Randomized Controlled Trial of an Intervention to Improve Nurses’ Hazardous Drug Handling
Objectives: To evaluate whether a web-based educational intervention improved personal protective equipment (PPE) use among oncology nurses who handle hazardous drugs.
Sample & Setting: From 2015 to 2017, the authors partnered with 12 ambulatory oncology settings in the United States to enroll 396 nurses, 257 of whom completed baseline and primary endpoint surveys.
Methods & Variables: In a cluster randomized controlled trial, 136 nurses in control settings received a one-hour educational module on PPE use with quarterly reminders, and 121 nurses in treatment settings received the control intervention plus tailored messages to address perceived barriers and quarterly data gathered on hazardous drug spills across all study settings. The primary outcome was nurse-reported PPE use.
Results: Control and intervention sites had suboptimal PPE use before and after the intervention. No significant differences were observed in PPE use knowledge or perceived barriers. Participants reported high satisfaction with the study experience.
Implications for Nursing: Hazardous drug exposure confers notable health risks to healthcare workers. To improve hazardous drug handling, occupational healthcare workers, health systems, and professional organizations should consider coordinated efforts to implement policy and practice changes.
Jump to a section
For more than 40 years, healthcare workers have administered drugs known to be hazardous to human health (Connor & McDiarmid, 2006). Antineoplastic drugs, principally used to treat cancer, comprise the largest group of drugs classified by the National Institute for Occupational Safety and Health ([NIOSH], 2016) as hazardous. People who handle hazardous drugs in their routine work report higher rates of adverse reproductive effects (Lawson et al., 2012), rare cancers (NIOSH, 2017), and an array of ill-defined respiratory and skin ailments (Couch & West, 2012; West & Beaucham, 2014). Studies that establish either a causal relationship between exposure and health effects or a dose-response relationship are missing. NIOSH (2004), in addition to other provider organizations, such as the American Society of Health-System Pharmacists (2006) and Oncology Nursing Society (Polovich & Olsen, 2018), have published recommendations to reduce hazardous drug exposures, including the consistent use of personal protective equipment (PPE) when handling hazardous drugs. Despite this evidence, workers continue to report exposure, and documented adherence to risk-reduction actions remains suboptimal. In a multistate survey of oncology nurses, 17% reported unintentional exposure to a hazardous drug in the prior year (Friese, Himes-Ferris, Frasier, McCullagh, & Griggs, 2012). Oncology nurses, who administer the majority of these drugs, report persistently low adoption of PPE use to minimize potential exposure (Polovich & Clark, 2012). Few interventions designed to increase PPE use have undergone systematic study (Crickman, 2017; Keat, Sooaid, Yun, & Sriraman, 2013). To date, no published intervention studies have adopted a randomized controlled trial design.
Evidence exists that, when provided with data collected in oncology nurses’ own practice settings, policy and equipment changes can occur swiftly, and worker-level adoption of PPE use can increase (Friese, McArdle, et al., 2015). Motivated by this pilot work, the current study team hypothesized that nurses who received feedback about hazardous drug exposures, coupled with messages intentionally designed to address known barriers, would increase PPE use when handling hazardous drugs.
In this context, the authors conducted a cluster randomized controlled trial to evaluate the efficacy of audit and feedback of hazardous drug exposures, coupled with tailored messages to address known barriers to optimal PPE use. The study’s primary outcome was nurse-reported PPE use during hazardous drug administration.
Methods
Settings, Study Participants, and Recruitment
The current authors previously published the study protocol and corresponding conceptual model for the study and related intervention development (Friese, Mendelsohn-Victor, et al., 2015). A convenience sample of 12 academic health center ambulatory oncology settings with high patient volume participated after chief nurse executive endorsement of the study. Participant sites were enrolled from March 2015 to March 2017. Settings were included if they had 20 or more employed RNs who met participant eligibility criteria.
Eligible participants included RNs employed an average of 16 hours per week or more in ambulatory chemotherapy infusion settings. As part of the authors’ efforts to quantify exposures and provide feedback, blood samples were obtained to analyze for participant exposure to hazardous drugs. To reduce the risk of contaminated results, participants treated with an antineoplastic drug in the past year were excluded. Pregnant workers could participate in surveys but did not participate in blood draws. Definitive results from the plasma analyses were inconclusive; therefore, the study team had no results to share with participants.
To recruit participants, the study team conducted site visits to present a study overview and answer questions. Selected on-site employees served as study champions and shared information with potential participants. All eligible participants received an informational pamphlet, a cover letter, and a $10 no-obligation, upfront cash incentive. The coordinating center sent two reminder emails to eligible participants. Interested participants received a unique study identifier with instructions to register, complete informed consent, and complete a baseline survey on the project’s website. Participants also used the project website to view control or treatment educational interventions. At primary endpoint assessment, enrolled participants received as many as three email invitations and reminders from on-site study champions to complete the final survey with another $10 no-obligation, upfront cash incentive.
Randomization occurred at the site level to prevent the likelihood of contamination across study arms. Randomization occurred after participants enrolled and completed the baseline survey. Given the variability of setting size and the potential for baseline differences in PPE use, the authors used the nonbimatch function from the nbpMatching package in R software to conduct stratified randomization to achieve group balances between the number of nurse participants and their baseline PPE use (Lu, Greevy, Xu, & Beck, 2011).
Control Intervention
Participants in settings randomly assigned to the control intervention received access to a one-hour educational module on the project website. The module included audio and video content synchronized to a slide presentation that summarized principles of safer hazardous drug handling, congruent with Oncology Nursing Society chemotherapy guidelines, recommendations from NIOSH, and recommendations from the American Society of Health-System Pharmacists. After completing the module and a quiz, participants could receive one contact hour of continuing education. Each quarter, participants received an email reminder that reinforced content in the educational module. To measure fidelity to the control intervention, study personnel monitored participants’ website login attempts and whether participants viewed the video to completion.
Treatment Intervention
Participants in settings randomly assigned to the treatment intervention also received access to the control intervention described previously. The treatment intervention added two components. First, participants received a tailored intervention of as many as three short videos that addressed the barriers to PPE use they individually reported on the baseline survey. Second, participants individually reported chemotherapy drug spills they experienced during the study period and submitted plasma samples for analysis. Subsequently, they received email prompts quarterly that directed them to the study website. Each quarter, the study team prepared a brief video that summarized the hazardous drug spill reports across all participating sites during the past quarter. The reports included (a) the number of drug(s) spilled, (b) the context of the spill occurrences, and (c) any pertinent results from the plasma sample analyses. Results from plasma analyses were aggregated across all participants. To assess fidelity to the treatment intervention, study personnel used website paradata to monitor the number of participant login attempts, the number of video(s) viewed to completion, and whether a post-video attestation was completed (see Figure 1). 
Modifications were made to the protocol at some sites. Three sites identified challenges with accessing the project website from their setting because of organizational-level privacy restrictions, outdated web browsers, or authentication challenges. To enable easier viewing of content, the coordinating center sent email messages to all participants with the content embedded directly in the message, as well as a link to the website. The email distribution platform also enabled the coordinating center to track the number of participants who viewed educational materials directly from the email message. The change in quarterly video distribution was made starting with the second quarterly video.
Data Collection and Measures
All study data were collected from participants on the project website, which was user-authenticated, password-protected, and encrypted. Study measures were selected in accordance with the authors’ published conceptual framework. Details regarding the development, testing, reliability, and validation of these measures were published previously (Friese, Mendelsohn-Victor, et al., 2015). The study was approved by the principal investigator’s institutional review board at the University of Michigan in Ann Arbor, and participants completed informed consent using the web-based platform. The principal investigator established a data safety monitoring board to review any adverse events; none arose during the study period.
The primary outcome was PPE use, as measured by the previously published Revised Drug Handling Questionnaire (Martin & Larson, 2003; Polovich & Clark, 2012). The questionnaire measures PPE use as a five-item measure on a six-point Likert-type scale from 0 (never) to 5 (always) related to percentage of time PPE is worn. For each participant, the mean score is calculated for the five items worn during hazardous drug administration: chemotherapy gloves, double gloves, single-use disposable gowns, disposable eye protection, and respirators.
Participants self-reported demographic factors on the baseline survey. Factors included oncology nursing experience (years), education (bachelor’s degree or higher), and oncology nursing certification (yes or no), in addition to race/ethnicity.
The authors hypothesized that three potential organizational factors (i.e., workloads, practice environments, and safety behaviors) would be associated with the primary outcome of PPE use. The authors aggregated these organizational factors to the setting level by constructing an average score for all participants in their respective settings. To measure workload, participants were asked to report the number of patients to whom they delivered chemotherapy on their last shift as a continuous measure (Friese et al., 2012). The nursing practice environment was measured by the Practice Environment Scale of the Nursing Work Index, as revised for ambulatory settings (Friese, 2012). Researchers have published reliability and validity studies for this measure. For each participant, the authors calculated a mean score across the 23 items that use a six-point Likert-type scale from 0 (strongly disagree) to 5 (strongly agree) that a favorable element is present in their current practice environment (e.g., nurses participate in decision making, collegial relationships between nurses and physicians).
To measure safety behaviors, participants completed the nine-item Safety Organizing Scale that assesses the observable actions of clinicians to maintain a safety culture, in congruence with high-reliability organization principles (Vogus & Sutcliffe, 2007). Previously studied for validity and reliability, the Safety Organizing Scale is scored on a seven-point Likert-type scale from 1 (not at all) to 7 (to a very great extent) evaluating the extent to which nurses and other unit-based coworkers engage in safety behaviors.
The authors measured knowledge of hazardous drug handling using a team-generated, pilot-tested 10-item questionnaire that assessed knowledge of existing hazardous drug handling science and recommendations. Participants answered 10 questions using a four-item multiple-choice format, with a range of scores from 0 (no knowledge) to 10 (full knowledge).
Participants completed the three-item Occupational Dermal Survey to measure perceived risk (Geer, Curbow, Anna, Lees, & Buckley, 2006). Using a four-point Likert-type scale from 1 (strongly disagree) to 4 (strongly agree), values assess the degree to which nurses perceive risk of exposure to hazardous drugs in their workplace.
At the primary endpoint survey, the authors asked participants to rate their satisfaction with study participation on a five-point Likert-type scale from 1 (very dissatisfied) to 5 (very satisfied), the usefulness of the content to their clinical practice on a five-point Likert-type scale from 1 (strongly disagree) to 5 (strongly agree), and their willingness to participate in similar studies in the future (yes or no). Participants could also provide free-text feedback on their study experience.
Statistical Analysis
Univariate analyses examined each variable listed previously, followed by examination of variation in these measures across participating practices. To evaluate the efficacy of the treatment intervention on PPE use, the authors used linear mixed models with random intercepts at the individual and site levels to adjust for repeated measurements from each nurse and cluster effects because of intervention assignment. The response variable of the models was PPE use, and the explanatory variable was the intervention. The covariates related to personal factors and organizational factors were also included in the models to increase precision.
Regarding sensitivity, participating practices varied in their PPE policies and equipment availability. In some practices, eye protection and respirators were not routinely available or required by policy. Therefore, the authors examined the primary outcome using two versions of the measure: a three-item measure of PPE use (chemotherapy-tested gloves, double gloves, and single-use disposable gowns) and a five-item measure (gloves, double gloves, gowns, eye shield, and respirator). The authors used the five-item measure for primary analyses and the three-item measure as a sensitivity analysis. The second sensitivity analysis restricted attention to the 175 participants who viewed the web-based materials at least one time during the study period.
Results
Of 439 RNs eligible to participate across 12 practice sites, 415 (95%) enrolled in the study, 189 from practice sites assigned to the treatment arm and 226 from practice sites assigned to the control arm. Of enrolled participants, 378 (91%) completed the baseline survey and 257 (62%) completed the baseline and primary endpoint surveys; 121 participants were in treatment arm–assigned practices, and 136 participants were in control arm–assigned practices.
The authors observed differences between participants employed in treatment arm–assigned settings and control arm–assigned setting participants by race/ethnicity, years of experience, and workload of patients receiving chemotherapy (see Table 1). There were more Asian nurses in the control arm (12% versus 6% in the treatment arm), more Hispanic nurses in the control arm (9% versus 12% in the treatment arm), and fewer White nurses in the control arm (79% versus 92% in the treatment arm). Nurses in the control arm reported slightly more years in current role (control arm: mean = 6.7, SD = 7.2; treatment arm: mean = 5.1, SD = 4.5). Nurses in the treatment arm reported higher workloads of patients receiving chemotherapy than nurses in the control arm (6.2 patients on the last shift versus 5 patients). Initial fidelity intervention, defined as viewing the webinar, was 50% for the intervention arm and 73% for the control arm. In addition, 57% of intervention participants watched one or more feedback videos. 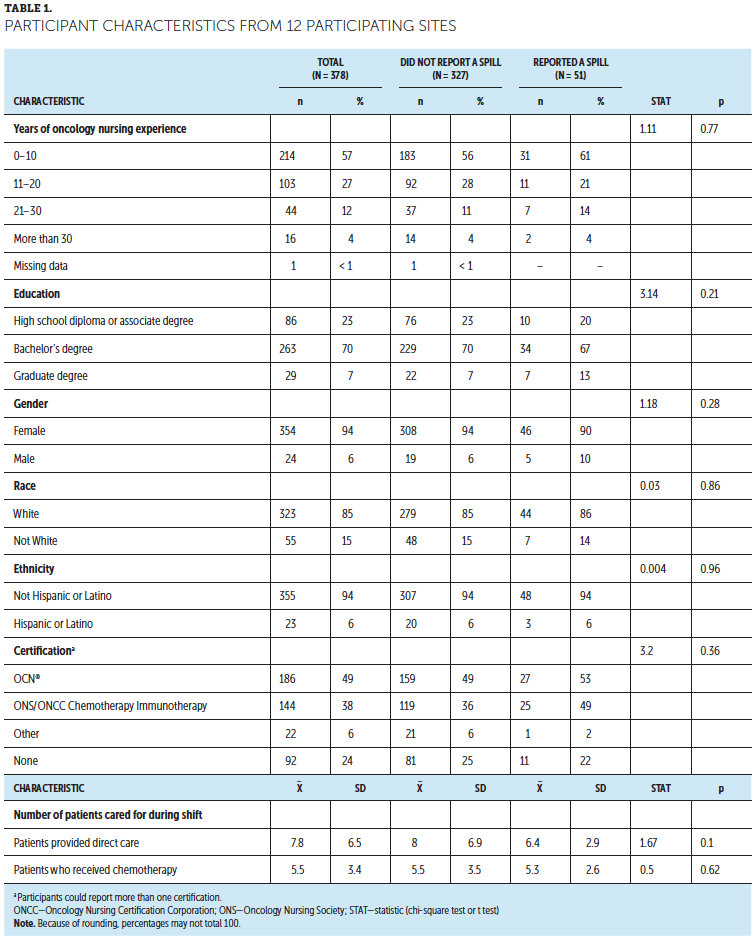
For the intervention and control arms, differences in PPE use score did not change between study initiation and primary endpoint assessment (see Table 2). At baseline, the mean five-item PPE use score was 2.4 (SD = 0.8) in the treatment arm and 2.4 (SD = 0.7) in the control arm. At the one-year follow-up, the PPE use score was similar in the treatment and control arms (mean = 2.3, SD = 0.9 for both groups). Hazardous drug knowledge scores and reported barriers to PPE use did not change significantly from baseline to follow-up for nurses in either arm. 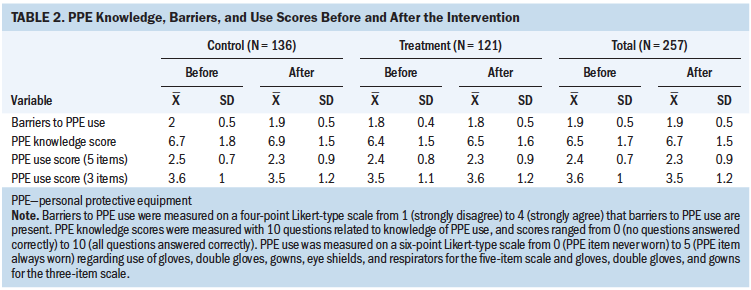
Results from a linear mixed model show that PPE use scores from baseline to follow-up did not change significantly in the intervention arm after adjustment for PPE use at baseline (beta = 0.1, SE = 0.4, p = 0.75).
In sensitivity analyses using the three-item PPE use score (chemotherapy-tested gloves, double gloves, and single-use disposable gowns), results obtained did not differ significantly from those reported previously when all five PPE items were considered (see Table 3). Results reported previously also did not change significantly when analyses were restricted to participants who had viewed the web-based materials at least once during the study period. 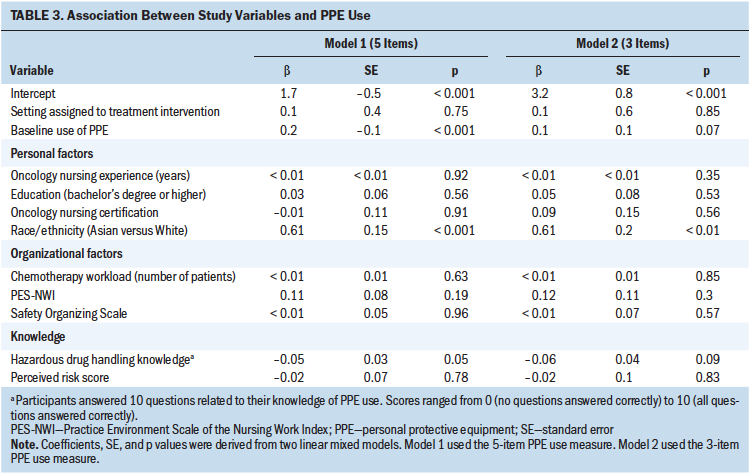
In the process evaluation, 71% of participants reported that they were satisfied or very satisfied with participating in the study, 5% were dissatisfied or very dissatisfied, and 24% endorsed a neutral assessment. Sixty-five percent agreed or strongly agreed that the educational content was useful to their clinical practice, 2% disagreed or strongly disagreed, and 23% endorsed a neutral assessment. Eighty-one percent would be willing to receive future invitations for study participation. Thirty-nine nurses provided open-text feedback; 23 were positive (principally focused on importance of the topic and feedback received), 12 were negative (principally focused on time involved and website difficulties), and 4 were neutral.
Discussion
In this cluster randomized controlled trial, receipt of a web-based educational intervention that included ongoing feedback on study results and tailored messages to reduce barriers, when compared to a static educational module, did not result in improved PPE use among oncology nurses employed in ambulatory settings. These findings suggest that pervasive challenges exist for nurses to fully implement the recommendations for hazardous drug handling from NIOSH and other professional organizations.
Baseline PPE use scores across multiple participating clinical settings demonstrated suboptimal use of PPE among nurses in ambulatory infusion settings. This was a discouraging finding, given that the study took place in settings where multiple organizational factors favor excellence in safety, such as academic health centers with high-volume cancer programs. These findings underscore the ongoing risks that healthcare workers take when providing patient care and the accompanying need for novel interventions to mitigate risks associated with significant health effects, such as reproductive problems and rare cancers.
The current intervention did not improve PPE use among participants. One possible reason for this finding includes suboptimal content in the intervention. Additional possible explanations include too few interactions with participants and structural barriers to adopting desired behaviors. Prior work among oncology nurses has shown low perceived risk from hazardous drug exposure (Friese, McArdle, et al., 2015). In addition, the intervention did not personalize feedback to individuals but reported similar content to all participants. Individualized feedback may have been more effective in influencing participant behavior change. Participation in the educational activities waned during the course of the study period, suggesting that participants did not find additional content useful. The quarterly feedback for participants in the treatment arm may have been too infrequent to challenge existing practice norms and support participants to adopt behavior change.
During site visits, study personnel anecdotally identified structural barriers to participation in the educational activities. These barriers included difficulties accessing web-based content outside the institution; workload demands that limited time for participants to view materials during their scheduled shifts; and vague or unclear institutional policies on gowns, eye protection, and respirator use when handling hazardous drugs. Study follow-up efforts have included sharing institution-specific data with leaders and clinicians to develop specific strategies within each organization to reduce hazardous drug exposure risks. This is a promising area for future intervention development and testing.
Although exposure remains an important occupational challenge in oncology settings, hazardous drugs are administered with increasing frequency outside of cancer settings. Antineoplastic drugs and biologic agents have expanded approvals in conditions such as rheumatoid arthritis, psoriasis, and solid organ transplantation (Friese, McCullagh, & Sutcliffe, 2015). As such, the target audience for education and outreach interventions has expanded beyond cancer center providers. Providers in these emerging areas may need different strategies to increase awareness of hazardous drug exposures and proficiency with PPE use, including ongoing training specific to hazardous drug handling.
Limitations
Despite high participation and response rates for nurses, coupled with a controlled experimental design informed by a theory-based framework, the study has several limitations worthy of comment. First, the study took place in a convenience sample of academic health centers with high-volume cancer programs. Results may not generalize to smaller or community-based oncology settings. Second, the calculated reliability of the outcome measure in the current sample was relatively low (0.46 for the three-item measure and 0.5 for the five-item measure considered in the sensitivity analysis). Fidelity to the intervention was high soon after study activation and decayed over time. Therefore, assessing the primary endpoint one year after study activation may have limited the ability to detect meaningful changes in PPE use. Future research efforts would benefit from development and testing of novel measures of PPE use and evaluation of optimal measurement times after delivering educational interventions and delivering study reminders. Novel study designs, such as sequential multiple-assignment randomized trials, may address the ongoing challenges of reaching non-engaged participants and titrating interventions based on behavioral response. Implementation science techniques could elucidate factors associated with increased adherence to study protocols and/or PPE recommendations. Participants’ knowledge of chemotherapy administration safety was measured using a team-designed instrument. Psychometric testing of the instrument in diverse samples will increase confidence in measure validity. The study focused on nurses who handle hazardous drugs but did not include other workers routinely exposed to hazardous drugs.
Implications for Nursing
The current study findings have important implications for nursing from various perspectives: individual health systems, professional organizations, and regulatory efforts. The challenges that characterize influencing nurses’ use of PPE found in this and previous studies underscore the importance of higher-order hazard control strategies, such as engineering and administrative controls (Hon & Abusitta, 2016). During the enrollment period, the authors noted inconsistencies in existing institutional policies on hazardous drug handling across participating institutions, despite similar patient populations and care processes. Nursing leaders could standardize educational content and policies on PPE use across oncology settings with leadership endorsement and accountability to address existing confusion among healthcare workers. Although nursing and other professional organizations have attempted to address this issue, differences in opinion remain across these organizations (Connor, Celano, Frame, & Zon, 2017; Zon, 2018), and recent efforts to strengthen oversight of hazardous drug handling across cancer settings have been delayed to 2019 (United States Pharmacopeial Convention, 2017). These delays will hamper efforts to improve PPE use. Several states have passed legislation aimed to improve hazardous drug handling, but delayed implementation has hindered effectiveness (Walton, Eisenberg, & Friese, 2017). When placed in the context of the current study findings, it is clear that education and engagement of nursing personnel is not sufficient to improve PPE use; systematic approaches may result in improved practice. 
Conclusion
Despite four decades of evidence to suggest adverse health effects for workers who handle hazardous drugs, nurses persistently do not wear PPE as recommended. An educational intervention tailored to address documented barriers and targeted to practicing nurses did not improve PPE use. When considering the hierarchy of controls, efforts should focus on developing novel and reliable engineering controls, improving existing engineering controls, strengthening clinician adherence to efficacious engineering controls, and developing and evaluating system-level interventions to address pervasive gaps in hazardous drug handling practice. To minimize the risk of hazardous drug exposure, healthcare workers must receive adequate training and equipment. Policymakers, clinical experts, and health-system leaders should encourage clinical settings to adopt guideline-concordant PPE policies and activities.
The authors gratefully acknowledge Marylee Scherdt, BS, for manuscript assistance, as well as the DEFENS study investigators: Penny Moore, MSN, RN, OCN®, Susan Cobb, PhD, RN-BC, Theresa Rudnitzki, MS, RN, ACNS-BC, AOCNS®, Susan R. Hartranft, PhD, ARNP, CNL, Clara Beaver, MSN, RN, AOCNS®, ACNS-BC, Christen Hughes, MSN, RN, OCN®, Judy Delmonte, MS, CSSGB, CPHQ, Catherine A. Lyons, RN, MS, MEA-BC, D. Kathryn Tierney, RN, PhD, Catherine Glennon, RN, MHS, OCN®, NE-BC, and Jill Dobias, RN, APRN.
About the Author(s)
Christopher R. Friese, PhD, RN, AOCN®, FAAN, is the Elizabeth Tone Hosmer Professor of Nursing, Health Management, and Policy, James Yang, PhD, is an associate research scientist, Kari Mendelsohn-Victor, MPH, is a clinical research coordinator, and Marjorie C. McCullagh, PhD, RN, APHN-BC, COHN-S, FAAOHN, FAAN, is a professor, all in the School of Nursing at the University of Michigan in Ann Arbor. This research was funded by a grant (1 R01 OH 010582-01) from the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention. This article’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. All authors contributed to the conceptualization and design. Friese and Mendelsohn-Victor completed the data collection. Friese and Yang provided statistical support. Friese, Yang, and McCullagh provided the analysis. Friese, Mendelsohn-Victor, and McCullagh contributed to the manuscript preparation. Friese can be reached at cfriese@umich.edu, with copy to ONFEditor@ons.org. (Submitted August 2018. Accepted October 9, 2018.)
References
American Society of Health-System Pharmacy. (2006). ASHP guidelines on handling hazardous drugs. American Journal of Health-System Pharmacy, 63, 1172–1191. https://doi.org/10.2146/ajhp050529
Connor, T.H., Celano, P., Frame, J.N., & Zon, R.T. (2017). Summary of the workshop on the safe handling of hazardous drugs cohosted by the National Institute for Occupational Safety and Health and the American Society of Clinical Oncology. Journal of Oncology Practice, 13, 199–205. https://doi.org/10.1200/JOP.2016.017384
Connor, T.H., & McDiarmid, M.A. (2006). Preventing occupational exposures to antineoplastic drugs in health care settings. CA: A Cancer Journal for Clinicians, 56, 354–365.
Couch, J., & West, C. (2012). Chemotherapy drug exposures at an oncology clinic—Florida (Report HETA 2009-0148-3158). Retrieved from https://www.cdc.gov/niosh/hhe/reports/pdfs/2009-0148-3158.pdf
Crickman, R. (2017). Chemotherapy safe handling: Limiting nursing exposure with a hazardous drug control program. Clinical Journal of Oncology Nursing, 21, 73–78. https://doi.org/10.1188/17.CJON.73-78
Friese, C.R. (2012). Practice environments of nurses employed in ambulatory oncology settings: Measure refinement. Oncology Nursing Forum, 39, 166–172. https://doi.org/10.1188/12.ONF.166-172
Friese, C.R., Himes-Ferris, L., Frasier, M.N., McCullagh, M.C., & Griggs, J.J. (2012). Structures and processes of care in ambulatory oncology settings and nurse-reported exposure to chemotherapy. BMJ Quality and Safety, 21, 753–759. https://doi.org/10.1136/bmjqs-2011-000178
Friese, C.R., McArdle, C., Zhao, T., Sun, D., Spasojevic, I., Polovich, M., & McCullagh, M.C. (2015). Antineoplastic drug exposure in an ambulatory setting: A pilot study. Cancer Nursing, 38, 111–117. https://doi.org/10.1097/NCC.0000000000000143
Friese, C.R., McCullagh, M.C., & Sutcliffe, K.M. (2015). Handle with care: The known and unknown risks to nurses who handle hazardous drugs. American Nurse Today, 10, 41–42.
Friese, C.R., Mendelsohn-Victor, K., Wen, B., Sun, D., Sutcliffe, K., Yang, J.J., . . . McCullagh, M.C. (2015). DEFENS—Drug Exposure Feedback and Education for Nurses’ Safety: Study protocol for a randomized controlled trial. Trials, 16, 171. https://doi.org/10.1186/s13063-015-0674-5
Geer, L.A., Curbow, B.A., Anna, D.H., Lees, P.S., & Buckley, T.J. (2006). Development of a questionnaire to assess worker knowledge, attitudes and perceptions underlying dermal exposure. Scandinavian Journal of Work, Environment and Health, 32, 209–218.
Hon, C.Y., & Abusitta, D. (2016). Causes of health care workers’ exposure to antieoplastic drugs: An exploratory study. Canadian Journal of Hospital Pharmacy, 69, 216–223. https://doi.org/http://dx.doi.org/10.4212/cjhp.v69i3.1558
Keat, C.H., Sooaid, N.S., Yun, C.Y., & Sriraman, M. (2013). Improving safety-related knowledge, attitude and practices of nurses handling cytotoxic anticancer drug: Pharmacists’ experience in a general hospital, Malaysia. Asian Pacific Journal of Cancer Prevention, 14, 69–73.
Lawson, C.C., Rocheleau, C.M., Whelan, E.A., Lividoti Hibert, E.N., Grajewski, B., Spiegelman, D., & Rich-Edwards, J.W. (2012). Occupational exposures among nurses and risk of spontaneous abortion. American Journal of Obstetrics and Gynecology, 206, 327. https://doi.org/10.1016/j.ajog.2011.12.030
Lu, B., Greevy, R., Xu, X., & Beck, C. (2011). Optimal nonbipartite matching and its statistical applications. American Statistician, 65, 21–30. https://doi.org/10.1198/tast.2011.08294
Martin, S., & Larson, E. (2003). Chemotherapy-handling practices of outpatient and office-based oncology nurses. Oncology Nursing Forum, 30, 575–581. https://doi.org/10.1188/03.ONF.575-581
National Institute for Occupational Safety and Health. (2004). NIOSH alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. Retrieved from http://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf
National Institute for Occupational Safety and Health. (2016). NIOSH list of antineoplastic and other hazardous drugs in healthcare settings, 2016. Retrieved from https://www.cdc.gov/niosh/docs/2016-161/pdfs/2016-161.pdf
National Institute for Occupational Safety and Health. (2017). Hazardous drug exposures in healthcare: Effects of occupational exposure. Retrieved from https://www.cdc.gov/niosh/topics/hazdrug/effects.html
Polovich, M., & Clark, P.C. (2012). Factors influencing oncology nurses’ use of hazardous drug safe-handling precautions [Online exclusive]. Oncology Nursing Forum, 39, E299–E309. https://doi.org/10.1188/12.ONF.E299-E309
Polovich, M., & Olsen, M.M. (Eds.). (2018). Safe handling of hazardous drugs (3rd ed.). Pittsburgh, PA: Oncology Nursing Society.
United States Pharmacopeial Convention. (2017). USP general chapter <800> hazardous drugs—Handling in healthcare settings. Retrieved from http://www.usp.org/compounding/general-chapter-hazardous-drugs-handling…
Vogus, T.J., & Sutcliffe, K.M. (2007). The Safety Organizing Scale: Development and validation of a behavioral measure of safety culture in hospital nursing units. Medical Care, 45, 46–54. https://doi.org/10.1097/01.mlr.0000244635.61178.7a
Walton, A.L., Eisenberg, S., & Friese, C.R. (2017). Hazardous drugs: Legislative and regulatory efforts to improve safe handling. Clinical Journal of Oncology Nursing, 21, 254–256. https://doi.org/10.1188/17.CJON.254-256
West, C., & Beaucham, C. (2014). Health hazard evaluation program: Evaluation of chemotherapy drug exposure in an outpatient infusion center. Retrieved from http://www.cdc.gov/niosh/hhe/reports/pdfs/2013-0019-3205.pdf
Zon, R.T. (2018). USP3: United in safeguarding patients, personnel, and providers. Journal of Oncology Practice, 13, 145–148. https://doi.org/10.1200/JOP.2016.020453




