Cardiovascular Disease Incidence and Cardiovascular Health Among Diverse Women With Breast and Gynecologic Cancers
Objectives: To examine if racial differences in cardiovascular health (CVH) are associated with cardiovascular disease (CVD) disparities among women with breast and gynecologic cancers.
Sample & Setting: The sample consisted of 252 Black women and 93 White women without a self-reported history of cancer or CVD who developed a breast or gynecologic malignancy. Women who developed CVD before their cancer diagnosis were excluded.
Methods & Variables: CVH was classified using metrics of the American Heart Association’s Life’s Simple 7 framework. Metrics were summed to create a total CVH score (0–7). Associations among race, ideal CVH (score of 5–7), and CVD incidence following cancer diagnosis were estimated with Cox proportional hazards models.
Results: Ideal CVH was similar between Black women (33%) and White women (37%). Race and CVH were not associated with CVD incidence.
Implications for Nursing: In a small sample of women diagnosed with breast and gynecologic cancers, racial disparities in CVH and CVD incidence were not observed. Additional investigation of potential confounders relating to social determinants of health tied to the construct of race is warranted.
Jump to a section
Individuals with cancer experience a higher risk of developing cardiovascular disease (CVD) after diagnosis. Large studies conducted with U.K. and U.S. populations have demonstrated increased risks of several CVD outcomes, such as heart failure and coronary artery disease, among cancer survivors compared with individuals without a cancer diagnosis (Armenian et al., 2016; Strongman et al., 2019). These increased risks are likely related to receipt of cardiotoxic cancer treatments (Greenlee et al., 2022; Okwuosa et al., 2017; Sutton et al., 2023) and etiologic factors common to cancer and CVD (Koene et al., 2016). In studies that examine sex-specific risks of CVD following a cancer diagnosis, elevated CVD risk following a breast cancer diagnosis is well established (Florido et al., 2022; Gernaat et al., 2017), with some evidence of increased CVD risk following a diagnosis of endometrial or ovarian cancer (Anderson et al., 2022; Felix et al., 2017; Soisson et al., 2018; Strongman et al., 2019). There are also known CVD mortality disparities among Black women with and without breast cancer (Williams et al., 2023), as well as compared to White women (Gallicchio et al., 2017; Lu et al., 2016; Troeschel et al., 2019). Although CVD represents an important burden among women with breast and gynecologic cancers, the factors associated with racial differences in CVD among women with breast and gynecologic cancers are unknown. It is important to gain clarity about such factors to support additional research toward the elimination of racial disparities in CVD, particularly related to risk of cardiotoxicity associated with targeted cancer therapies, among breast and gynecologic cancer survivors (Chen et al., 2021).
Importantly, in 2010, the American Heart Association (AHA) outlined goals for reaching ideal cardiovascular health (CVH) through the Life’s Simple 7 (LS7) framework (Lloyd-Jones et al., 2010). Biometric and lifestyle factors, such as body mass index (BMI), physical activity, smoking status, total cholesterol, diet, blood glucose, and blood pressure comprise this framework. Multiethnic cohorts including Black people (Dong et al., 2012; Ommerborn et al., 2016; Polonsky et al., 2017) and studies focused specifically on Black people (Ommerborn et al., 2016) showed that better CVH is associated with significantly lower risk of CVD and cancer (Lloyd-Jones et al., 2010; Ogunmoroti et al., 2016; Rasmussen-Torvik et al., 2013). However, Black women are less likely to meet LS7 metrics for CVH compared to White, Chinese American, and Hispanic women (Bambs et al., 2011; Joseph et al., 2019; Mujahid et al., 2017). Still, it is currently unknown if and to what extent racial differences in ideal CVH in women with gynecologic and breast cancer may contribute to CVD disparities.
Among breast and endometrial cancer survivors, Black women tend to have a significantly higher number of CVD risk factors (e.g., hypertension, diabetes, obesity) and worse overall survival compared to White women (Du & Song, 2023; Florido et al., 2022; Gallicchio et al., 2017; Ruterbusch et al., 2014). Using data from 8,641 women enrolled in the Women’s Health Initiative, Simon et al. (2018) reported no racial differences in associations between cardiometabolic risk factors and death from CVD, breast cancer, or other causes. Although cardiometabolic diseases, including hypertension, diabetes, and obesity, are prevalent in women with cancer, prevention and treatment of CVD-related risk factors are limited in this population (Price et al., 2023; Weaver et al., 2013).
Despite the preventable and treatable nature of comorbidities, many women with cancer report that their healthcare providers do not effectively communicate with them about managing their CVD risk factors (Christian et al., 2017). In addition, many oncologists and other healthcare providers acknowledge that they may not address CVH in routine follow-up (Kelley et al., 2019; Stump et al., 2019). Identification of comorbid risk factors affecting disparate survival provides an opportunity to target interventions to reduce CVH disparities and improve survival, which supports the goal of cardio-oncology to reduce, prevent, and manage CVD in patients with cancer (Zamorano et al., 2016). Beyond traditional cardio-oncology practice and its focus on exercise and pharmacologic management of comorbid disease, the LS7 framework may provide a communicable metric to consider CVH and address CVD risk factors during the entire cancer continuum, including diagnosis, treatment, and long-term survivorship (Denlinger et al., 2018; Mehta et al., 2018; Zamorano et al., 2016). This study examined if racial differences in the association between CVH and CVD risk exist in a sample of women with breast and gynecologic cancers, noting that women with gynecologic cancers have not been the major focus of other analyses.
Methods
Study Population and Design
This study used data from the Southern Community Cohort Study (SCCS), a large prospective cohort study of about 85,000 men and women aged 40–79 years, enrolled between 2002 and 2009 (Signorello et al., 2010). The SCCS was approved by institutional review boards at Vanderbilt University and Meharry Medical College in Nashville, Tennessee. Participants were recruited via mail-based sampling of individuals in 12 states in the southeastern United States or via community health center–based primary care providers catering to underserved populations. At study enrollment, participants completed a self-administered questionnaire (general population participants) or a standardized computer-assisted personal interview (community health center participants) to assess information on demographic, socioeconomic, anthropometric, lifestyle, and personal medical history (Signorello et al., 2010).
Analysis was limited to Black and White women without a self-reported baseline history of cancer or CVD who subsequently developed one of the following cancers (identified through linkage with state cancer registries) during follow-up as identified by the International Classification of Diseases (ICD-10) coding (World Health Organization, 2011): breast, ovary, uterus, cervix, or cancers of other female genital organs without developing CVD before the cancer diagnosis (N = 676). In addition, the analysis was restricted to women who were either aged 65 years or older at the time of SCCS enrollment, or aged younger than age 65 years at enrollment and either reported being covered by Medicaid or Medicare on the baseline questionnaire or had a Centers for Medicare and Medicaid Services (CMS) claim within 90 days of SCCS enrollment (N = 421). These requirements increased the probability that participants had continuous coverage in Medicare or Medicaid from the time of cohort enrollment to the time that CVD incidence was ascertained (Akwo et al., 2017). Individuals without information relevant to all seven LS7 metrics and several covariates of interest (N = 59) were excluded from the study, as well as patients with no follow-up time after cancer diagnosis (N = 17). The final sample (N = 345) consisted of 93 White women and 252 Black women diagnosed with breast or gynecologic cancer.
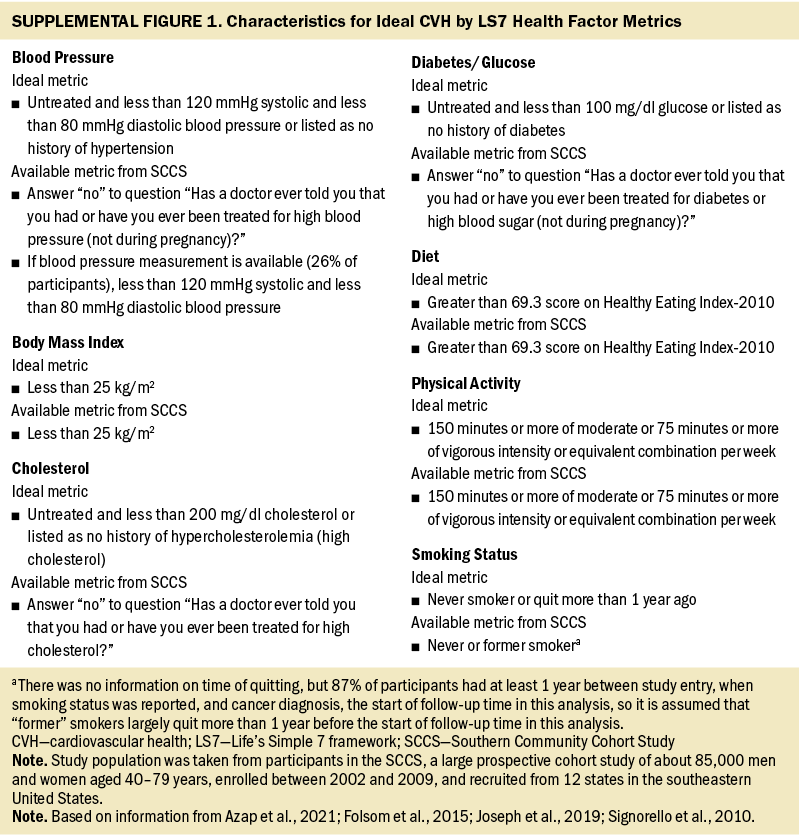
Ideal CVH and other covariates: All demographic and epidemiologic factors, comprising age at cohort entry; household income; education; insurance; marital status; smoking status; BMI; presence of hypertension, diabetes, or hypercholesterolemia; and depression, were collected via self-report at the time of entry into the SCCS cohort. CVH was categorized using the following seven baseline variables: smoking status, BMI, Healthy Eating Index-2010 (indicator of healthy diet), physical activity, presence of hypercholesterolemia (indicator of total cholesterol), presence of hypertension (indicator of blood pressure), and presence of diabetes (indicator of blood glucose) (Joseph et al., 2019). Each factor was categorized as not ideal (0 points) or ideal (1 point) based on the AHA guidelines (Azap et al., 2021) (see Supplemental Figure 1 online). Scores on these seven predictors were summed to generate a total CVH score (0–7), and CVH was categorized as nonideal (0–4) or ideal (5–7) based on cut points commonly used in the literature (Folsom et al., 2015).
CVD ascertainment: CVD diagnoses made after the breast or gynecologic cancer diagnosis in SCCS participants were confirmed after the study began by linking electronic health records to CMS Research Identifiable Files. Diagnoses of CVD were tracked and defined in this study according to participants’ medical claims that used the following ICD-10 codes:
- I00–I09; I11; I13; I20–I51: diseases of heart
- I60–I69: cerebrovascular disease
- I70: atherosclerosis
- I71: aortic aneurysm
- I72–I78: other diseases of arteries, arterioles, or capillaries
These codes were identified through review of various Medicare and Medicaid reviews, base files, and claims files made from the date of enrollment in SCCS.
Statistical Analysis
Baseline characteristics of the study population were compared according to race and CVH using Wilcoxon rank-sum tests for continuous variables and chi-square tests for categorical variables. Time between cancer diagnosis and incident CVD was computed for analyses of incident CVD risk. Among women who did not develop CVD, the end of follow-up was calculated using the last measured date in CMS. Kaplan–Meier estimates and Cox proportional hazards models were used to compare survival distributions according to race and CVH category, individually and combined. Unadjusted and multivariable-adjusted Cox models were both fit, and the multivariable models were adjusted for time from study entry to cancer diagnosis, age at cancer diagnosis, marital status, education, household income, insurance, depression, cancer site, stage, grade, and treatment type (surgery, radiation therapy, chemotherapy, and hormone treatment). The missing indicator method was used for missing data for the stage, grade, and treatment type variables.
Various sensitivity analyses were performed. In each setting, the tables and figures were rerun to assess for differences from the main analysis. First, patients with missing values on one or two of the LS7 metrics were included; for these patients, scores were rescaled and rounded to the 0–7 CVH LS7 scale, and categories (ideal or nonideal) were assigned. Second, because assessments of diabetes, hypercholesterolemia, and hypertension were based on self-report, and individuals may have had these conditions without knowing it, analyses were repeated based on an alternate CVH score using only the other four LS7 components (with ideal CVH defined as a score of 3–4 of 4). Third, because hypertensive disease is a potential CVD outcome according to the CMS codes, as well as a component of the LS7 assessment, it was not included as a CVD outcome in the main analysis. However, hypertension without heart disease (ICD-10: I10, I12) was included as a CVD outcome in this sensitivity analysis. SAS, version 9.4, and R, version 4.1.1, were used for statistical analyses. All p values were two-sided, and a p value of less than 0.05 was considered statistically significant.
Results
Study Population
Table 1 shows baseline, tumor, and LS7 characteristics of the study population according to race and CVH categorization. Relative to White women, Black women tended to be younger at enrollment, be younger at cancer diagnosis, have a lower household income, more frequently do not live with a partner, and less commonly report a diagnosis of depression. In terms of tumor characteristics, Black women tended to have cancers of higher stage and grade. Surgery, radiation therapy, and chemotherapy were used as treatments at similar rates among Black and White women, but hormone treatment was used somewhat less frequently among Black women compared with White women. Most demographic characteristics did not differ substantially by CVH category, except that individuals with more than a high school education more frequently had ideal CVH, and hormone treatment was less common among those with ideal CVH.
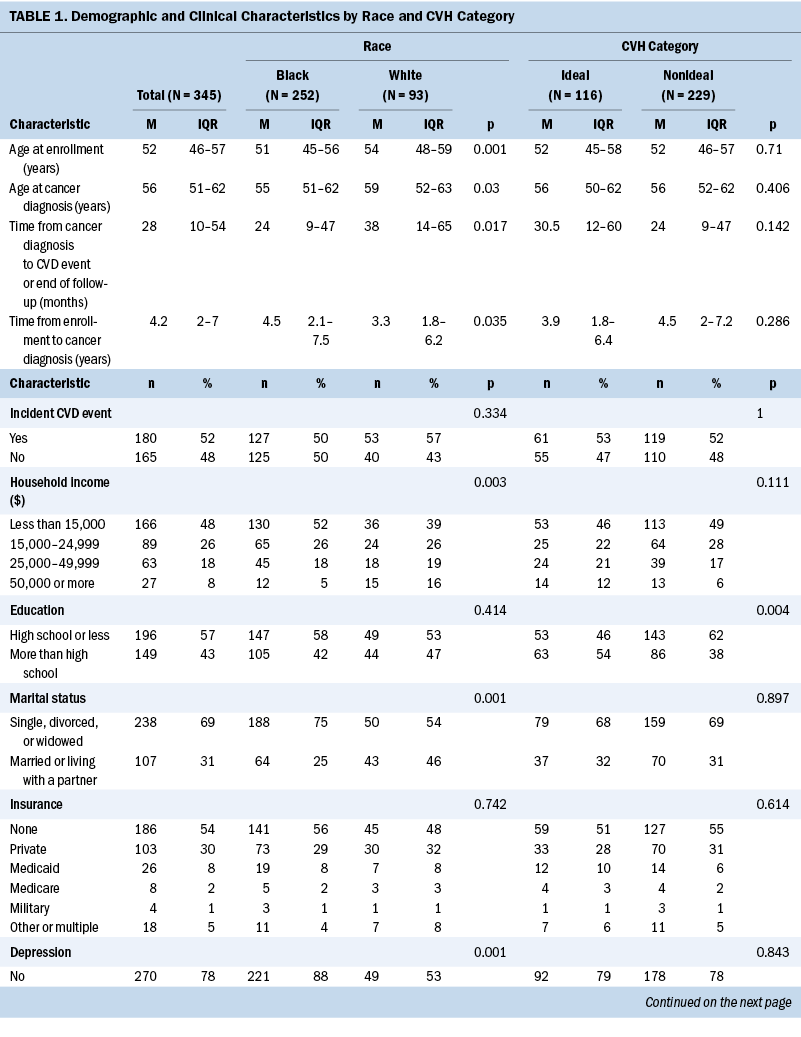
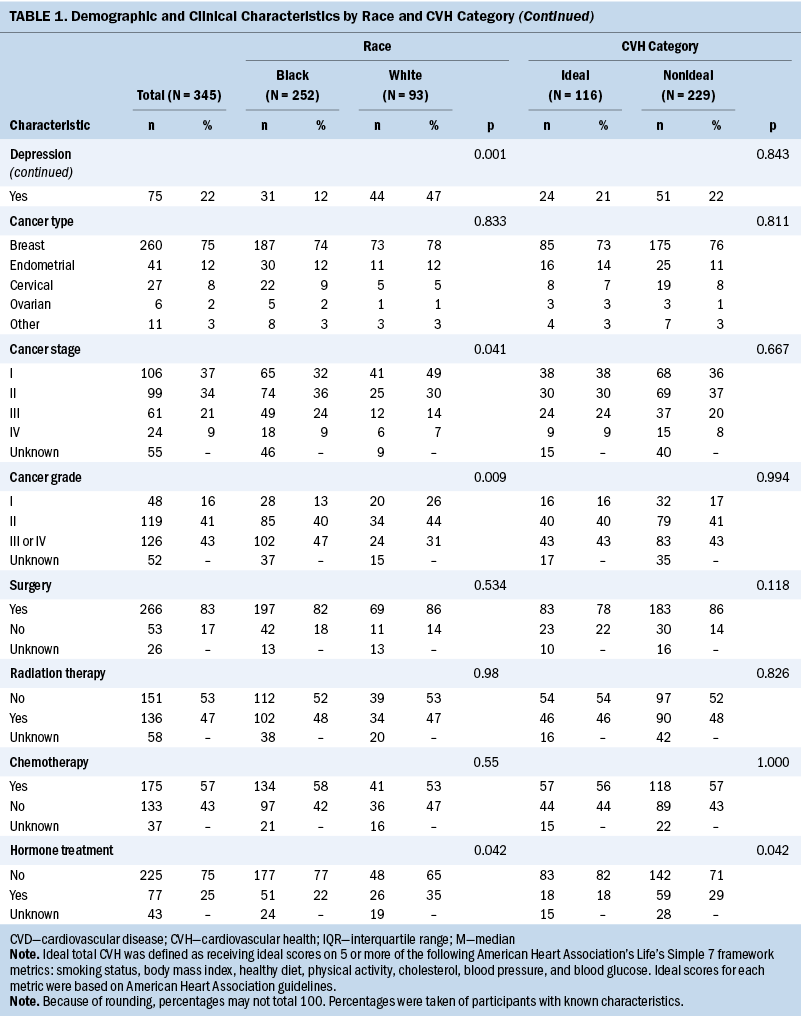
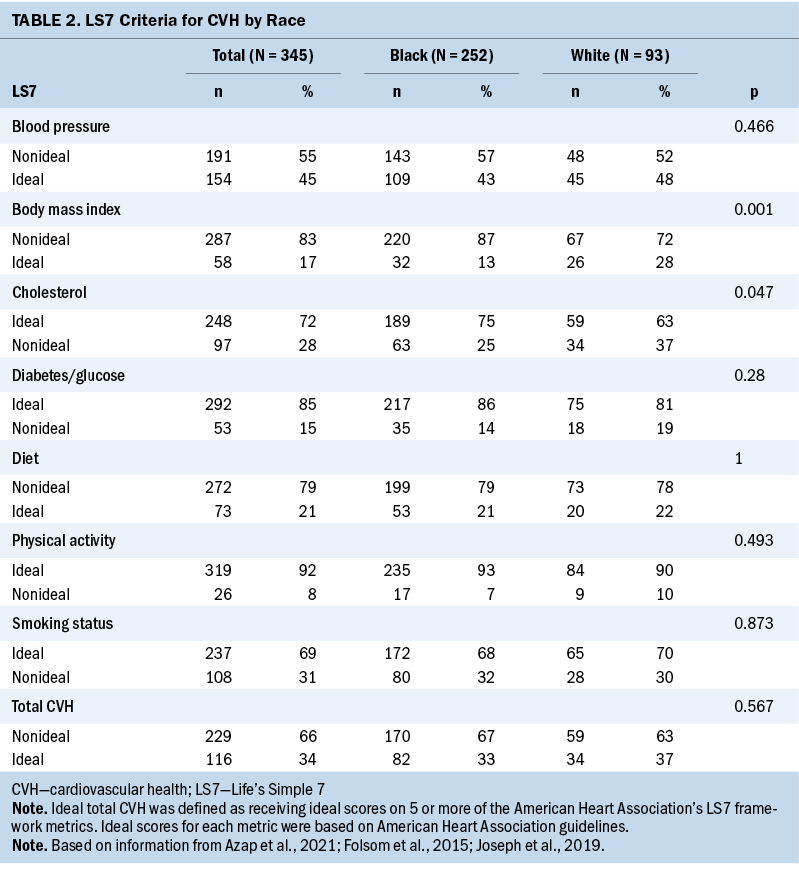
Focusing on the LS7 metrics, Black women had higher BMI and lower rates of reported hypercholesterolemia; other metrics of the LS7 were not statistically different between races (see Table 2). The distribution of nonideal versus ideal CVH was not statistically different between Black and White women.
Race, CVH, and CVD Risk
Figure 1 displays Kaplan–Meier curves for time from cancer diagnosis to incident CVD, separately by race, by CVH category, and by race within CVH categories. In these unadjusted comparisons, race was not significantly associated with differential CVD risk (log-rank p values > 0.05).
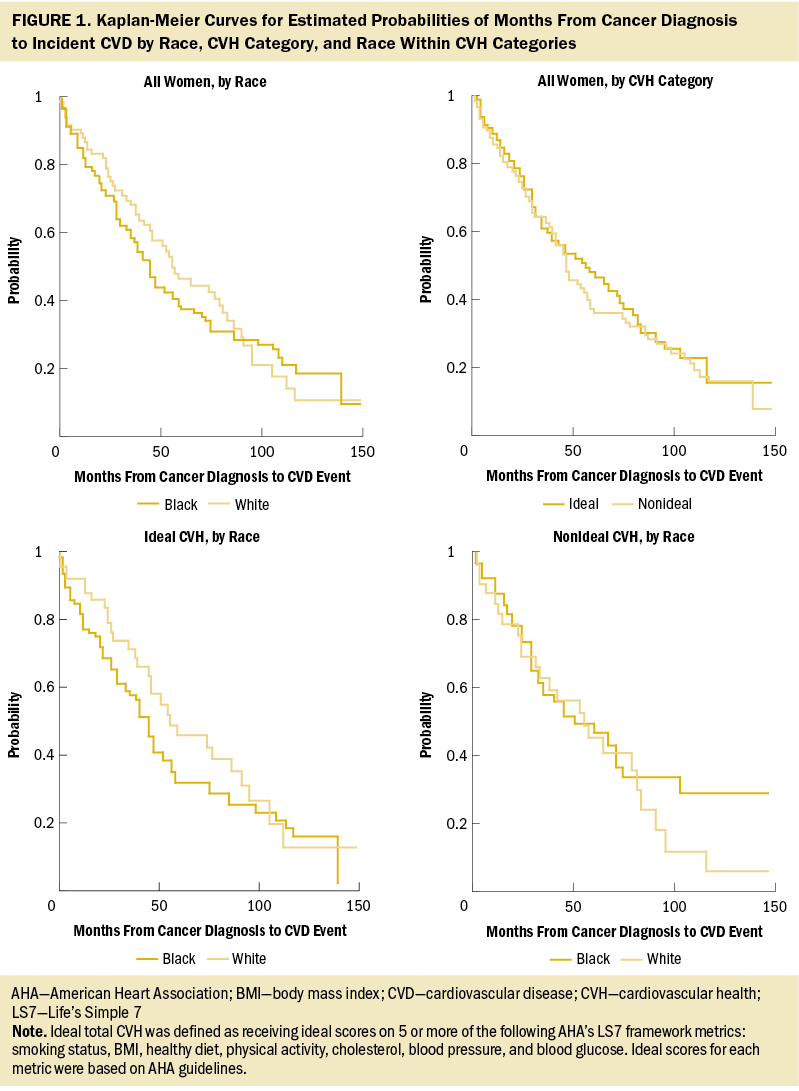
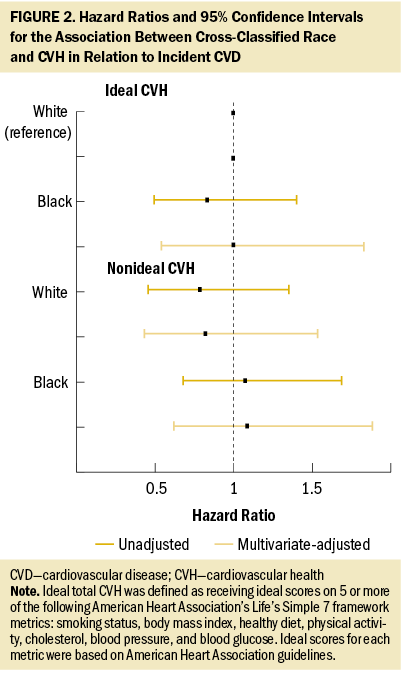
In a multivariable Cox regression model, neither race (hazard ratio [HR] = 1.14, 95% confidence interval [CI] [0.83, 1.57]) nor CVH (HR = 1.11, 95% CI [0.81, 1.51]) was independently associated with CVD risk (data not tabled). Figure 2 shows associations for cross-classified race and CVH groups from unadjusted and multivariable-adjusted models. Using White women with ideal CVH as the reference category, no significant differences in CVD risk were observed for Black women with ideal CVH (HR = 1, 95% CI [0.54, 1.83]), White women with nonideal CVH (HR = 0.82, 95% CI [0.44, 1.54]), or Black women with nonideal CVH (HR = 1.09, 95% CI [0.63, 1.89]).
Sensitivity Analyses
First, individuals with data for only five or six of the LS7 metrics were included in the first sensitivity analysis, with CVH scores rescaled and rounded to classify them as having nonideal or ideal CVH, which increased the sample size by 34. The results were similar to the main analysis. Second, CVH categories were classified based on only the BMI, diet, physical activity, and smoking status LS7 metrics. The results were very similar to the main analysis. Third, hypertension (ICD-10: I10, I12) was added to the types of CVD events that could be counted as incident CVD. The relationship between race and this new definition was similar, but the relationship between CVH according to the full set of LS7 metrics and CVD incidence was stronger (unadjusted HR = 1.4, 95% CI [1.07, 1.83]), perhaps driven by the 103 individuals with hypertension at baseline who were classified as having nonideal CVH and who went on to experience hypertensive disease as an outcome (47 individuals did not report hypertension at baseline and developed hypertensive disease; 15 individuals were hypertensive at baseline but still categorized as ideal CVH and later had hypertensive disease as an outcome). Otherwise, the main results were similar, although the nominal HRs for the cross-classification of race and CVH were more expected, in that Black and White women with nonideal CVH were at higher risk of incident CVD.
Discussion
In this sample of Black and White women diagnosed with breast or gynecologic cancers, no significant differences in ideal CVH by race were observed. In addition, the relationship between CVH category and CVD did not significantly differ by race. These findings, although not statistically significant, indicate a need for additional research into the roles of other potential influences stemming from race as a social construct, including racism, discrimination, and healthcare mistrust, that were not captured in this study’s analyses. More work is needed to elucidate pathways that drive CVH disparities and social vulnerabilities in Black women with a history of breast and gynecologic cancers toward the elimination of these disparities.
The finding of no differences in CVH category by race contrasts with previous studies of women without cancer. In the Women’s Health Initiative, among study participants without cancer, there was a significant difference in the attainment of ideal LS7 metrics by race and ethnicity, in that Black women were less likely to present with ideal CVH compared to White and Hispanic women (Foraker et al., 2016). CVD disparities among Black individuals are facilitated by a high prevalence of multiple risk factors, such as obesity, hypertension, and diabetes mellitus (Coughlin et al., 2015; Kurian & Cardarelli, 2007; Williams et al., 2020), in addition to the interaction of social and behavioral health factors (Min et al., 2017), which are metrics within the LS7 framework. The higher prevalence of comorbid conditions at the time of a cancer diagnosis contributes to not only CVD disparities, but also to cancer survivorship disparities (Jemal et al., 2018; Silber et al., 2013). In addition, there is evidence that poorer CVH affects survival in Black women with cancer in a racially disparate manner. In a study of 1,582 cancer survivors (394 breast cancer survivors; 372 Black Americans) that assessed CVH on five metrics (BMI, physical activity, smoking status, hypertension, and diabetes), Black cancer survivors were more likely to report having more CVD risk factors compared to White cancer survivors in adjusted models (Weaver et al., 2013). Other studies have also reported that Black women with poor CVH had significantly higher odds of developing CVD over time. In an electronic health records analysis of 203,488 men and women from eight health systems, there were noted racial and ethnic disparities between White and non-White patients. Findings illustrated that ideal CVH improved from 2012 to 2015 for White patients, but declined among non-White patients (Rudy et al., 2019). Black women with and without cancer experience a higher CVD burden than their White counterparts (Garcia et al., 2016) and experience a higher prevalence of negative social determinants of health, which account for a small portion of this burden that may not be modifiable (Mujahid et al., 2017).
A growing body of literature indicates that assessing racial and ethnic background without the acknowledgment of institutional or structural racism is inadequate (Panza et al., 2019). For example, it is well documented that allostatic load (cumulative life stressors inducing psychoneural cascades that ultimately influence CVD) is higher in Black Americans compared to their White counterparts (Chyu & Upchurch, 2011; McEwen, 1998; Moore et al., 2022), and a study of weathering expands this concept to include structural stressors like discrimination and racism (Geronimus et al., 2006). Others have reported Black Americans having higher odds of CVD compared to White Americans not only because of the previously mentioned clinical risk factors but also in association with environmental pollutants like particulate matter and neighborhood factors that are more concentrated in areas where Black people live (Peters, 2005; Sung et al., 2023). Unfortunately, the current study could not examine this line of inquiry. Additional study of this phenomenon should include interrogation of CVH and CVD risk with attention to other social determinants of health tied to the construct of race.
Efforts to activate and engage large numbers of Black people to attain ideal CVH with the goal of reducing disparities in CVD and cancer are vastly needed. In particular, for Black women who may present with lower CVH at the time of and following a cancer diagnosis, cardio-oncology guidelines suggest a multimodal approach to cardiovascular rehabilitation (Gilchrist et al., 2019). Given the ability to easily characterize CVH with the components of LS7, early intervention applying cultural humility to targeted education and rehabilitation may reduce future burden of CVD among women with breast and gynecologic cancers (Cousin et al., 2021; Williams & Anyimukwu, 2020). Studies identifying best practices to enable and motivate patients most vulnerable to CVD after a cancer diagnosis to attain ideal CVH is warranted. Community-based participatory research practices are promising, but, to date, have been limited in their application among this specific population (Brewer et al., 2017; Elgazzar et al., 2020; Israel et al., 2010).
Limitations
Findings from this study should be taken with considerations. A major limitation is the low overall small sample size of Black and White women who developed a breast or gynecologic cancer diagnosis with no history of CVD; in particular, it may be limited by the small number of White women (n = 93, 34 with ideal CVH). Other limitations include lack of granular cancer treatment data (because many common treatments are cardiotoxic); lack of data on ideal metrics to characterize CVH; the characterization of glucose, cholesterol, and blood pressure by diagnosis rather than biometric data; and the potential for nondifferential misclassification resulting from coding errors. Results may be biased because of availability of data from those who survived their diagnoses. Despite these limitations, this study addressed a unique question using the LS7 framework as a model to categorize ideal CVH among a diverse sample of cancer survivors. In addition, the longitudinal data allowed for an exploration of how a cancer diagnosis and existing CVH may be associated with a future CVD diagnosis in a diverse sample.
Implications for Nursing
Although the results of this study did not show racial disparities in CVH or CVD incidence, they showed that the majority of breast and gynecologic cancer survivors have nonideal CVH. Because nonideal CVH and a cancer history are associated with CVD incidence and mortality, nurses have a responsibility to teach patients with cancer about risk reduction techniques (e.g., physical activity, healthy diet) and to assess for and alleviate barriers to engaging in risk reduction. Future nursing research is necessary to elucidate potential confounders relating to the social determinants of health, including race as a social construct.
Conclusion
In summary, Black breast and gynecologic cancer survivors, in comparison to White breast and gynecologic cancer survivors, did not experience elevated risk of CVD despite CVH status. Given that available data showed no significant differences between the groups, additional investigation of other potential confounders related to the social determinants of health tied to the construct of race is warranted.
About the Authors
Timiya S. Nolan, PhD, APRN-CNP, ANP-BC, is an associate professor in the Department of Medicine in the Marnix E. Heersink School of Medicine in the Division of Preventive Medicine and the associate director of community outreach and engagement at the O’Neal Comprehensive Cancer Center, both at the University of Alabama at Birmingham; Jennifer A. Sinnott, PhD, is an associate professor in the Department of Statistics, Jessica L. Krok-Schoen, PhD, is an assistant professor in the School of Health and Rehabilitation Sciences in the College of Medicine, and Elizabeth K. Arthur, PhD, APRN-CNP, AOCNP®, is a nurse scientist in the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, all at the Ohio State University in Columbus; Emily Ridgway-Limle, MPH, MS, APRN-CNP, is a nurse practitioner at the Columbus Public Health Ben Franklin Tuberculosis Program; Darrell M. Gray II, MD, MPH, is the founder of Gray Area Strategies, LLC, in Columbus, OH; Daniel Addison, MD, is an associate professor in the Department of Internal Medicine in the Division of Cardiovascular Medicine in the College of Medicine at the Ohio State University Wexner Medical Center and the director of the Cardio-Oncology Program in the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute at the Ohio State University; Sakima Smith, MD, MPH, is an investigator in the Dorothy M. Davis Heart and Lung Research Institute and an associate professor in the Department of Internal Medicine in the Division of Cardiovascular Medicine in the College of Medicine at the Ohio State University Wexner Medical Center; Karen Patricia Williams, PhD, is a Nursing Distinguished Professor of Women’s Health and the inaugural executive director of the Martha S. Pitzer Center for Women, Children, and Youth in the College of Nursing and Darryl B. Hood, PhD, is a professor in the Division of Environmental Health Sciences in the College of Public Health, both at the Ohio State University; Joshua J. Joseph, MD, MPH, is an associate professor in the Department of Internal Medicine in the Division of Endocrinology, Diabetes, and Metabolism in the College of Medicine at the Ohio State University Wexner Medical Center; and Ashley Felix, PhD, is an associate professor in the Division of Epidemiology in the College of Public Health at the Ohio State University. This research was funded, in part, by a National Cancer Institute award (K08CA245208 and L60CA264455; principal investigator: Nolan). Southern Community Cohort Study data collection was performed by the Survey and Biospecimen Shared Resource, which is supported, in part, by the Vanderbilt-Ingram Cancer Center (P30 CA68485). Nolan, Sinnott, Krok-Schoen, Arthur, Ridgway-Limle, Gray, and Joseph contributed to the conceptualization and design. Sinnott provided statistical support. Nolan, Sinnott, Gray, Addison, Hood, and Joseph provided the analysis. Nolan, Sinnott, Krok-Schoen, Arthur, Ridgway-Limle, Gray, Smith, Williams, Hood, Joseph, and Felix contributed to the manuscript preparation. Nolan can be reached at tsnolan@uabmc.edu, with copy to ONFEditor@ons.org. (Submitted June 2023. Accepted November 29, 2023.)
References
Akwo, E.A., Kabagambe, E.K., Wang, T.J., Harrell, F.E., Jr., Blot, W.J., Mumma, M., . . . Lipworth, L. (2017). Heart failure incidence and mortality in the Southern Community Cohort Study. Circulation: Heart Failure, 10(3), e003553. https://doi.org/10.1161/circheartfailure.116.003553
Anderson, C., Olshan, A.F., Bae-Jump, V.L., Brewster, W.R., Lund, J.L., & Nichols, H.B. (2022). Cardiovascular disease diagnoses among older women with endometrial cancer. Gynecologic Oncology, 167(1), 51–57. https://doi.org/10.1016/j.ygyno.2022.08.014
Armenian, S.H., Xu, L., Ky, B., Sun, C., Farol, L.T., Pal, S.K., . . . Chao, C. (2016). Cardiovascular disease among survivors of adult-onset cancer: A community-based retrospective cohort study. Journal of Clinical Oncology, 34(10), 1122–1130. https://doi.org/10.1200/JCO.2015.64.0409
Azap, R.A., Nolan, T.S., Gray, D.M., II, Lawson, K., Gregory, J., Capers, Q.T., IV, . . . Joseph, J.J. (2021). Association of socioeconomic status with ideal cardiovascular health in Black men. Journal of the American Heart Association, 10(23), e020184. https://doi.org/10.1161/jaha.120.020184
Bambs, C., Kip, K.E., Dinga, A., Mulukutla, S.R., Aiyer, A.N., & Reis, S.E. (2011). Low prevalence of “ideal cardiovascular health” in a community-based population: The heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation, 123(8), 850–857. https://doi.org/10.1161/circulationaha.110.980151
Brewer, L.C., Balls-Berry, J.E., Dean, P., Lackore, K., Jenkins, S., & Hayes, S.N. (2017). Fostering African-American Improvement in Total Health (FAITH!): An application of the American Heart Association’s Life’s Simple 7™ among Midwestern African-Americans. Journal of Racial and Ethnic Health Disparities, 4(2), 269–281. https://doi.org/10.1007/s40615-016-0226-z
Chen, D.H., Tyebally, S., Malloupas, M., Roylance, R., Spurrell, E., Raja, F., & Ghosh, A.K. (2021). Cardiovascular disease amongst women treated for breast cancer: Traditional cytotoxic chemotherapy, targeted therapy, and radiation therapy. Current Cardiology Reports, 23(3), 16. https://doi.org/10.1007/s11886-021-01446-x
Christian, A.H., O’Malley, D., Barac, A., Miller, S.M., & Hudson, S.V. (2017). Cardiovascular risk and communication among early stage breast cancer survivors. Patient Education Counseling, 100(7), 1360–1366. https://doi.org/10.1016/j.pec.2017.02.010
Chyu, L., & Upchurch, D.M. (2011). Racial and ethnic patterns of allostatic load among adult women in the United States: Findings from the National Health and Nutrition Examination Survey 1999–2004. Journal of Women’s Health, 20(4), 575–583. https://doi.org/10.1089/jwh.2010.2170
Coughlin, S.S., Yoo, W., Whitehead, M.S., & Smith, S.A. (2015). Advancing breast cancer survivorship among African-American women. Breast Cancer Research and Treatment, 153(2), 253–261. https://doi.org/10.1007/s10549-015-3548-3
Cousin, L., Roper, N., & Nolan, T.S. (2021). Cardio-oncology health disparities: Social determinants of health and care for Black breast cancer survivors. Clinical Journal of Oncology Nursing, 25(5), 36–41. https://doi.org/10.1188/21.CJON.s1.36-41
Denlinger, C.S., Sanft, T., Baker, K.S., Broderick, G., Demark-Wahnefried, W., Friedman, D.L., . . . Freedman-Cass, D.A. (2018). Survivorship, version 2.2018, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network, 16(10), 1216–1247. https://doi.org/10.6004/jnccn.2018.0078
Dong, C., Rundek, T., Wright, C.B., Anwar, Z., Elkind, M.S.V., & Sacco, R.L. (2012). Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across Whites, Blacks, and Hispanics: The Northern Manhattan Study. Circulation, 125(24), 2975–2984. https://doi.org/10.1161/circulationaha.111.081083
Du, X.L., & Song, L. (2023). Age and racial disparities in the utilization of anticancer, antihypertension, and anti-diabetes therapies, and in mortality in a large population-based cohort of older women with breast cancer. Journal of Racial and Ethnic Health Disparities, 10(1), 446–461. https://doi.org/10.1007/s40615-022-01235-4
Elgazzar, R., Nolan, T.S., Joseph, J.J., Aboagye-Mensah, E.B., Azap, R.A., & Gray, D.M., II. (2020). Community-engaged and community-based participatory research to promote American Heart Association Life’s Simple 7 among African American adults: A systematic review. PLOS ONE, 15(9), e0238374. https://doi.org/10.1371/journal.pone.0238374
Felix, A.S., Bower, J.K., Pfeiffer, R.M., Raman, S.V., Cohn, D.E., & Sherman, M.E. (2017). High cardiovascular disease mortality after endometrial cancer diagnosis: Results from the Surveillance, Epidemiology, and End Results (SEER) database. International Journal of Cancer, 140(3), 555–564. https://doi.org/10.1002/ijc.30470
Florido, R., Daya, N.R., Ndumele, C.E., Koton, S., Russell, S.D., Prizment, A., . . . Selvin, E. (2022). Cardiovascular disease risk among cancer survivors: The Atherosclerosis Risk in Communities (ARIC) study. Journal of the American College of Cardiology, 80(1), 22–32. https://doi.org/10.1016/j.jacc.2022.04.042
Folsom, A.R., Shah, A.M., Lutsey, P.L., Roetker, N.S., Alonso, A., Avery, C.L., . . . Solomon, S.D. (2015). American Heart Association’s Life’s Simple 7: Avoiding heart failure and preserving cardiac structure and function. American Journal of Medicine, 128(9), 970–976.e2. https://doi.org/10.1016/j.amjmed.2015.03.027
Foraker, R.E., Abdel-Rasoul, M., Kuller, L.H., Jackson, R.D., Van Horn, L., Seguin, R.A., . . . Tindle, H.A. (2016). Cardiovascular health and incident cardiovascular disease and cancer: The Women’s Health Initiative. American Journal of Preventive Medicine, 50(2), 236–240. https://doi.org/10.1016/j.amepre.2015.07.039
Gallicchio, L., Calhoun, C., Riseberg, D., & Helzlsouer, K. (2017). Cardiovascular health among Black and White breast cancer patients initiating aromatase inhibitor therapy. Breast Journal, 23(2), 206–209. https://doi.org/10.1111/tbj.12709
Garcia, M., Mulvagh, S.L., Merz, C.N.B., Buring, J.E., & Manson, J.E. (2016). Cardiovascular disease in women: Clinical perspectives. Circulation Research, 118(8), 1273–1293. https://doi.org/10.1161/circresaha.116.307547
Gernaat, S.A.M., Ho, P.J., Rijnberg, N., Emaus, M.J., Baak, L.M., Hartman, M., . . . Verkooijen, H.M. (2017). Risk of death from cardiovascular disease following breast cancer: A systematic review. Breast Cancer Research and Treatment, 164(3), 537–555. https://doi.org/10.1007/s10549-017-4282-9
Geronimus, A.T., Hicken, M., Keene, D., & Bound, J. (2006). “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health, 96(5), 826–833. https://doi.org/10.2105/ajph.2004.060749
Gilchrist, S.C., Barac, A., Ades, P.A., Alfano, C.M., Franklin, B.A., Jones, L.W., . . . Wright, J.S. (2019). Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: A scientific statement from the American Heart Association. Circulation, 139(21), e997–e1012. https://doi.org/10.1161/CIR.0000000000000679
Greenlee, H., Iribarren, C., Rana, J.S., Cheng, R., Nguyen-Huynh, M., Rillamas-Sun, E., . . . Kwan, M.L. (2022). Risk of cardiovascular disease in women with and without breast cancer: The Pathways Heart Study. Journal of Clinical Oncology, 40(15), 1647–1658. https://doi.org/10.1200/JCO.21.01736
Israel, B.A., Coombe, C.M., Cheezum, R.R., Schulz, A.J., McGranaghan, R.J., Lichtenstein, R., . . . Burris, A. (2010). Community-based participatory research: A capacity-building approach for policy advocacy aimed at eliminating health disparities. American Journal of Public Health, 100(11), 2094–2102. https://doi.org/10.2105/ajph.2009.170506
Jemal, A., Robbins, A.S., Lin, C.C., Flanders, W.D., DeSantis, C.E., Ward, E.M., & Freedman, R.A. (2018). Factors that contributed to Black–White disparities in survival among nonelderly women with breast cancer between 2004 and 2013. Journal of Clinical Oncology, 36(1), 14–24. https://doi.org/10.1200/jco.2017.73.7932
Joseph, J.J., Bennett, A., Echouffo Tcheugui, J.B., Effoe, V.S., Odei, J.B., Hidalgo, B., . . . Carson, A.P. (2019). Ideal cardiovascular health, glycaemic status and incident type 2 diabetes mellitus: The REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Diabetologia, 62(3), 426–437. https://doi.org/10.1007/s00125-018-4792-y
Kelley, M., Foraker, R., Lin, E.-J.D., Kulkarni, M., Lustberg, M., & Weaver, K.E. (2019). Oncologists’ perceptions of a digital tool to improve cancer survivors’ cardiovascular health. ACI Open, 3(2), e78–e87. https://doi.org/10.1055/s-0039-1696732
Koene, R.J., Prizment, A.E., Blaes, A., & Konety, S.H. (2016). Shared risk factors in cardiovascular disease and cancer. Circulation, 133(11), 1104–1114. https://doi.org/10.1161/circulationaha.115.020406
Kurian, A.K., & Cardarelli, K.M. (2007). Racial and ethnic differences in cardiovascular disease risk factors: A systematic review. Ethnicity and Disease, 17, 143–152.
Lloyd-Jones, D.M., Hong, Y., Labarthe, D., Mozaffarian, D., Appel, L.J., Van Horn, L., . . . Rosamond, W.D. (2010). Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic impact goal through 2020 and beyond. Circulation, 121(4), 586–613. https://doi.org/10.1161/circulationaha.109.192703
Lu, Y., Ezzati, M., Rimm, E.B., Hajifathalian, K., Ueda, P., & Danaei, G. (2016). Sick populations and sick subpopulations: Reducing disparities in cardiovascular disease between Blacks and Whites in the United States. Circulation, 134(6), 472–485. https://doi.org/10.1161/circulationaha.115.018102
McEwen, B.S. (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44. https://doi.org/10.1111/j.1749-6632.1998.tb09546.x
Mehta, L.S., Watson, K.E., Barac, A., Beckie, T.M., Bittner, V., Cruz-Flores, S., . . . Volgman, A.S. (2018). Cardiovascular disease and breast cancer: Where these entities intersect: A scientific statement from the American Heart Association. Circulation, 137(8), e30–e66. https://doi.org/10.1161/cir.0000000000000556
Min, Y.-I., Anugu, P., Butler, K.R., Hartley, T.A., Mwasongwe, S., Norwood, A.F., . . . Correa, A. (2017). Cardiovascular disease burden and socioeconomic correlates: Findings from the Jackson Heart Study. Journal of the American Heart Association, 6(8). https://doi.org/10.1161/jaha.116.004416
Moore, J.X., Andrzejak, S.E., Bevel, M.S., Jones, S.R., & Tingen, M.S. (2022). Exploring racial disparities on the association between allostatic load and cancer mortality: A retrospective cohort analysis of NHANES, 1988 through 2019. SSM Population Health, 19, 101185. https://doi.org/10.1016/j.ssmph.2022.101185
Mujahid, M.S., Moore, L.V., Petito, L.C., Kershaw, K.N., Watson, K., & Diez Roux, A.V. (2017). Neighborhoods and racial/ethnic differences in ideal cardiovascular health (The Multi-Ethnic Study of Atherosclerosis). Health and Place, 44, 61–69. https://doi.org/10.1016/j.healthplace.2017.01.005
Ogunmoroti, O., Allen, N.B., Cushman, M., Michos, E.D., Rundek, T., Rana, J.S., . . . Nasir, K. (2016). Association between Life’s Simple 7 and noncardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association, 5(10), e003954. https://doi.org/10.1161/jaha.116.003954
Okwuosa, T.M., Anzevino, S., & Rao, R. (2017). Cardiovascular disease in cancer survivors. Postgraduate Medical Journal, 93(1096), 82–90. https://doi.org/10.1136/postgradmedj-2016-134417
Ommerborn, M.J., Blackshear, C.T., Hickson, D.A., Griswold, M.E., Kwatra, J., Djoussé, L., & Clark, C.R. (2016). Ideal cardiovascular health and incident cardiovascular events: The Jackson Heart Study. American Journal of Preventive Medicine, 51(4), 502–506. https://doi.org/10.1016/j.amepre.2016.07.003
Panza, G.A., Puhl, R.M., Taylor, B.A., Zaleski, A.L., Livingston, J., & Pescatello, L.S. (2019). Links between discrimination and cardiovascular health among socially stigmatized groups: A systematic review. PLOS ONE, 14(6), e0217623. https://doi.org/10.1371/journal.pone.0217623
Peters, A. (2005). Particulate matter and heart disease: Evidence from epidemiological studies. Toxicology and Applied Pharmacology, 207(2, Suppl.), 477–482. https://doi.org/10.1016/j.taap.2005.04.030
Polonsky, T.S., Ning, H., Daviglus, M.L., Liu, K., Burke, G.L., Cushman, M., . . . Lloyd-Jones, D.M. (2017). Association of cardiovascular health with subclinical disease and incident events: The Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association, 6(3). https://doi.org/10.1161/jaha.116.004894
Price, S., Perkins, S., Berini, C., & Diaz, V. (2023). Perception and counseling for cardiac health in breast cancer survivors. Annals of Family Medicine, 21(Suppl. 1), 3760. https://doi.org/10.1370/afm.21.s1.3760
Rasmussen-Torvik, L.J., Shay, C.M., Abramson, J.G., Friedrich, C.A., Nettleton, J.A., Prizment, A.E., & Folsom, A.R. (2013). Ideal cardiovascular health is inversely associated with incident cancer: The Atherosclerosis Risk in Communities Study. Circulation, 127(12), 1270–1275. https://doi.org/10.1161/circulationaha.112.001183
Rudy, J.E., Khan, Y., Bower, J.K., Patel, S., & Foraker, R.E. (2019). Cardiovascular health trends in electronic health record data (2012–2015): A cross-sectional analysis of the Guideline Advantage™. EGEMS, 7(1), 30. https://doi.org/10.5334/egems.268
Ruterbusch, J.J., Ali-Fehmi, R., Olson, S.H., Sealy-Jefferson, S., Rybicki, B.A., Hensley-Alford, S., . . . Cote, M.L. (2014). The influence of comorbid conditions on racial disparities in endometrial cancer survival. American Journal of Obstetrics and Gynecology, 211(6), 627.E1–627.E9. https://doi.org/10.1016/j.ajog.2014.06.036
Signorello, L.B., Hargreaves, M.K., & Blot, W.J. (2010). The Southern Community Cohort Study: Investigating health disparities. Journal of Health Care for the Poor and Underserved, 21(1, Suppl.), 26–37. https://doi.org/10.1353/hpu.0.0245
Silber, J.H., Rosenbaum, P.R., Clark, A.S., Giantonio, B.J., Ross, R.N., Teng, Y., . . . Fox, K.R. (2013). Characteristics associated with differences in survival among Black and White women with breast cancer. JAMA, 310(4), 389–397. https://doi.org/10.1001/jama.2013.8272
Simon, M.S., Beebe-Dimmer, J.L., Hastert, T.A., Manson, J.E., Cespedes Feliciano, E.M., Neuhouser, M.L., . . . Caan, B. (2018). Cardiometabolic risk factors and survival after breast cancer in the Women’s Health Initiative. Cancer, 124(8), 1798–1807. https://doi.org/10.1002/cncr.31230
Soisson, S., Ganz, P.A., Gaffney, D., Rowe, K., Snyder, J., Wan, Y., . . . Hashibe, M. (2018). Long-term cardiovascular outcomes among endometrial cancer survivors in a large, population-based cohort study. Journal of the National Cancer Institute, 110(12), 1342–1351.https://doi.org/10.1093/jnci/djy070
Strongman, H., Gadd, S., Matthews, A., Mansfield, K.E., Stanway, S., Lyon, A.R., . . . Bhaskaran, K. (2019). Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: A population-based cohort study using multiple linked UK electronic health records databases. Lancet, 394(10203), 1041–1054. https://doi.org/10.1016/s0140-6736(19)31674-5
Stump, T.K., Robinson, J.K., Yanez, B., Penedo, F., Ezeofor, A., Kircher, S., & Spring, B. (2019). Physicians’ perspectives on medication adherence and health promotion among cancer survivors. Cancer, 125(23), 4319–4328. https://doi.org/10.1002/cncr.32410
Sung, H., Hyun, N., Ohman, R.E., Yang, E.H., Siegel, R.L., & Jemal, A. (2023). Mediators of Black–White inequities in cardiovascular mortality among survivors of 18 cancers in the USA. International Journal of Epidemiology, dyad097. https://doi.org/10.1093/ije/dyad097
Sutton, A.L., Felix, A.S., Wahl, S., Franco, R.L., Leicht, Z., Williams, K.P., . . . Sheppard, V.B. (2023). Racial disparities in treatment-related cardiovascular toxicities amongst women with breast cancer: A scoping review. Journal of Cancer Survivorship, 17(6), 1596–1605. https://doi.org/10.1007/s11764-022-01210-2
Troeschel, A.N., Liu, Y., Collin, L.J., Bradshaw, P.T., Ward, K.C., Gogineni, K., & McCullough, L.E. (2019). Race differences in cardiovascular disease and breast cancer mortality among US women diagnosed with invasive breast cancer. International Journal of Epidemiology, 48(6), 1897–1905. https://doi.org/10.1093/ije/dyz108
Weaver, K.E., Foraker, R.E., Alfano, C.M., Rowland, J.H., Arora, N.K., Bellizzi, K.M., . . . Aziz, N.M. (2013). Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: A gap in survivorship care? Journal of Cancer Survivorship, 7(2), 253–261. https://doi.org/10.1007/s11764-013-0267-9
Williams, K.P., Lin, C.J., Felix, A.S., Addison, D., Sheppard, V.B., Sutton, A.L., . . . Hood, D.B. (2023). The association between cardiovascular disease and breast and gynecologic cancers among Black female patients. Journal of the National Medical Association, 115(5), 466–474. https://doi.org/10.1016/j.jnma.2023.07.004
Williams, M.S., & Anyimukwu, C. (2020). The association between cardiovascular disease knowledge and risk factors among African American breast cancer survivors in the deep South [Abstract]. Journal of Health Disparities Research and Practice, 13(4), 7.
Williams, M.S., Beech, B.M., Griffith, D.M., & Thorpe, R.J., Jr. (2020). The association between hypertension and race/ethnicity among breast cancer survivors. Journal of Racial and Ethnic Health Disparities, 7(6), 1172–1177. https://doi.org/10.1007/s40615-020-00741-7
World Health Organization. (2011). 2011 high level meeting on prevention and control of non-communicable diseases. http://www.un.org/en/ga/ncdmeeting2011
Zamorano, J.L., Lancellotti, P., Rodriguez Munoz, D., Aboyans, V., Asteggiano, R., Galderisi, M., . . . Suter, T.M. (2016). 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). European Heart Journal, 37(36), 2768–2801. https://doi.org/10.1093/eurheartj/ehw211



