Effects of Physical Exercise Interventions for Individuals With Gynecologic Cancer: A Systematic Review and Meta-Analysis
Problem Identification: Data on the efficacy of physical exercise interventions for individuals with gynecologic cancer are limited and discordant. The purpose of this review was to determine the benefits of exercise interventions in this population.
Literature Search: The PubMed®, Web of Science, Embase® (Ovid), and Cochrane Central Register of Controlled Trials databases were searched for studies published from January 1, 2010, to November 9, 2022.
Data Evaluation: 12 randomized controlled trials were included. A quantitative synthesis method was used to investigate the effects of exercise interventions on individuals with gynecologic cancer.
Synthesis: The findings indicate that physical exercise interventions may have beneficial effects on the fatigue, depression, and health-related quality of life of this patient population. However, because of the small group of studies available, the evidence must be regarded as preliminary.
Implications for Practice: Clinicians and oncology nurses should recommend and refer individuals with gynecologic cancer to clinic- or community-based physical exercise programs.
Jump to a section
Gynecologic cancer, which refers to a malignant condition of the female reproductive system, has become a major public health concern (Uwins et al., 2020). The incidence of various gynecologic cancers is 6.6–13.1 per 100,000 women globally, and the morbidity and mortality rates of gynecologic cancer have been steadily increasing (Ferlay et al., 2019; Jiang et al., 2018). The diagnosis of cancer and a wide range of treatment (e.g., chemotherapy, radiation therapy, surgery) side effects have profound impacts on patients, and they may experience physical and psychological symptom distress such as fatigue, pain, sexual problems, reduced cardiorespiratory fitness (CRF), malnutrition, and depression, which have significant impacts on their health-related quality of life (HRQOL) (Do et al., 2017; Maurer et al., 2022; Pin et al., 2018). The survival rates of gynecologic cancer gradually increase with the improvement of treatment techniques, and healthcare providers should implement some evidence-based interventions to manage these symptoms in clinical practice.
Exercise is a well-planned physical activity that can include aerobic exercise or resistance exercise, both of which aim to improve physical fitness (Campbell et al., 2019). The American College of Sports Medicine recommends that cancer survivors should achieve 150 minutes of moderate-intensity physical exercise or 75 minutes of high-intensity physical exercise per week (Piercy et al., 2018). Based on a number of supporting systematic reviews, exercise and physical activity interventions have the potential to decrease physical and psychological disorders and improve symptom management as well as HRQOL of patients with cancer (Buffart et al., 2017; Michael et al., 2021; Morishita et al., 2020; Wagoner et al., 2021). However, patients with breast cancer, lung cancer, colorectal cancer, and other cancers were predominantly represented in these reviews, with low representation from gynecologic cancer. Given that positive or negative effects of exercise interventions appear differently among various types of patients with cancer and survivors, it is necessary to examine the literature that has focused specifically on this population.
To date, to the authors’ knowledge, few systematic reviews have evaluated the effects of exercise interventions on gynecologic cancer (Lin et al., 2016; Maqbali et al., 2019), and these reviews included a limited randomized design, mixed cancer cohorts, or the same cohorts of participants. More importantly, the current evidence is limited. Considering that a growing number of studies about this topic are emerging constantly and may provide new evidence, making an update on the topic would be advisable. The aims of this systematic review and meta-analysis were to estimate the effects of exercise interventions on fatigue, HRQOL, CRF, and depression in this patient population based on the available evidence from randomized controlled trials (RCTs), thereby helping healthcare professionals make more informed decisions about the uptake of exercise programs in clinical settings.
Methods
This systematic review and meta-analysis was conducted and reported using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Liberati et al., 2009).
Search Strategy
The PubMed®, Web of Science, Embase® (Ovid), and Cochrane Central Register of Controlled Trials databases were searched for related studies from January 1, 2010, to November 9, 2022. The electronic search strategies were conducted by using the following combinations of search terms to find relevant articles: ([title] “cancer*” or “neoplas*” or “tumor” or “carcinoma” or “oncology” or “malignan*”) and ([title] “exercise*” or “training*” or “sport*” or “rehabilitation” or “activit*” or “yoga” or “tai chi” or “tai ji” or “qi gong” or “pilates”) and ([title] “cervi*” or “endometr*” or “uterus” or “uterine” or “ovar*” or “vagina*” or “vulva*” or “fallopian tube” or “genital”). In addition, a manual search of reference lists from relevant reviews and articles was also conducted to find additional studies on the subject.
Study Selection
Studies were included if they met each of the following criteria:
- Study design: prospective RCTs that were published in peer-reviewed journals
- Population: patients (aged 18 years or older) with any type of gynecologic cancer
- Interventions: exercise or physical activity interventions (e.g., walking, running, strength training)
- Comparators: no specific exercise intervention
- Outcomes: The outcomes of interest included fatigue, HRQOL, CRF, and depression; studies should include at least one of these outcomes.
- Language: studies published in English
The exclusion criteria were as follows:
- Conference abstracts, protocols, dissertations, theses, editorial letters, duplicate studies, reviews, and guidelines
- Studies with mixed cancer cohorts
- The same cohorts of participants reported by more than one publication
- Pelvic floor muscle exercise
Two reviewers selected the studies independently. Differing opinions were resolved through discussion with another reviewer.
Data Extraction
The following data were independently extracted from the included studies by two reviewers using Microsoft Excel 2010 database: first author, year published, country, age, sample size, types of cancer, intervention details (e.g., frequency, intensity, type, duration), and evaluated outcomes. Any disagreements in this process were resolved by discussing with a third reviewer to reach a consensus.
Risk-of-Bias Assessment
Study quality was evaluated using the risk-of-bias assessment tool following the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins & Green, 2008), including (a) random sequence generation and allocation concealment (selection bias), (b) blinding of participants and personnel (performance bias), (c) blinding of outcome assessors (detection bias), (d) incomplete outcome data (attrition bias), (e) selective outcome reporting (reporting bias), and (f) other biases. Each potential source of bias was graded as low, high, or unclear. The authors did not exclude poor-quality studies because of the limited number of articles available for inclusion.
Statistical Analysis
RevMan, version 5.3, was used for statistical analyses, and forest plots were constructed to clarify the effect sizes. All data are expressed as mean and SD. Given that some of the included studies had more than one follow-up time point, the data in the forest plots were selected from the first time point after the interventions because the effect sizes immediately after intervention periods are regarded as the most relevant (Frost et al., 2019; Hilfiker et al., 2018). The standard mean difference (SMD) was used because outcomes in this review were measured on different scales (Chang et al., 2013). The I2 statistic was used to assess statistical heterogeneity. The authors set the low, moderate, and high standards to I2 values of 25%, 50%, and 75%, respectively (Higgins et al., 2003). When I2 was greater than 50%, the random effects model was used and a sensitivity analysis was conducted to evaluate the stability of the outcomes. Otherwise, the fixed-effect model was used. The results were calculated as the SMDs with 95% confidence intervals (CIs). SMDs were categorized as small effects (0.2–0.5), moderate effects (0.5–0.8), or large effects (greater than 0.8) (Cohen, 1962). If the p value was less than 0.05, the results were determined to be statistically significant.
Findings
Study Selection
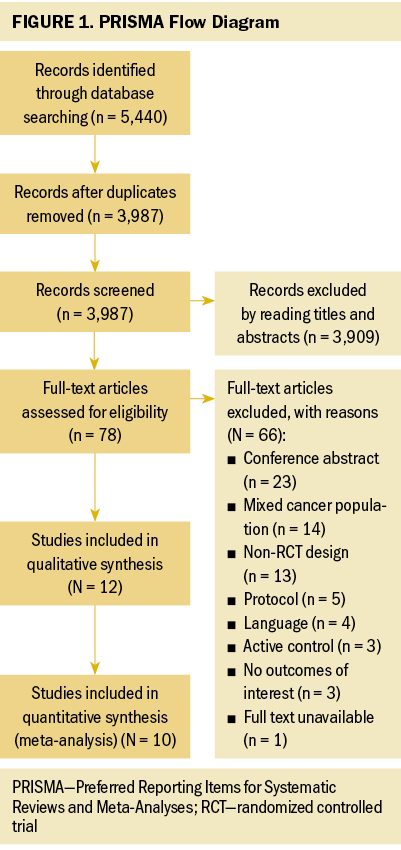
The four database searches retrieved 5,440 articles, which were reduced to 3,987 after excluding duplicate articles. After reading the titles and abstracts, another 3,909 articles were excluded because of irrelevant study issues with intervention. A full-text review was performed for 78 studies; afterward, 66 studies were excluded. The main reasons for exclusion were conference abstract, mixed cancer population, non-RCT design, and protocol. Finally, this systematic review consisted of 12 RCTs (Crawford et al., 2016; Do et al., 2017; Donnelly et al., 2011; Edbrooke et al., 2022; Gorzelitz et al., 2022; Koutoukidis et al., 2019; Lee et al., 2022; Maurer et al., 2022; McCarroll et al., 2014; Rossi et al., 2016; Zhang et al., 2018; Zhou et al., 2017), 2 of which could not be included in meta-analysis (Lee et al., 2022; McCarroll et al., 2014). The flow diagram of the study selection process is shown in Figure 1.
Study Characteristics
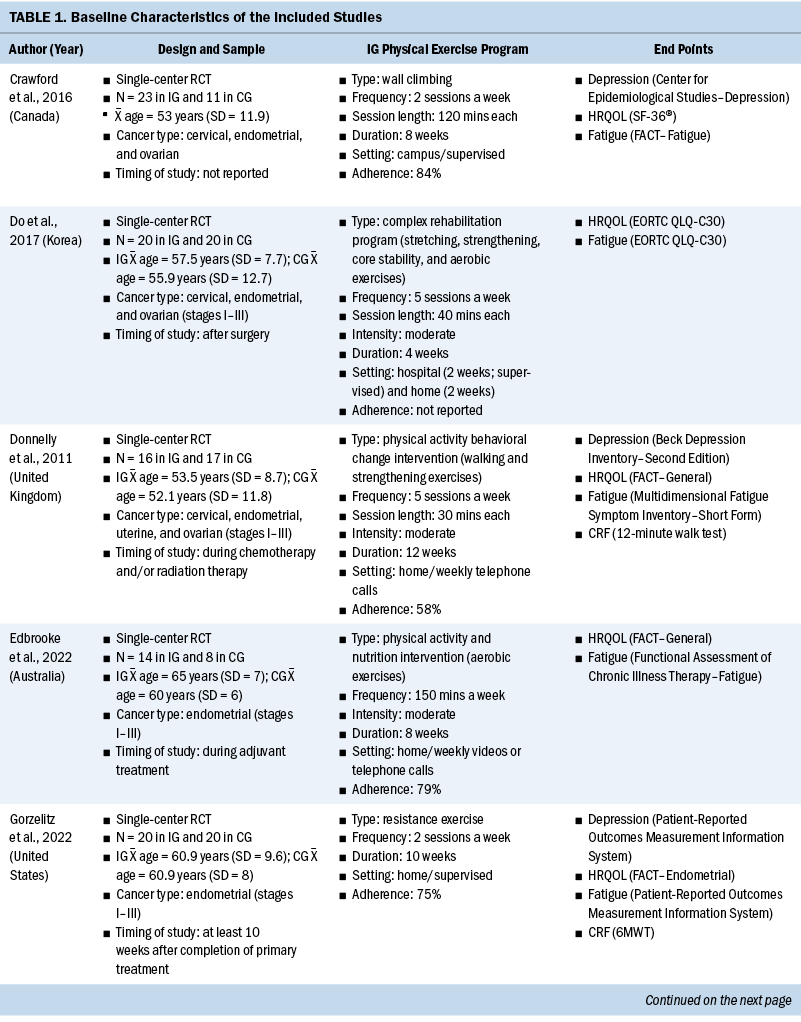
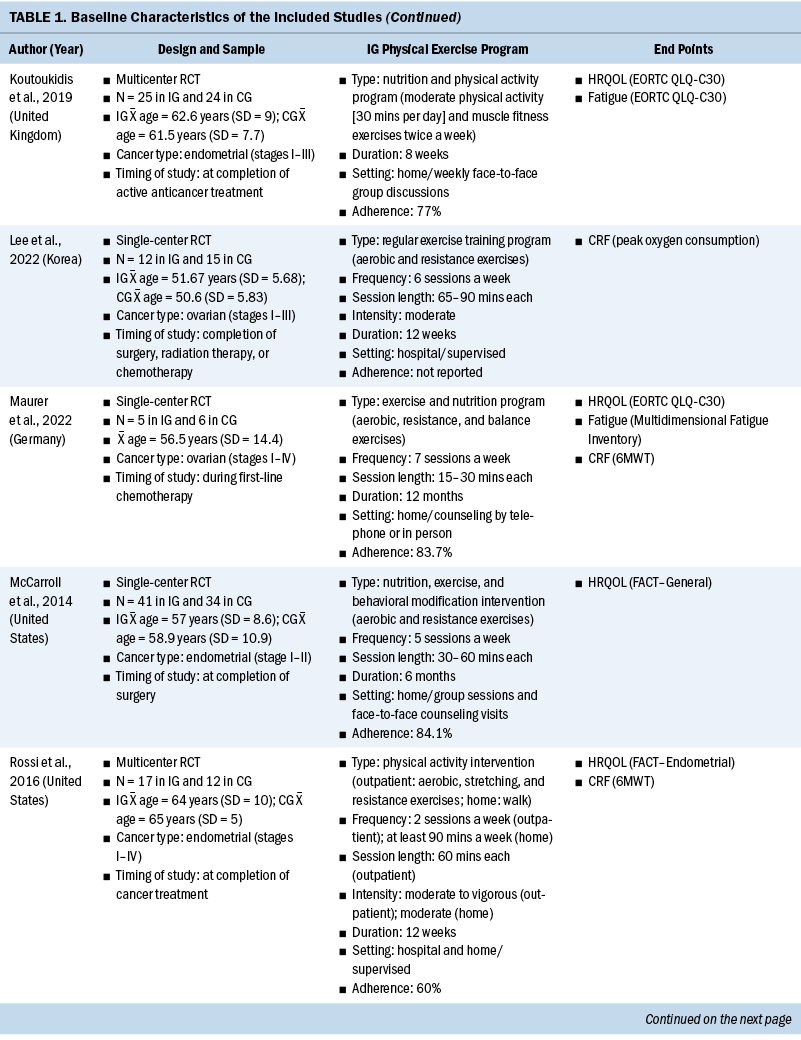
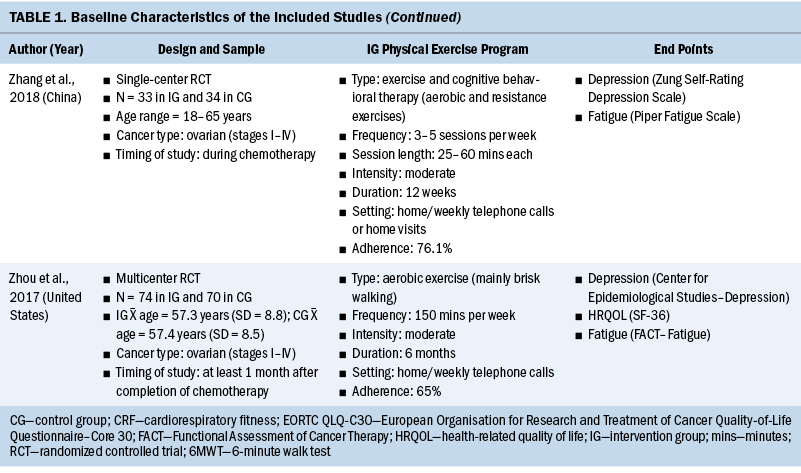
The systematic review included individuals with various gynecologic cancers at different stages of the disease and at different points of treatment, mainly including individuals with ovarian and endometrial cancers. The 12 studies were published from 2011 to 2022. A total of 574 participants were included, and sample sizes ranged from 15 to 144. The average age was 58 years, ranging from 50.6 years (SD = 5.83) to 65 years (SD = 7). Except for one study that did not specify, the majority of studies included participants with stage I–III disease (n = 6), four studies included participants with any stage of disease (stages I–IV), and one study included only participants in the early stages of disease (stages I–II). Among these studies, most of them were conducted in North America (n = 5) and Europe (n = 3).
All included studies used interventions with some form of physical exercise, and most of them were aerobic and/or resistance exercise. Seven studies assessed exercise training alone as an intervention, one study combined physical exercise with a nutritional intervention (Maurer et al., 2022) and one with a psychological intervention (Zhang et al., 2018), and three studies assessed lifestyle interventions with a physical exercise component (Edbrooke et al., 2022; Koutoukidis et al., 2019; McCarroll et al., 2014). Overall, the exercise programs varied in terms of the intensity, frequency, and duration. The duration of the physical exercise prescriptions ranged from 4 weeks to 12 months. In most studies, exercises were conducted two to five days a week, and the intensity was moderate. The exercise programs were conducted in different settings as follows: hospital, outpatient, outdoor, home, or mixed. The intervention was mainly performed after (n = 8) and during (n = 4) anticancer treatment. Among the seven studies that reported safety, only one minor exercise-related adverse event (skin irritation following accelerometer wear) was reported (Edbrooke et al., 2022). Overall, the adherence ranged from 58% to 84.1%. More details of the included studies are summarized in Table 1.
Quality Assessment
Table 2 shows the bias risks of the included studies. Each study reported that the individuals were randomly divided into an experimental group and a control group, but the randomization process was unclear in four studies. Six studies clearly reported the methods for allocation concealment, and the remaining studies had insufficient information to assess. None of the included studies clearly showed that participants were blinded. Because of the nature of the physical exercise interventions, the blinding of participants and personnel is difficult. Five studies reported blinding of outcome assessor, whereas others were either high bias risk or unclear risk regarding this criterion. Five studies reported the implementation of intention-to-treat analysis, indicating that the risk of attrition bias is low. There are seven studies whose study protocols were published online. In the other bias column, five studies were rated as having a high bias risk because of the lack of calculation of sample size.
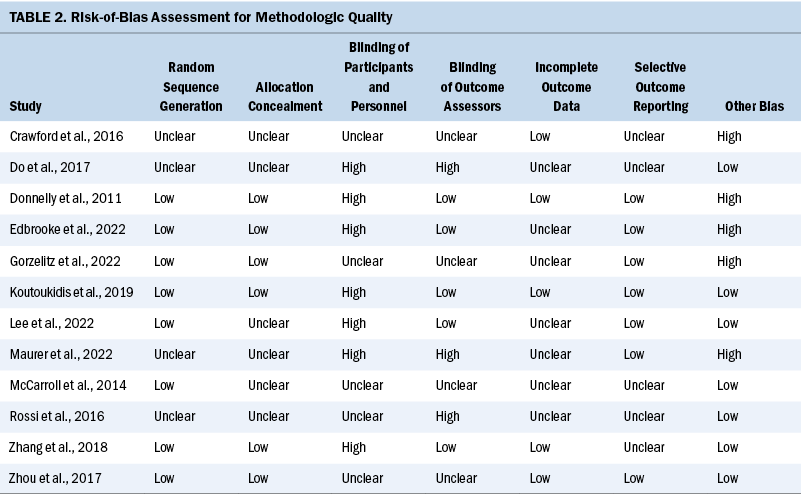
Fatigue
The fatigue outcome was measured in nine studies using different instruments. Table 3 shows that exercise interventions had no significant effect on fatigue when compared to control groups (SMD = –0.5; 95% CI [–1.15, 0.15]; p = 0.13; I2 = 89%). When the authors removed Zhou et al.’s (2017) study, the heterogeneity significantly decreased, which indicated that this study was the source of the heterogeneity. The adjusted pooled estimates changed significantly (SMD = –0.31; 95% CI [–0.55, –0.08]; p = 0.008; I2 = 0%).
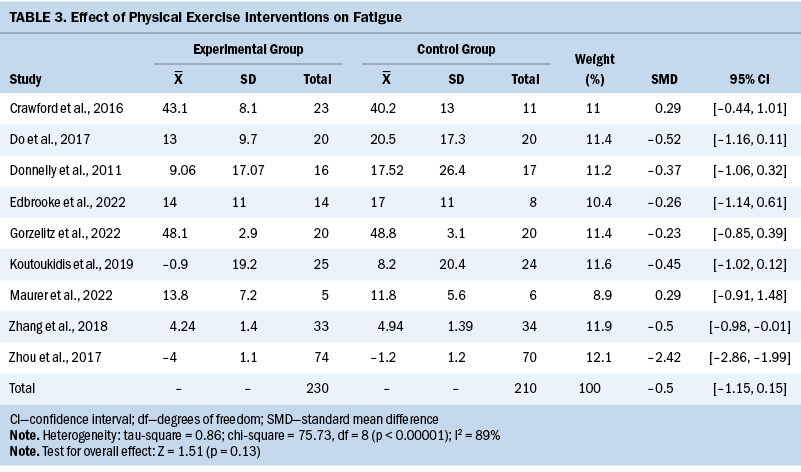
HRQOL
A total of 10 studies investigated global HRQOL, and the data of 9 studies could be included in meta-analysis. Results show that the overall effect of exercise interventions on HRQOL was not significant (SMD = 0.73; 95% CI [–0.24, 1.71]; p = 0.14; I2 = 94%) (see Table 4). By omitting one study (Zhou et al., 2017), the heterogeneity significantly decreased and the adjusted pooled estimates changed (SMD = 0.28; 95% CI [0.04, 0.53]; p = 0.03; I2 = 0%). The remaining study (McCarroll et al., 2014) found that exercise interventions did not significantly improve the HRQOL of patients compared with those who did not engage in exercise during the whole study period (p = not reported).
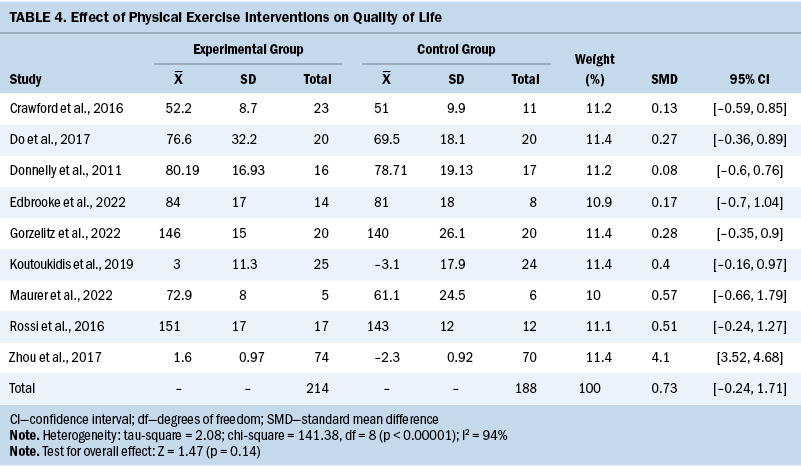
CRF
CRF was measured in five studies. Peak oxygen consumption (VO2peak) was used in one study, and 6-minute walking distance/12-minute walking distance was used in four studies. Using the walking test, the improvement of CRF in the experimental groups was not significant compared to the control groups (SMD = 0.19; 95% CI [–0.18, 0.57]; p = 0.31; I2 = 0%) (see Table 5). The remaining study (Lee et al., 2022) reported that VO2peak in the intervention group was statistically better than that in the control group after the intervention (p = 0.001).
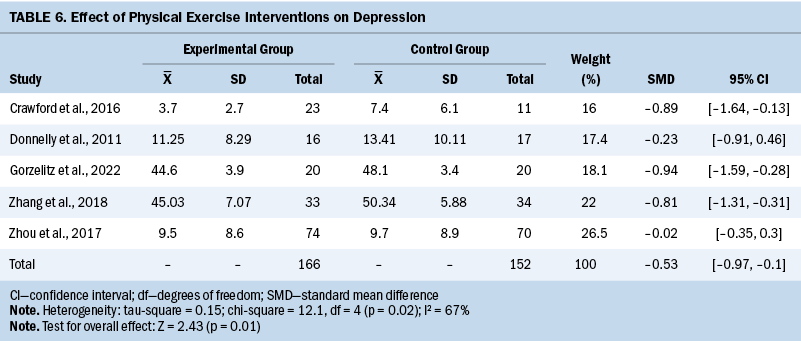
Depression
Five studies assessed depression levels using various questionnaires. Overall, exercise interventions led to an improvement in depression levels compared to the control groups (SMD = –0.53; 95% CI [–0.97, –0.1]; p = 0.01; I2 = 67%) (see Table 6).
Discussion
The goal of the current systematic review was to quantitatively evaluate the effects of physical exercise interventions on some physical and psychological outcomes in individuals with gynecologic cancer. The findings suggest that physical exercises seem safe and have the potential to improve fatigue, HRQOL, and depression. However, no unique effect on CRF measured by walking performance was found. In addition, no major adverse events caused by exercise interventions were found.
Different from fatigue of cancer-free individuals, the fatigue of individuals with cancer is an unusual, persistent, and subjective sense of tiredness related to cancer or cancer-related treatment (Thong et al., 2020). After removing one study (Zhou et al., 2017), the meta-analysis of the remaining eight studies showed that physical exercise interventions had small positive effects on fatigue. This result is similar to some reviews that showed that an increase in physical exercise was an effective strategy to combat fatigue (Wender et al., 2022). The majority of the included studies used aerobic exercise, which appears to combat fatigue by improving one or more of the following health-related fitness parameters: aerobic capacity, muscular strength and endurance, flexibility, and body composition (Hussey & Gupta, 2022). Zhou et al. (2017) reported a significant improvement in fatigue and HRQOL levels after intervention, which was the source of heterogeneity. The participants in their study had greater fatigue and lower HRQOL at baseline, so they could have greater benefit from an exercise intervention. Therefore, screening for participants with high symptom burden may help oncologists identify those who may have greater benefit from starting a physical exercise program.
HRQOL reflects patient-perceived evaluation of their own health, including physical, symptom, emotional, and social dimensions (Fiteni et al., 2016). The authors found that physical exercise interventions can improve global HRQOL, which is clinically important because HRQOL is a significant end point to determine the impact of cancer and its treatment (Duncan et al., 2017). The current authors’ findings are more consistent with the most recent studies, which indicated there was sufficient evidence to conclude that exercise improves HRQOL in cancer survivors (Gerritsen & Vincent, 2016; Sweegers et al., 2018). The mechanism of improving HRQOL by exercise interventions may be that exercise can indirectly change HRQOL by improving physical and physiological functioning. In the study that could not be included in meta-analysis, no significant differences in HRQOL were observed between groups (McCarroll et al., 2014). This result is not surprising given that this study reported higher baseline HRQOL, and it may indicate that the high baseline scores leave little room for an intervention to have much impact (McCarroll et al., 2014).
Cancer itself and cancer-related treatment are associated with the physical decline of patients, which is reflected in the decline of activities of daily living and the low levels of CRF (Manchola-González et al., 2020). During and after cancer treatment, physical exercise can increase plasma volume and muscle mass, improve pulmonary ventilation as well as pulmonary perfusion, and increase cardiac reserve. VO2 peak and walking distance are considered the most widely accepted measures of CRF (Piraux et al., 2018; Scott et al., 2018). Contrary to the current authors’ hypotheses, this review showed that physical exercise interventions had no significant improvement on CRF (walking distance). In fact, walking performance showed a trend of improvement postintervention, but the improvement did not reach statistical significance. Given that some authors of the included studies reported the lack of statistical power inherent within the study, the effectiveness of physical exercise on CRF in gynecologic cancer should be tested in larger-scale studies.
Depression is prevalent among individuals with gynecologic cancer, which may negatively affect their HRQOL. Among this patient population, comorbid conditions and functional limitations were strongly associated with depression (Klapheke et al., 2020). It was reported that individuals who experience poor mental health have poorer physical health and low levels of fitness (Knapen et al., 2015). This review provided evidence of positive effects of physical exercise interventions on depression levels in individuals with gynecologic cancer. Depression is usually considered a mental health problem that is most likely to be positively affected by exercise. The underlying mechanism of exercise’s antidepressant effect is that regular exercise can effectively reshape the central nervous system organization of patients with depression (Thomas et al., 2016). Exercise can also reduce depression by improving participants’ self-efficacy, reducing negative emotions, and stimulating positive behaviors (Jaffery et al., 2017).
Limitations
This review included only RCTs, which represent the gold standard in evaluating the most convincing evidence. However, certain limitations of this study should be acknowledged. First, because of the limited number of included RCTs, the authors did not conduct subgroup analysis based on exercise modes and dosage. Therefore, the optimal dosage and intensity of physical exercise programs for individuals with gynecologic cancer are still unknown. Second, the authors included only studies published in English, so studies published in languages other than English may have been missed. Third, the methodologic quality of some included studies was not high, which could exaggerate clinical improvements. For example, the blinding of the participants and outcome assessors was not implemented adequately. Finally, the long-term effects of the exercise interventions on this patient population remain uncertain, and they require longer study and further attention.
Implications for Nursing
This systematic review was focused on the effects of physical exercise interventions on individuals with gynecologic cancer. Adherence is a key component for any physical exercise intervention. The goal of exercise interventions should be to prescribe the optimal exercise amount for each patient to maximize the intervention effects. Additional studies are still needed to establish the optimal type and dosage (frequency, duration, intensity) of physical exercise for this population. Ideally, healthcare professionals should consider using a robust research design, adequate sample size, and validated instruments. In addition, future studies should not only use self-report questionnaires but also use objective measurements (such as pedometers and accelerometers) to check more outcomes in this population.
Aerobic exercise (e.g., walking, jogging, cycling, swimming) is the most studied exercise traditionally; it forces the body to burn the stored fat for energy, enhances the strength of respiratory muscles, and facilitates the body’s utilization of oxygen. Most of the included studies required patients to do moderate-intensity aerobic exercise. One explanation for the lack of other exercise modalities (such as resistance exercise) could be that aerobic exercise has fewer barriers to participate, which leads to better adherence levels. Resistance exercise uses muscle strength to work against a resistive load, causing isolated and brief activity of single muscle groups, thus increasing the size and strength of muscles. Compared with aerobic exercise, resistance exercise requires greater levels of coordination and sometimes requires the use of specialized facility and/or equipment (e.g., weight machines, barbells, dumbbells), which may make it difficult to effectively administer resistance training to these patients, particularly at home.
Center-based (i.e., inpatient or outpatient settings) exercise interventions and/or interventions requiring direct supervision may be difficult to scale. Therefore, home-based exercise interventions seem to be an attractive alternative. Home-based physical exercise programs can eliminate geographic barriers, make exercise more broadly accessible, and promote the participation of patients who are far away from traditional locations such as hospitals, clinics, community centers, or gyms. In addition, telecoaching systems, which provide individualized coaching via a distance-based resource and offer supervision and support without interacting in person, have been proven to be a key supportive element for home-based physical exercise interventions (Lopez et al., 2020). Oncology nurses should cooperate with physical therapists to integrate physical exercise interventions into routine clinical practice for individuals with gynecologic cancer and consider mHealth-based exercise programs for individuals who are unwilling or unable to exercise in the hospital setting.

Conclusion
Taken together, the current systematic review of 12 RCTs with 574 individuals with gynecologic cancer showed that physical exercise interventions were safe and hold potential for positive fatigue, HRQOL, and depression outcomes. However, the findings should be interpreted with caution because of the small sample sizes of the included studies and the large heterogeneity of the physical exercise program composition, participant characteristics, and measurement tools. More well-designed RCTs with large sample sizes are still needed to make progress in this field of study.
The authors gratefully acknowledge the Affiliated Cancer Hospital of Zhengzhou University for all valuable support.
About the Authors
Rui-Chen Ma, RN, MSN, Xiu-Jie Li, BSN, CNS, and Rui Liu, RN, MSN, are all RNs in the Department of Gynecological Oncology; and Xiao-Xia Xu, PhD, CNS, is a professor and the director of the Department of Nursing, all at the Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital in Zhengzhou, China. No financial relationships to disclose. Ma and Xu contributed to the conceptualization and design. Li and Liu completed the data collection and provided statistical support. Ma and Liu provided the analysis. Ma, Li, and Xu contributed to the manuscript preparation. Xu can be reached at hulibu758@126.com, with copy to ONFEditor@ons.org. (Submitted December 2022. Accepted March 17, 2023.)
References
Buffart, L.M., Kalter, J., Sweegers, M.G., Courneya, K.S., Newton, R.U., Aaronson, N.K., . . . Brug, J. (2017). Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treatment Reviews, 52, 91–104. https://doi.org/10.1016/j.ctrv.2016.11.010
Campbell, K.L., Winters-Stone, K.M., Wiskemann, J., May, A.M., Schwartz, A.L., Courneya, K.S., . . . Schmitz, K.H. (2019). Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Medicine and Science in Sports and Exercise, 51(11), 2375–2390. https://doi.org/10.1249/mss.0000000000002116
Chang, C.-W., Mu, P.-F., Jou, S.-T., Wong, T.-T., & Chen, Y.-C. (2013). Systematic review and meta-analysis of nonpharmacological interventions for fatigue in children and adolescents with cancer. Worldviews on Evidence-Based Nursing, 10(4), 208–217. https://doi.org/10.1111/wvn.12007
Cohen, J. (1962). The statistical power of abnormal-social psychological research: A review. Journal of Abnormal and Social Psychology, 65, 145–153. https://doi.org/10.1037/h0045186
Crawford, J.J., Vallance, J.K., Holt, N.L., Steed, H., & Courneya, K.S. (2016). A phase I/II pilot study assessing the preliminary efficacy of wall climbing for improving posttraumatic growth and quality of life in gynecologic cancer survivors. Mental Health and Physical Activity, 11, 60–66.
Do, J.H., Choi, K.H., Ahn, J.S., & Jeon, J.Y. (2017). Effects of a complex rehabilitation program on edema status, physical function, and quality of life in lower-limb lymphedema after gynecological cancer surgery. Gynecologic Oncology, 147(2), 450–455. https://doi.org/10.1016/j.ygyno.2017.09.003
Donnelly, C.M., Blaney, J.M., Lowe-Strong, A., Rankin, J.P., Campbell, A., McCrum-Gardner, E., & Gracey, J.H. (2011). A randomised controlled trial testing the feasibility and efficacy of a physical activity behavioural change intervention in managing fatigue with gynaecological cancer survivors. Gynecologic Oncology, 122(3), 618–624. https://doi.org/10.1016/j.ygyno.2011.05.029
Duncan, M., Moschopoulou, E., Herrington, E., Deane, J., Roylance, R., Jones, L., . . . Bhui, K. (2017). Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open, 7(11), e015860. https://doi.org/10.1136/bmjopen-2017-015860
Edbrooke, L., Khaw, P., Freimund, A., Carpenter, D., McNally, O., Joubert, L., . . . Denehy, L. (2022). Enhancing lifestyle behaviors in endometrial cancer (ENABLE): A pilot randomized controlled trial. Integrative Cancer Therapies, 21, 15347354211069885. https://doi.org/10.1177/15347354211069885
Ferlay, J., Colombet, M., Soerjomataram, I., Mathers, C., Parkin, D.M., Piñeros, M., . . . Bray, F. (2019). Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer, 144(8), 1941–1953. https://doi.org/10.1002/ijc.31937
Fiteni, F., Anota, A., Westeel, V., & Bonnetain, F. (2016). Methodology of health-related quality of life analysis in phase III advanced non-small-cell lung cancer clinical trials: A critical review. BMC Cancer, 16, 122. https://doi.org/10.1186/s12885-016-2152-1
Frost, R., Bauernfreund, Y., & Walters, K. (2019). Non-pharmacological interventions for depression/anxiety in older adults with physical comorbidities affecting functioning: Systematic review and meta-analysis. International Psychogeriatrics, 31(8), 1121–1136. https://doi.org/10.1017/s1041610218001564
Gerritsen, J.K.W., & Vincent, A.J.P.E. (2016). Exercise improves quality of life in patients with cancer: A systematic review and meta-analysis of randomised controlled trials. British Journal of Sports Medicine, 50(13), 796–803. https://doi.org/10.1136/bjsports-2015-094787
Gorzelitz, J.S., Stoller, S., Costanzo, E., Gangnon, R., Koltyn, K., Dietz, A.T., . . . Cadmus-Bertram, L. (2022). Improvements in strength and agility measures of functional fitness following a telehealth-delivered home-based exercise intervention in endometrial cancer survivors. Supportive Care in Cancer, 30(1), 447–455. https://doi.org/10.1007/s00520-021-06415-2
Higgins, J.P.T., & Green, S. (2008). Cochrane handbook for systematic reviews of interventions: Cochrane book series. Wiley-Blackwell.
Higgins, J.P.T., Thompson, S.G., Deeks, J.J., & Altman, D.G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. https://doi.org/10.1136/bmj.327.7414.557
Hilfiker, R., Meichtry, A., Eicher, M., Nilsson Balfe, L., Knols, R.H., Verra, M.L., & Taeymans, J. (2018). Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: A systematic review incorporating an indirect-comparisons meta-analysis. British Journal of Sports Medicine, 52(10), 651–658. https://doi.org/10.1136/bjsports-2016-096422
Hussey, C., & Gupta, A. (2022). Exercise interventions to combat cancer-related fatigue in cancer patients undergoing treatment: A review. Cancer Investigation, 40(9), 822–838. https://doi.org/10.1080/07357907.2022.2105349
Jaffery, A., Edwards, M.K., & Loprinzi, P.D. (2017). Randomized control intervention evaluating the effects of acute exercise on depression and mood profile: Solomon experimental design. Mayo Clinic Proceedings, 92(3), 480–481. https://doi.org/10.1016/j.mayocp.2016.12.017
Jiang, X., Tang, H., & Chen, T. (2018). Epidemiology of gynecologic cancers in China. Journal of Gynecologic Oncology, 29(1), e7. https://doi.org/10.3802/jgo.2018.29.e7
Klapheke, A.K., Keegan, T.H.M., Ruskin, R., & Cress, R.D. (2020). Depressive symptoms and health-related quality of life in older women with gynecologic cancers. Journal of Geriatric Oncology, 11(5), 820–827. https://doi.org/10.1016/j.jgo.2019.10.001
Knapen, J., Vancampfort, D., Moriën, Y., & Marchal, Y. (2015). Exercise therapy improves both mental and physical health in patients with major depression. Disability and Rehabilitation, 37(16), 1490–1495. https://doi.org/10.3109/09638288.2014.972579
Koutoukidis, D.A., Beeken, R.J., Manchanda, R., Burnell, M., Ziauddeen, N., Michalopoulou, M., . . . Lanceley, A. (2019). Diet, physical activity, and health-related outcomes of endometrial cancer survivors in a behavioral lifestyle program: The diet and exercise in uterine cancer survivors (DEUS) parallel randomized controlled pilot trial. International Journal of Gynecological Cancer, 29(3), 531–540. https://doi.org/10.1136/ijgc-2018-000039
Lee, J.-K., Park, S., & Jee, Y.-S. (2022). Immunoprotecting effects of exercise program against ovarian cancer: A single-blind, randomized controlled trial. Cancers, 14(11), 2808. https://doi.org/10.3390/cancers14112808
Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gøtzsche, P.C., Ioannidis, J.P.A., . . . Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ, 339, b2700. https://doi.org/10.1136/bmj.b2700
Lin, K.-Y., Frawley, H.C., Denehy, L., Feil, D., & Granger, C.L. (2016). Exercise interventions for patients with gynaecological cancer: A systematic review and meta-analysis. Physiotherapy, 102(4), 309–319. https://doi.org/10.1016/j.physio.2016.02.006
Lopez, C., McGarragle, K., Pritlove, C., Jones, J.M., Alibhai, S.M.H., Lenton, E., & Santa Mina, D. (2020). Variability and limitations in home-based exercise program descriptions in oncology: A scoping review. Supportive Care in Cancer, 28(9), 4005–4017. https://doi.org/10.1007/s00520-020-05453-6
Manchola-González, J.D., Bagur-Calafat, C., Girabent-Farrés, M., Serra-Grima, J.R., Álvarez Pérez, R., Garnacho-Castaño, M.V., . . . Ramírez-Vélez, R. (2020). Effects of a home-exercise programme in childhood survivors of acute lymphoblastic leukaemia on physical fitness and physical functioning: Results of a randomised clinical trial. Supportive Care in Cancer, 28(7), 3171–3178. https://doi.org/10.1007/s00520-019-05131-2
Maqbali, M., Hughes, C., Dunwoody, L., Rankin, J.P., Hacker, E.D., & Gracey, J. (2019). Exercise interventions to manage fatigue in women with gynecologic cancer: A systematic review. Oncology Nursing Forum, 46(1), 71–82. https://doi.org/10.1188/19.ONF.71-82
Maurer, T., Belau, M.H., von Grundherr, J., Schlemmer, Z., Patra, S., Becher, H., . . . Chang-Claude, J. (2022). Randomised controlled trial testing the feasibility of an exercise and nutrition intervention for patients with ovarian cancer during and after first-line chemotherapy (BENITA-study). BMJ Open, 12(2), e054091. https://doi.org/10.1136/bmjopen-2021-054091
McCarroll, M.L., Armbruster, S., Frasure, H.E., Gothard, M.D., Gil, K.M., Kavanagh, M.B., . . . von Gruenigen, V.E. (2014). Self-efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): A randomized controlled trial. Gynecologic Oncology, 132(2), 397–402. https://doi.org/10.1016/j.ygyno.2013.12.023
Michael, C.M., Lehrer, E.J., Schmitz, K.H., & Zaorsky, N.G. (2021). Prehabilitation exercise therapy for cancer: A systematic review and meta-analysis. Cancer Medicine, 10(13), 4195–4205. https://doi.org/10.1002/cam4.4021
Morishita, S., Hamaue, Y., Fukushima, T., Tanaka, T., Fu, J.B., & Nakano, J. (2020). Effect of exercise on mortality and recurrence in patients with cancer: A systematic review and meta-analysis. Integrative Cancer Therapies, 19, 1534735420917462. https://doi.org/10.1177/1534735420917462
Piercy, K.L., Troiano, R.P., Ballard, R.M., Carlson, S.A., Fulton, J.E., Galuska, D.A., . . . Olson, R.D. (2018). The physical activity guidelines for Americans. JAMA, 320(19), 2020–2028. https://doi.org/10.1001/jama.2018.14854
Pin, F., Barreto, R., Kitase, Y., Mitra, S., Erne, C.E., Novinger, L.J., . . . Bonetto, A. (2018). Growth of ovarian cancer xenografts causes loss of muscle and bone mass: A new model for the study of cancer cachexia. Journal of Cachexia, Sarcopenia and Muscle, 9(4), 685–700. https://doi.org/10.1002/jcsm.12311
Piraux, E., Caty, G., & Reychler, G. (2018). Effects of preoperative combined aerobic and resistance exercise training in cancer patients undergoing tumour resection surgery: A systematic review of randomised trials. Surgical Oncology, 27(3), 584–594. https://doi.org/10.1016/j.suronc.2018.07.007
Rossi, A., Garber, C.E., Ortiz, M., Shankar, V., Goldberg, G.L., & Nevadunsky, N.S. (2016). Feasibility of a physical activity intervention for obese, socioculturally diverse endometrial cancer survivors. Gynecologic Oncology, 142(2), 304–310. https://doi.org/10.1016/j.ygyno.2016.05.034
Scott, J.M., Zabor, E.C., Schwitzer, E., Koelwyn, G.J., Adams, S.C., Nilsen, T.S., . . . Jones, L.W. (2018). Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: A systematic review and meta-analysis. Journal of Clinical Oncology, 36(22), 2297–2305. https://doi.org/10.1200/jco.2017.77.5809
Sweegers, M.G., Altenburg, T.M., Chinapaw, M.J., Kalter, J., Verdonck-de Leeuw, I.M., Courneya, K.S., . . . Buffart, L.M. (2018). Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. British Journal of Sports Medicine, 52(8), 505–513. https://doi.org/10.1136/bjsports-2017-097891
Thomas, A.G., Dennis, A., Rawlings, N.B., Stagg, C.J., Matthews, L., Morris, M., . . . Johansen-Berg, H. (2016). Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. NeuroImage, 131, 162–170. https://doi.org/10.1016/j.neuroimage.2015.10.090
Thong, M.S.Y., van Noorden, C.J.F., Steindorf, K., & Arndt, V. (2020). Cancer-related fatigue: Causes and current treatment options. Current Treatment Options in Oncology, 21(2), 17. https://doi.org/10.1007/s11864-020-0707-5
Uwins, C., Bhandoria, G.P., Shylasree, T.S., Butler-Manuel, S., Ellis, P., Chatterjee, J., . . . Michael, A. (2020). COVID-19 and gynecological cancer: A review of the published guidelines. International Journal of Gynecological Cancer, 30(9), 1424–1433. https://doi.org/10.1136/ijgc-2020-001634
Wagoner, C.W., Lee, J.T., & Battaglini, C.L. (2021). Community-based exercise programs and cancer-related fatigue: A systematic review and meta-analysis. Supportive Care in Cancer, 29(9), 4921–4929. https://doi.org/10.1007/s00520-021-06135-7
Wender, C.L.A., Manninen, M., & O’Connor, P.J. (2022). The effect of chronic exercise on energy and fatigue states: A systematic review and meta-analysis of randomized trials. Frontiers in Psychology, 13, 907637. https://doi.org/10.3389/fpsyg.2022.907637
Zhang, Q., Li, F., Zhang, H., Yu, X., & Cong, Y. (2018). Effects of nurse-led home-based exercise & cognitive behavioral therapy on reducing cancer-related fatigue in patients with ovarian cancer during and after chemotherapy: A randomized controlled trial. International Journal of Nursing Studies, 78, 52–60. https://doi.org/10.1016/j.ijnurstu.2017.08.010
Zhou, Y., Cartmel, B., Gottlieb, L., Ercolano, E.A., Li, F., Harrigan, M., . . . Irwin, M.L. (2017). Randomized trial of exercise on quality of life in women with ovarian cancer: Women’s activity and lifestyle study in Connecticut (WALC). Journal of the National Cancer Institute, 109(12), djx072.

