. Despite this rigorous methodology, this article faces some limitations regarding heterogeneity and generalizability. The study population was heterogeneous in type of cancer and OAM regimen. In addition, because of heterogeneity in how outcomes were reported, pooled analysis was not always possible. Interventions were also delivered in a variety of ways, introducing heterogeneity among studies. The studies included in this review included complex interventions with several components delivered by multiple types of healthcare providers. Findings reporting on diverse interventions may have limitations when generalized to different programs or interventions. A standardized and transparent process to assess COE was informed by these concerns about heterogeneity and indirectness. Another limitation is that only studies published in English were included in this systematic review. It is possible that studies published in a different language may have been overlooked by this review.
Relation to Other Studies
Other reviews focused on OAM adherence identified findings consistent with this review. A systematic review by Zerillo et al. (2018) identified several interventions for adherence, including education and monitoring. Interventions that found a statistically significant improvement in care delivery included telephone contact within the first days after treatment initiation and standardized toxicity management protocols. Technology-based interventions to increase contact between the patient and care team were not effective (Zerillo et al., 2018). Greer et al. (2016) identified 12 intervention studies to improve adherence. The interventions included education, treatment monitoring, pharmacy-based programs, counseling, and automated voice response systems. Only treatment monitoring and intensified interprofessional pharmaceutical care were associated with higher adherence rates (Greer et al., 2016). Two reviews that focused on adherence to endocrine therapy for people with breast cancer identified that education alone was not effective, but that a combination of education and bidirectional communication was associated with improved adherence (Finitsis et al., 2019; Heiny et al., 2019).
Implications for Nursing
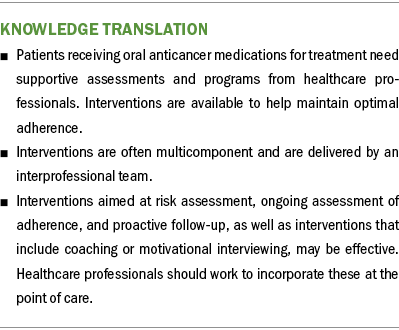
This review serves as the evidence base for a clinical practice guideline on the necessity for tailored interventions to support adherence to OAMs (Belcher et al., 2022) and has important implications for clinicians. Research on interventions to support patients on OAM regimens are emerging, but work remains to identify and test the most promising interventions. The paradigm change from in-person IV therapy to at-home oral therapy for many cancers requires nurses and other healthcare professionals to develop systems to support patients (Given, 2016). Interventions aimed at risk assessment, ongoing assessment of adherence, and proactive follow-up, as well as interventions that include coaching or motivational interviewing, may be effective, and healthcare professionals should work to incorporate these at the point of care. Patients tasked with managing their treatment at home while taking OAMs are at risk for fragmented care, and well-defined, supportive programs backed by research can be effective at bridging gaps in care.
This review identified types of interventions that could improve adherence to OAMs. Future research is needed to standardize terminology, measurements, definitions, and outcomes to compare findings on OAM adherence across studies (Heiney et al., 2019). Standardizing reporting will aid in identifying effective interventions that can then be translated into clinical practice more successfully. The European Society for Patient Adherence has established a validated set of reporting guidelines to enhance the quality of medication adherence research reporting (De Geest et al., 2018). A scoping review conducted in parallel to this technical review distilled programmatic considerations for implementing an OAM program, and findings can be considered when prioritizing a research agenda in this area (Sivakumaran et al., 2022).
Conclusion
Patients prescribed an OAM for cancer treatment require support and collaboration from healthcare professionals to ensure optimal treatment adherence. Although no single intervention proved to be the most clinically relevant, studies demonstrated that multiple interventions are available for use in the care of patients taking OAMs and can be incorporated into clinical practice. Oncology healthcare professionals need to incorporate processes and guidelines to support patients on oral therapy similar to those used for patients undergoing IV therapy. These interventions and processes may be refined, but the foundation to build from is available and ready to be implemented.
About the Authors
Haya Waseem, MPH, is a research assistant at the Evidence Foundation in Cleveland Heights, OH; Pamela K. Ginex, EdD, MPH, RN, OCN®, is an assistant professor in the School of Nursing at Stony Brook University with a joint appointment in the Division of Population Science at the Stony Brook Cancer Center, both in New York, and was, at the time of writing, the senior manager of evidence-based practice and inquiry at the Oncology Nursing Society in Pittsburgh, PA; Kapeena Sivakumaran, MPH, is a research assistant at the Evidence Foundation; Gina M. DeGennaro, DNP, RN, CNS, AOCN®, CNL, is a professor in the School of Nursing at the University of Virginia in Charlottesville; Sarah Lagler-Clark, MPH, is a practicum student at the Evidence Foundation and a graduate research assistant in the Department of Health Research Methods, Evidence and Impact at McMaster University in Hamilton, Ontario, Canada; Kristine B. LeFebvre, MSN, RN, NPD-BC, AOCN®, is an oncology clinical specialist at the Oncology Nursing Society in Pittsburgh, PA; Nicole Palmer, MPH, is a research assistant at the Evidence Foundation; Tejanth Pasumarthi is a research student at the Evidence Foundation and a student in the School of Interdisciplinary Science at McMaster University; Paula Rieger, DNP, RN, FAAN, is retired and previously was the chief executive officer at the Oncology Nursing Society; Kelli Thoele, PhD, RN, ACNS-BC, BMTCN®, OCN®, is a clinical research scientist at the Eli Lilly and Company in Indianapolis, IN; and Rebecca L. Morgan, PhD, MPH, is the executive director at the Evidence Foundation and an assistant professor in the Department of Health Research Methods, Evidence and Impact at McMaster University. Development of this review was wholly funded by the Oncology Nursing Society, a nonprofit organization that represents oncology nurses. No honoraria were provided. Waseem, Ginex, Sivakumaran, DeGennaro, Thoele, and Morgan contributed to the conceptualization and design. Waseem, Ginex, Sivakumaran, Lagler-Clark, LeFebvre, Palmer, Pasumarthi, and Morgan completed the data collection. Waseem, Ginex, and Morgan provided statistical support. Waseem, Ginex, LeFebvre, Palmer, Rieger, Thoele, and Morgan provided the analysis. All authors contributed to the manuscript preparation. Ginex can be reached at pamela.ginex@stonybrookmedicine.edu, with copy to ONFEditor@ons.org. (Submitted December 2021. Accepted February 17, 2022.)
References
Atkinson, T.M., Rodríguez, V.M., Gordon, M., Avildsen, I.K., Emanu, J.C., Jewell, S.T., . . . Ginex, P.K. (2016). The association between patient-reported and objective oral anticancer medication adherence measures: A systematic review. Oncology Nursing Forum, 43(5), 576–582. https://doi.org/10.1188/16.ONF.576-582
Belcher, S.M., Mackler, E., Muluneh, B., Ginex, P.K., Anderson, J.K., Bettencourt, E. . . . Morgan, R.L. (2022). ONS Guidelines™ to support patient adherence to oral anticancer medications. Oncology Nursing Forum, 49(4), 279–295. https://doi.org/10.1188/22.ONF.279-295
Berry, D.L., Blonquist, T.M., Hong, F., Halpenny, B., & Partridge, A.H. (2015). Self-reported adherence to oral cancer therapy: Relationships with symptom distress, depression, and personal characteristics. Patient Preference and Adherence, 9, 1587–1592. https://doi.org/10.2147/ppa.S91534
Bordonaro, S., Raiti, F., Di Mari, A., Lopiano, C., Romano, F., Pumo, V., . . . Tralongo, P. (2012). Active home-based cancer treatment. Journal of Multidisciplinary Healthcare, 5, 137–143. https://doi.org/10.2147/jmdh.S31494
Bordonaro, S., Romano, F., Lanteri, E., Cappuccio, F., Indorato, R., Butera, A., . . . Tralongo, P. (2014). Effect of a structured, active, home-based cancer-treatment program for the management of patients on oral chemotherapy. Patient Preference and Adherence, 8, 917–923. https://doi.org/10.2147/PPA.S62666
Bouleftour, W., Muron, T., Guillot, A., Tinquaut, F., Rivoirard, R., Jacquin, J.P., . . . Vassal, C. (2021). Effectiveness of a nurse-led telephone follow-up in the therapeutic management of patients receiving oral antineoplastic agents: A randomized, multicenter controlled trial (ETICCO study). Supportive Care in Cancer, 29(8), 4257–4267. https://doi.org/10.1007/s00520-020-05955-3
Byrne, A.E., Redmayne, G.M., Lam, T., Tran, J., & Chan, D.K. (2018). Implementation and evaluation of a pharmacist-led oral anticancer medication management clinic. Journal of Pharmacy Practice and Research, 48(6), 509–515. https://doi.org/10.1002/jppr.1429
Collado-Borrell, R., Escudero-Vilaplana, V., Ribed, A., Gonzalez-Anleo, C., Martin-Conde, M., Romero-Jimenez, R., . . . Sanjurjo-Saez, M. (2020). Effect of a mobile app for the pharmacotherapeutic follow-up of patients with cancer on their health outcomes: Quasi-experimental study. JMIR mHealth and uHealth, 8(10), e20480. https://doi.org/10.2196/20480
Curry, M.A., Chineke, I., Redelico, T., Terrell, C., Bell, W., Flood, D., . . . Bernal-Mizrachi, L. (2020). Adherence to oral anticancer medications after implementation of an ambulatory adherence program at a large urban academic hospital. JCO Oncology Practice, 16(4), e350–e356. https://doi.org/10.1200/jop.19.00167
De Geest, S., Zullig, L.L., Dunbar-Jacob, J., Helmy, R., Hughes, D.A., Wilson, I.B., & Vrijens, B. (2018). ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Annals of Internal Medicine, 169(1), 30–35. https://doi.org/10.7326/m18-0543
Dennison, T., Deal, A.M., Foster, M., Valgus, J., & Muluneh, B. (2021). A pharmacist-led oral chemotherapy program’s impact on chronic myeloid leukemia patient satisfaction, adherence, and outcomes. Journal of the Advanced Practitioner in Oncology, 12(2), 148–157. https://doi.org/10.6004/jadpro.2021.12.2.3
DerSimonian, R., & Laird, N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Eldeib, H.K., Abbassi, M.M., Hussein, M.M., Salem, S.E., & Sabry, N.A. (2019). The effect of telephone-based follow-up on adherence, efficacy, and toxicity of oral capecitabine-based chemotherapy. Telemedicine and e-Health, 25(6), 462–470. https://doi.org/10.1089/tmj.2018.0077
Eton, D.T., Cella, D., Yost, K.J., Yount, S.E., Peterman, A.H., Neuberg, D.S., . . . Wood, W.C. (2004). A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. Journal of Clinical Epidemiology, 57(9), 898–910. https://doi.org/10.1016/j.jclinepi.2004.01.012
Fernandez, S., Chaplin, W., Schoenthaler, A.M., & Ogedegbe, G. (2008). Revision and validation of the medication adherence self-efficacy scale (MASES) in hypertensive African Americans. Journal of Behavioral Medicine, 31(6), 453–462. https://doi.org/10.1007/s10865-008-9170-7
Finitsis, D.J., Vose, B.A., Mahalak, J.G., & Salner, A.L. (2019). Interventions to promote adherence to endocrine therapy among breast cancer survivors: A meta-analysis. Psycho-Oncology, 28(2), 255–263. https://doi.org/10.1002/pon.4959
Fischer, N., Agboola, S., Palacholla, R., Atif, M., Jethwani, K., & Kvedar, J. (2018). A 2-arm randomized pilot study to evaluate the impact of a mobile health application on medication adherence in patients on oral anti-cancer medications. Value in Health, 21(5, Suppl.), S35. https://doi.org/10.1016/j.jval.2018.04.296
Gebbia, V., Bellavia, M., Banna, G.L., Russo, P., Ferraù, F., Tralongo, P., & Borsellino, N. (2013). Treatment monitoring program for implementation of adherence to second-line erlotinib for advanced non-small-cell lung cancer. Clinical Lung Cancer, 14(4), 390–398. https://doi.org/10.1016/j.cllc.2012.11.007
Given, B. (2016). Oral oncolytic agents—A need for research to enhance patient centeredness? Cancer Nursing, 39(1), 84–85. https://doi.org/10.1097/NCC.00000000000000316
Gönderen Çakmak, H.S., & Kapucu, S. (2021). The effect of educational follow-up with the motivational interview technique on self-efficacy and drug adherence in cancer patients using oral chemotherapy treatment: A randomized controlled trial. Seminars in Oncology Nursing, 37(2), 151140. https://doi.org/10.1016/j.soncn.2021.151140
Greer, J.A., Amoyal, N., Nisotel, L., Fishbein, J.N., MacDonald, J., Stagl, J., . . . Pirl, W.F. (2016). A systematic review of adherence to oral antineoplastic therapies. Oncologist, 21(3), 354–376. https://doi.org/10.1634/theoncologist.2015-0405
Greer, J.A., Jacobs, J.M., Pensak, N., Nisotel, L.E., Fishbein, J.N., MacDonald, J.J., . . . Temel, J.S. (2020). Randomized trial of a smartphone mobile app to improve symptoms and adherence to oral therapy for cancer. Journal of the National Comprehensive Cancer Network, 18(2), 133–141. https://doi.org/10.6004/jnccn.2019.7354
Guyatt, G., Oxman, A.D., Akl, E.A., Kunz, R., Vist, G., Brozek, J., . . . Schünemann, H.J. (2011). GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology, 64(4), 383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
Heiney, S.P., Parker, P.D., Felder, T.M., Adams, S.A., Omofuma, O.O., & Hulett, J.M. (2019). A systematic review of interventions to improve adherence to endocrine therapy. Breast Cancer Research and Treatment, 173(3), 499–510. https://doi.org/10.1007/s10549-018-5012-7
Hendricks, C.B. (2015). Improving adherence with oral antiemetic agents in patients with breast cancer receiving chemotherapy. Journal of Oncology Practice, 11(3), 216–218. https://doi.org/10.1200/JOP.2015.004234
Hershman, D.L., Unger, J.M., Hillyer, G.C., Moseley, A., Arnold, K.B., Dakhil, S.R., . . . Neugut, A.I. (2020). Randomized trial of text messaging to reduce early discontinuation of adjuvant aromatase inhibitor therapy in women with early-stage breast cancer: SWOG S1105. Journal of Clinical Oncology, 38(19), 2122–2129. https://doi.org/10.1200/jco.19.02699
Higgins, J.P., Savović, J., Page, M.J., Elbers, R.G., & Sterne, J.A. (2021). Assessing risk of bias in a randomized trial. In J.P. Higgins & S. Green (Eds.), Cochrane handbook for systematic reviews of interventions version 6.2. Wiley. https://training.cochrane.org/handbook/archive/v6.2/chapter-08
Khandelwal, N., Duncan, I., Ahmed, T., Rubinstein, E., & Pegus, C. (2012). Oral chemotherapy program improves adherence and reduces medication wastage and hospital admissions. Journal of the National Comprehensive Cancer Network, 10(5), 618–625. https://doi.org/10.6004/jnccn.2012.0063
Kim, H.J., Kim, S.M., Shin, H., Jang, J.-S., Kim, Y.I., & Han, D.H. (2018). A mobile game for patients with breast cancer for chemotherapy self-management and quality-of-life improvement: Randomized controlled trial. Journal of Medical Internet Research, 20(10), e273. https://doi.org/10.2196/jmir.9559
King, M.T., Cella, D., Osoba, D., Stockler, M., Eton, D., Thompson, J., & Eisenstein, A. (2010). Meta-analysis provides evidence-based interpretation guidelines for the clinical significance of mean differences for the FACT-G, a cancer-specific quality of life questionnaire. Patient Related Outcome Measures, 1, 119–126. https://doi.org/10.2147/PROM.S10621
Komatsu, H., Yagasaki, K., Yamaguchi, T., Mori, A., Kawano, H., Minamoto, N., . . . Tamura, K. (2020). Effects of a nurse-led medication self-management programme in women with oral treatments for metastatic breast cancer: A mixed-method randomised controlled trial. European Journal of Oncology Nursing, 47, 101780. https://doi.org/10.1016/j.ejon.2020.101780
Krikorian, S., Pories, S., Tataronis, G., Caughey, T., Chervinsky, K., Lotz, M., . . . Weissmann, L. (2019). Adherence to oral chemotherapy: Challenges and opportunities. Journal of Oncology Pharmacy Practice, 25(7), 1590–1598. https://doi.org/10.1177/1078155218800384
Krok-Schoen, J.L., Naughton, M.J., Young, G.S., Moon, J., Poi, M., Melin, S.A., . . . Post, D.M. (2019). Increasing adherence to adjuvant hormone therapy among patients with breast cancer: A smart phone app-based pilot study. Cancer Control, 26(1). https://doi.org/10.1177/1073274819883287
Krolop, L., Ko, Y.D., Schwindt, P.F., Schumacher, C., Fimmers, R., & Jaehde, U. (2013). Adherence management for patients with cancer taking capecitabine: A prospective two-arm cohort study. BMJ Open, 3(7). https://doi.org/10.1136/bmjopen-2013-003139
Lam, M.S.H., & Cheung, N. (2016). Impact of oncology pharmacist-managed oral anticancer therapy in patients with chronic myelogenous leukemia. Journal of Oncology Pharmacy Practice, 22(6), 741–748. https://doi.org/10.1177/1078155215608523
Lin, M., Hackenyos, D., Savidge, N., Weidner, R.A., Murphy-Banks, R., Fleckner, T., . . . Rodday, A.M. (2020). Enhancing patients’ understanding of and adherence to oral anticancer medication: Results of a longitudinal pilot intervention. Journal of Oncology Pharmacy Practice, 27(6), 1409–1421. https://doi.org/10.1177/1078155220960800
Mackler, E., Segal, E.M., Muluneh, B., Jeffers, K., & Carmichael, J. (2019). 2018 Hematology/Oncology Pharmacist Association best practice for the managmeent of oral oncolytic therapy: Pharmacy practice standard. Journal of Oncology Practice, 15(4), e364–e355. https://doi.org/10.1200/JOP.18.00581
Maringwa, J., Quinten, C., King, M., Ringash, J., Osoba, D., Coens, C., . . . Bottomley, A. (2011). Minimal clinically meaningful differences for the EORTC QLQ-C30 and EORTC QLQ-BN20 scales in brain cancer patients. Annals of Oncology, 22(9), 2107–2112. https://doi.org/10.1093/annonc/mdq726
Mauro, J., Mathews, K.B., & Sredzinski, E.S. (2019). Effect of a smart pill bottle and pharmacist intervention on medication adherence in patients with multiple myeloma new to lenalidomide therapy. Journal of Managed Care and Specialty Pharmacy, 25(11), 1244–1254. https://doi.org/10.18553/jmcp.2019.25.11.1244
McClure, N.S., Sayah, F.A., Xie, F., Luo, N., & Johnson, J.A. (2017). Instrument-defined estimates of the minimally important difference for EQ-5D-5L index scores. Value in Health, 20(4), 644–650. https://doi.org/10.1016/j.jval.2016.11.015
McKay, R., Mills, H., Werner, L., Choudhury, A., Choueiri, T., Jacobus, S., . . . Taplin, M.E. (2019). Evaluating a video-based, personalized webpage in genitourinary oncology clinical trials: A phase 2 randomized trial. Journal of Medical Internet Research, 21(5), e12044. https://doi.org/10.2196/12044
Mendoza, T.R., Williams, L.A., Keating, K.N., Siegel, J., Elbi, C., Nowak, A.K., . . . Cleeland, C.S. (2019). Evaluation of the psychometric properties and minimally important difference of the MD Anderson Symptom Inventory for malignant pleural mesothelioma (MDASI-MPM). Journal of Patient-Reported Outcomes, 3(1), 34. https://doi.org/10.1186/s41687-019-0122-5
Middendorff, G., Elsey, R., Lounsbery, B., & Chadwell, R. (2018). Impact of a specialty pharmacy case management service on adherence in patients receiving oral antineoplastic agents. Journal of Oncology Pharmacy Practice, 24(5), 371–378. https://doi.org/10.1177/1078155217708022
Milata, J.L., Otte, J.L., & Carpenter, J.S. (2016). Oral endocrine therapy nonadherence, adverse effects, decisional support, and decisional needs in women with breast cancer. Cancer Nursing, 41(1), E9–E18. https://doi.org/10.1097/NCC.0000000000000430
Mir, O., Ferrua, M., Fourcade, A., Mathivon, D., Duflot-Boukobza, A., Dumont, S.N., . . . Minvielle, E. (2020). Intervention combining nurse navigators (NNs) and a mobile application versus standard of care (SOC) in cancer patients (pts) treated with oral anticancer agents (OAA): Results of CapRI, a single-center, randomized phase III trial. Journal of Clinical Oncology, 38(15, Suppl.), 2000. https://doi.org/10.1200/JCO.2020.38.15_suppl.2000
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D.G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ, 339, b2535. https://doi.org/10.1136/bmj.b2535
Morgan, K.P., Muluneh, B., Deal, A.M., & Amerine, L.B. (2018). Impact of an integrated oral chemotherapy program on patient adherence. Journal of Oncology Pharmacy Practice, 24(5), 332–336. https://doi.org/10.1177/1078155217703792
Muluneh, B., Schneider, M., Faso, A., Amerine, L., Daniels, R., Crisp, B., . . . Savage, S. (2018). Improved adherence rates and clinical outcomes of an integrated, closed-loop, pharmacist-led oral chemotherapy management program. Journal of Oncology Practice, 14(6), e324–e334. https://doi.org/10.1200/jop.17.00039
Musoro, J.Z., Coens, C., Fiteni, F., Katarzyna, P., Cardoso, F., Russell, N.S., . . . Bottomley, A. (2019). Minimally important differences for interpreting EORTC QLQ-C30 scores in patients with advanced breast cancer. JNCI Cancer Spectrum, 3(3), pkz037. https://doi.org/10.1093/jncics/pkz037
Neuss, M.N., Gilmore, T.R., Belderson, K.M., Billett, A.L., Conti-Kalchik, T., Harvey, B.E., . . . Polovich, M. (2016). 2016 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards, including standards for pediatric oncology. Oncology Nursing Forum, 44(1), 31–43. https://doi.org/10.1188/17.ONF.31-43
Neuss, M.N., Polovich, M., McNiff, K., Esper, P., Gilmore, T.R., LeFebvre, K.B., . . . Jacobson, J.O. (2013). 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards including standards for the safe administration and management of oral chemotherapy. Journal of Oncology Practice, 9(2, Suppl.), S5–S13. https://doi.org/10.1200/JOP.2013.000874
Patel, J.M., Holle, L.M., Clement, J.M., Bunz, T., Niemann, C., & Chamberlin, K.W. (2016). Impact of a pharmacist-led oral chemotherapy-monitoring program in patients with metastatic castrate-resistant prostate cancer. Journal of Oncology Pharmacy Practice, 22(6), 777–783. https://doi.org/10.1177/1078155215612541
Ribed, A., Romero-Jiménez, R.M., Escudero-Vilaplana, V., Iglesias-Peinado, I., Herranz-Alonso, A., Codina, C., & Sanjurjo-Sáez, M. (2016). Pharmaceutical care program for onco-hematologic outpatients: Safety, efficiency and patient satisfaction. International Journal of Clinical Pharmacy, 38(2), 280–288. https://doi.org/10.1007/s11096-015-0235-8
Rodriguez, G., Utate, M.A., Joseph, G., & St Victor, T. (2017). Oral chemotherapy adherence: A novel nursing intervention using an electronic health record workflow. Clinical Journal of Oncology Nursing, 21(2), 165–167. https://doi.org/10.1188/17.CJON.165-167
Salgado, T.M., Mackler, E., Severson, J.A., Lindsay, J., Batra, P., Petersen, L., & Farris, K.B. (2017). The relationship between patient activation, confidence to self-manage side effects, and adherence to oral oncolytics: A pilot study with Michigan oncology practices. Supportive Care in Cancer, 25(6), 1797–1807. https://doi.org/10.1007/s00520-017-3584-0
Schneider, S.M., Adams, D.B., & Gosselin, T. (2014). A tailored nurse coaching intervention for oral chemotherapy adherence. Journal of the Advanced Practitioner in Oncology, 5(3), 163–172. https://doi.org/10.6004/jadpro.2014.5.3.2
Sikorskii, A., Given, C.W., Given, B.A., Vachon, E., Krauss, J.C., Rosenzweig, M., . . . Majumder, A. (2018). An automated intervention did not improve adherence to oral oncolytic agents while managing symptoms: Results from a two-arm randomized controlled trial. Journal of Pain and Symptom Management, 56(5), 727–735. https://doi.org/10.1016/j.jpainsymman.2018.07.021
Simons, S., Ringsdorf, S., Braun, M., Mey, U.J., Schwindt, P.F., Ko, Y.D., . . . Jaehde, U. (2011). Enhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical care. Supportive Care in Cancer, 19(7), 1009–1018. https://doi.org/10.1007/s00520-010-0927-5
Sivakumaran, K., Ginex, P.K., Waseem, H., Belcher, S.M., Lagler-Clark, S., LeFebvre K.B., . . . Morgan, R.L. (2022). Domains of structured oral anticancer medication programs: A scoping review. Oncology Nursing Forum, 49(4), 296–306. https://doi.org/10.1188/22.ONF.296-306
Spoelstra, S.L., Given, B.A., Given, C.W., Grant, M., Sikorskii, A., You, M., & Decker, V. (2013). An intervention to improve adherence and management of symptoms for patients prescribed oral chemotherapy agents: An exploratory study. Cancer Nursing, 36(1), 18–28. https://doi.org/10.1097/NCC.0b013e3182551587
Spoelstra, S.L., Given, C.W., Sikorskii, A., Coursaris, C.K., Majumder, A., DeKoekkoek, T., . . . Given, B.A. (2015). Feasibility of a text messaging intervention to promote self-management for patients prescribed oral anticancer agents. Oncology Nursing Forum, 42(6), 647–657. https://doi.org/10.1188/15.ONF.647-657
Spoelstra, S.L., Given, C.W., Sikorskii, A., Coursaris, C.K., Majumder, A., DeKoekkoek, T. . . . Given, B.A. (2016). Proof of concept of a mobile health short message service text message intervention that promotes adherence to oral anticancer agent medications: A randomized controlled trial. Telemedicine and e-Health, 22(6), 497–506. https://doi.org/10.1089/tmj.2015.0126
Spoelstra, S.L., Sikorskii, A., Majumder, A., Burhenn, P.S., Schueller, M., & Given, B. (2017). Oral anticancer agents: An intervention to promote medication adherence and symptom management. Clinical Journal of Oncology Nursing, 21(2), 157–160. https://doi.org/10.1188/17.CJON.157-160
Sterne, J.A., Hernán, M.A., Reeves, B.C., Savović, J., Berkman, N.D., Viswanathan, M., . . . Higgins, J.P. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ, 355, i4919. https://doi.org/10.1136/bmj.i4919
Stokes, M., Reyes, C., Xia, Y., Alas, V., Goertz, H.P., & Boulanger, L. (2017). Impact of pharmacy channel on adherence to oral oncolytics. BMC Health Services Research, 17(1), 414. https://doi.org/10.1186/s12913-017-2373-2
Suttmann, H., Gleissner, J., Huebner, A., Mathes, T., Baurecht, W., Krützfeldt, K., . . . Feyerabend, S. (2020). Adherence measures for patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate plus prednisone: Results of a prospective, cluster-randomized trial. Cancers, 12(9), 2550. https://doi.org/10.3390/cancers12092550
Tschida, S.J., Aslam, S., Lal, L., Khan, T.T., Shrank, W.H., Bhattarai, G., . . . Newcomer, L.N. (2012). Outcomes of a specialty pharmacy program for oral oncology medications. American Journal of Pharmacy Benefits, 4(4), 165–174. https://www.pharmacytimes.com/view/outcomes-of-a-specialty-pharmacy-pro…
Vacher, L., Thivat, E., Poirier, C., Mouret-Reynier, M.-A., Chollet, P., Devaud, H., . . . Chevrier, R. (2020). Improvement in adherence to capecitabine and lapatinib by way of a therapeutic education program. Supportive Care in Cancer, 28(7), 3313–3322. https://doi.org/10.1007/s00520-019-05144-x
Weingart, S.N., Brown, E., Bach, P.B., Eng, K., Johnson, S.A., Kuzel, T.M., . . . Walters, R.S. (2008). NCCN Task Force Report: Oral chemotherapy. Journal of the National Comprehensive Cancer Network, 6(Suppl. 3), S1–S14. https://doi.org/10.6004/jnccn.2008.2003
Weingart, S.N., Li, J.W., Zhu, J., Morway, L., Stuver, S.O., Shulman, L.N., & Hassett, M.J. (2012). US cancer center implementation of ASCO/Oncology Nursing Society Chemotherapy Administration Safety Standards. Journal of Oncology Practice, 8(1), 7–12. https://doi.org/10.1200/JOP.2011.000379
Yost, K.J., & Eton, D.T. (2005). Combining distribution- and anchor-based approaches to determine minimally important differences: The FACIT experience. Evaluation and the Health Professions, 28(2), 172–191. https://doi.org/10.1177/0163278705275340
Yost, K.J., Thompson, C.A., Eton, D.T., Allmer, C., Ehlers, S.L., Habermann, T.M., . . . Cerhan, J.R. (2013). The Functional Assessment of Cancer Therapy–General (FACT-G) is valid for monitoring quality of life in patients with non-Hodgkin lymphoma. Leukemia and Lymphoma, 54(2), 290–297. https://doi.org/10.3109/10428194.2012.711830
Zerbit, J., Chevret, S., Bernard, S., Kroemer, M., Ablard, C., Harel, S., . . . Thieblemont, C. (2020). Improved time to treatment failure and survival in ibrutinib-treated malignancies with a pharmaceutical care program: An observational cohort study. Annals of Hematology, 99(7), 1615–1625. https://doi.org/10.1007/s00277-020-04045-y
Zerillo, J.A., Goldenberg, B.A., Kotecha, R.R., Tewari, A.K., Jacobson, J.O., & Krzyzanowska, M.K. (2018). Interventions to improve oral chemotherapy safety and quality: A systematic review. JAMA Oncology, 4(1), 105–117. https://doi.org/10.1001/jamaoncol.2017.0625
Ziller, V., Kyvernitakis, I., Knöll, D., Storch, A., Hars, O., & Hadji, P. (2013). Influence of a patient information program on adherence and persistence with an aromatase inhibitor in breast cancer treatment: The COMPAS study. BMC Cancer, 13, 407. https://doi.org/10.1186/1471-2407-13-407


