Associations Between Cholecalciferol Supplementation and Self-Reported Symptoms Among Women With Metastatic Breast Cancer and Vitamin D Deficiency: A Pilot Study
Objectives: To assess the potential effect of cholecalciferol supplementation to reduce symptom burden for women with metastatic breast cancer (MBC).
Sample & Setting: 11 clinically stable women with estrogen receptor–positive MBC were recruited from a single cancer center for this phase 1, nonrandomized study (NCT02186015).
Methods & Variables: Women with insufficient serum 25-hydroxyvitamin D (25[OH]D) levels qualified to receive high-dose repletion therapy. Clinical and questionnaire data on common symptoms and quality of life were obtained prior to and following supplementation.
Results: Serum 25(OH)D increased significantly pre- versus postintervention. Trends for improvements in endocrine symptoms, bone pain, and fatigue were observed following the intervention.
Implications for Nursing: Women achieved normal serum 25(OH)D levels after eight weeks of supplementation and reported reduced symptom burden. Vitamin D may be a low-cost supportive care therapy; however, future studies should be considered.
Jump to a section
Vitamin D has long been known for its role in bone health, working with the parathyroid hormone to regulate calcium homeostasis (Holick, 2007). It is only naturally found in a limited number of foods in the human diet (e.g., tuna, salmon, egg yolk), necessitating fortification efforts since the 1930s to help decrease the occurrence of bone disease. Vitamin D is considered a prohormone, requiring ultraviolet radiation for conversion to its active forms (Holick, 2007). Because of concerns regarding melanoma or cutaneous hypersensitivity secondary to specific medications, healthcare professionals do not advocate treating or preventing vitamin D deficiency with sunlight exposure. Not surprisingly, the use of vitamin D supplementation among cancer survivors, particularly women with breast cancer, is highly prevalent (Marian, 2017).
Eighty percent of breast cancers are hormone sensitive (estrogen receptor–positive [ER+]) (American Cancer Society, 2020), necessitating the use of medications to decrease the production or absorption of estrogen (i.e., aromatase inhibitors). Arthralgias and myalgias are highly prevalent within weeks of estrogen blockade and may result in medication discontinuation (Castel et al., 2013). The etiology of these musculoskeletal disturbances is not well understood; however, the analgesic role of estrogen (Niravath, 2013), as well as heightened levels of inflammation (Bauml et al., 2015) and cytokines (interleukins [ILs] or tumor necrosis factor alpha [TNF-a]) (Islander et al., 2011), may further contribute. In addition, the combination of efficient estrogen deprivation combined with low serum 25-hydroxyvitamin D (25[OH]D) may also accentuate muscle and joint pain (Napoli et al., 2010). To better address these clinical phenomena, several clinical trials have investigated if ergocalciferol (vitamin D2 sourced from plants) or cholecalciferol (vitamin D3 derived from animal sources) dosed at 10,000–50,000 IU per week (recommended levels are greater than 600 IU per day) can improve treatment-related musculoskeletal side effects (Khan et al., 2017; Rastelli et al., 2011; Shapiro et al., 2016). For unknown reasons, these trials exclude women with metastatic breast cancer, a rapidly expanding sector of cancer survivors. Women with ER+ metastatic breast cancer are prescribed aromatase inhibitors in addition to other sequential and ongoing systemic therapies, resulting in significant treatment-related side effects of a persistent nature. Considering that quality of life and symptom burden dictate all treatment decisions in the metastatic setting (Irvin et al., 2011), finding supportive therapies that improve treatment tolerance and are cost-effective is an ongoing challenge. This has yet to be reported in this clinical population. Therefore, the goal of this pilot study was to preliminarily evaluate the systemic effects of cholecalciferol (vitamin D3) supplementation in women with ER+ metastatic breast cancer with vitamin D insufficiency.
Methods
Sample and Setting
After acquiring ethical approval, adult women aged 18 years or older were recruited from the Cardinal Bernardin Cancer Center, part of the Loyola University Medical Center in Maywood, Illinois. Eligible women were required to have histologically confirmed ER+ metastatic breast cancer, have an Eastern Cooperative Oncology Group Performance Status of 0–2 (Oken et al., 1982), and be deemed clinically stable and cleared by their medical oncologist at the time of study consent (i.e., no changes in antineoplastic agents within the past 30 days, no new clinical or radiologic evidence of disease progression, no unexplained weight loss, no uncontrolled pain, and/or six months or greater of life anticipated). Women meeting these criteria were approached during a routine oncology clinic visit to assess their interest in study participation. If they agreed, informed consent was obtained in a private room. Women were then referred for screening phlebotomy, completed by trained phlebotomists as part of their routine clinical care. Women with early-stage breast cancer (stage I–III), normal serum 25(OH)D levels (30 ng/ml or greater), treatment-unresponsive metastatic breast cancer, or a history of renal calculi, renal failure, hyperparathyroidism, or vitamin D hypersensitivity were deemed ineligible. No upper age restriction was mandated.
Design
A phase 1, open-label, nonrandomized trial was conducted to evaluate safety and demonstrate proof of concept. Per Endocrine Society guidelines (Holick et al., 2011), a serum 25(OH)D less than 75 nmol/L (less than 30 ng/ml) was used to classify vitamin D insufficiency. This study is registered on ClinicalTrials.gov (NCT02186015).
Intervention
Cholecalciferol was purchased and sent directly to the medical center investigational drug service for inventory and dispensing purposes. Following the treatment guidelines of the Endocrine Society (Holick et al., 2011), the intervention entailed 50,000 IU of cholecalciferol once per week for eight weeks. Cholecalciferol is the preferred form for vitamin D supplementation for raising serum 25(OH)D levels (Tripkovic et al., 2012). An investigational new drug application was approved for this trial by the U.S. Food and Drug Administration (IND 123378). Women were provided with eight study pills in a study vial, with instructions to take the vitamin D on the same day each week, starting at their research appointment.
Measures
Guided by the Wilson and Cleary (1995) health-related quality-of-life conceptual model, several levels of patient outcomes were examined, including (a) biologic and physiologic, (b) symptoms, (c) functional status, (d) overall quality of life, and (e) characteristics of the individual.
Clinical: Sociodemographic (age, race/ethnicity, employment) and medical information (breast cancer surgery, previous and current cancer treatments, metastatic sites) were gathered from the participant and the electronic health record at baseline, respectively. At a research visit, anthropometric data, as well as hand grip strength (reflecting functional status and upper-body strength), were measured following established procedures (National Institutes of Health, 2019).
Laboratory: Two additional tubes of blood were obtained for study purposes (about 30 cc). In the first year of the study, vitamin D was routinely outsourced to a local laboratory using liquid chromatography–mass spectroscopy to determine serum 25(OH)D levels. For the remainder of the study, all blood samples were processed by the hospital’s central laboratory using a radioimmune assay to obtain serum 25(OH)D levels. To quantify serum biomarkers of inflammation, concentrations of high-sensitivity C-reactive protein, TNF-alpha, and IL-6 were quantified by an internal research laboratory using kits purchased from Aniara Diagnostica and R&D Systems. All serum biomarker samples were stored at –80°C until the time of batch analyses and run in duplicate with controls. Only participants in the intervention arm returned for follow-up phlebotomy.
Quality of life, symptom assessment, and lifestyle factors: To assess symptom burden, participants completed the Brief Pain Inventory (Cleeland & Ryan, 1994), the Piper Fatigue Scale (Piper et al., 1998), the Hospital Anxiety and Depression Scale (Herrmann, 1997), and the Pittsburgh Sleep Quality Index (Carpenter & Andrykowski, 1998). To capture multidimensional quality of life, the Functional Assessment of Cancer Therapy (FACT)–General (Cella et al., 1993), as well as the –Bone Pain (Broom et al., 2009), –Breast (Reed et al., 2012), and –Endocrine Symptoms (Fallowfield et al., 1999) subscales, were completed pre- and postintervention. To quantify vitamin D from sun exposure and dietary sources, the authors administered a sun habits questionnaire (Glanz et al., 2008) and a calcium/vitamin D screener (Cummings et al., 1987). Physical activity and social support were measured using the Godin Leisure-Time Exercise Questionnaire (Godin & Shephard, 1985) and the Medical Outcomes Study Social Support Survey (Sherbourne & Stewart, 1991). These valid and reliable tools were selected, taking into account availability, time to complete, and comparability across studies because they have been previously used in patient populations with breast cancer or other cancers.
Statistical Analyses
Descriptive statistics were conducted to examine differences in sociodemographics, medical characteristics, and vitamin D levels. Data are presented as medians, interquartile ranges (IQRs), or counts and percentages. Statistical differences between groups were assessed with Wilcoxon rank sum tests for continuous variables and chi-square or Fisher’s exact test for nominal variables. Analyses were performed with SAS, version 9.4. By design, this was a proof of concept study, and formal power calculations were not performed a priori. A p value of less than 0.05 was used to denote statistical significance, and a p value of less than 0.1 was used to designate statistical trends.
Results
A total of 52 women with clinically stable ER+ metastatic breast cancer were approached for inclusion over 30 months from February 2015 to October 2017. Of these, 31 were screen failures, 9 were not interested, 1 withdrew prior to completing the questionnaires, and 11 qualified for the intervention. The prevalence of vitamin D insufficiency was 26% (n = 11 of 42). March through April yielded the greatest time of intervention eligibility. The study was stopped because of lack of participant accrual and budgetary restrictions. As described in Table 1, women who were classified as serum 25(OH)D insufficient tended to be younger (p = 0.09), Black (p = 0.003), more recently diagnosed with metastatic breast cancer (p = 0.06), and not vitamin D supplement users (n = 10 of 11). They also experienced a greater frequency of brain metastases (p = 0.01), were more likely to present with de novo disease (p = 0.04), and had lower dietary intakes of calcium (p = 0.06). Although not different between groups, generally high levels of social support were reported overall. 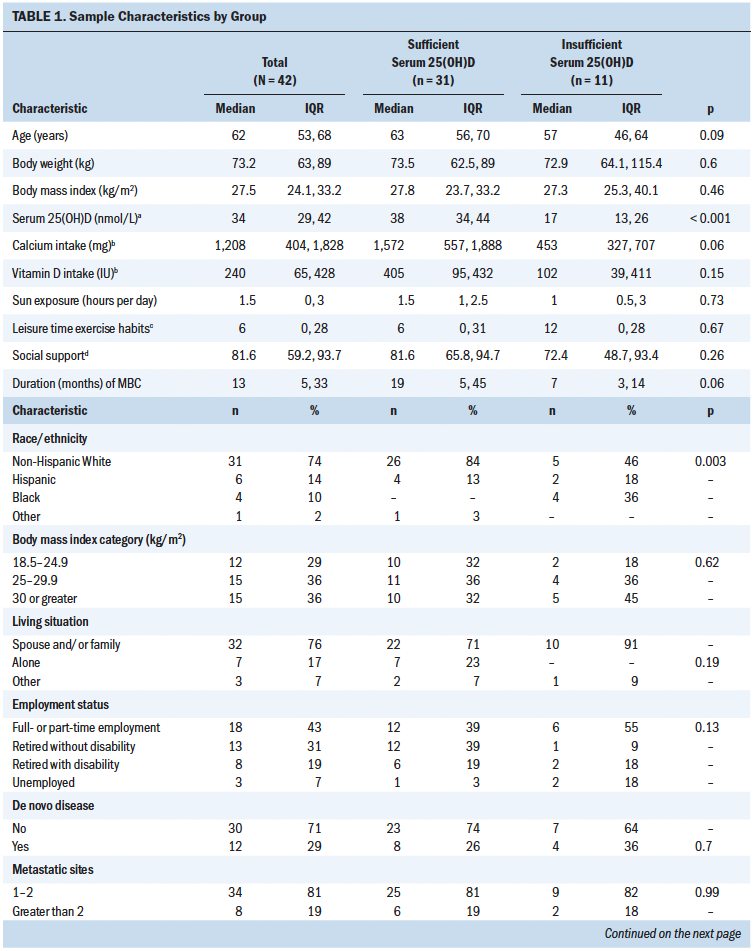
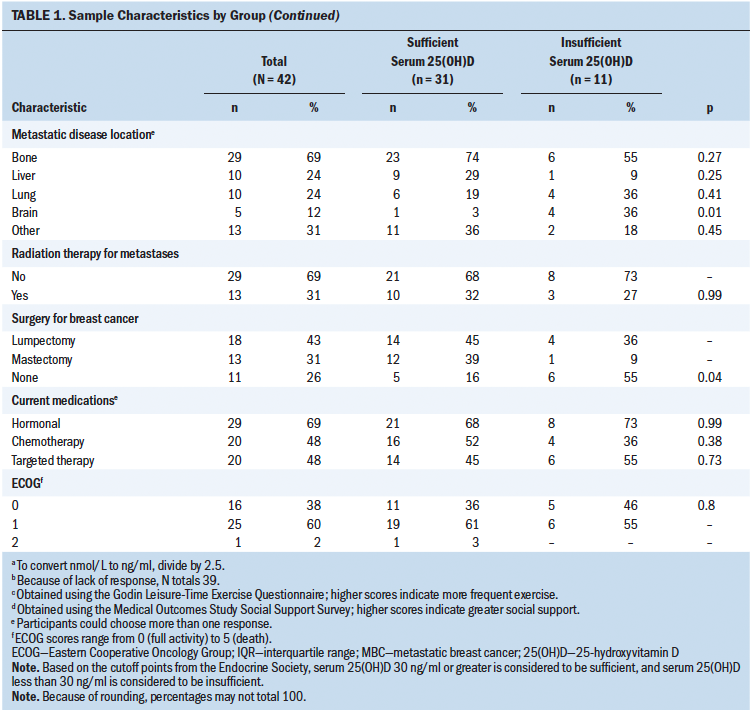
In total, 11 women initiated the high-dose cholecalciferol intervention. One participant was medically withdrawn after several weeks on study because of disease progression. As seen in Table 2, serum 25(OH)D increased significantly from baseline to eight weeks, reflecting a median change of 80 nmol/L (IQR = 55, 100; p = 0.002). No participants needed additional vitamin D3 supplementation to achieve normal serum levels. During the time of the study, a total of 14 adverse events were recorded. None of the adverse events were classified as serious. Increasing scores on the FACT–Endocrine Symptoms subscale reflect better symptom control, as indicated by the median positive change of 6 points (IQR = –2, 14; p = 0.09). In addition, there was a tendency to show improvements in fatigue (p = 0.09), with significant decreases in fatigue related to behavior and severity (p = 0.02). Women with higher levels of baseline fatigue demonstrated the greatest declines in fatigue scores (data not shown). Average pain scores showed overall declines, with significant improvements in bone pain specifically (p = 0.05). No trends or significant changes were noted for inflammatory biomarkers pre- versus postintervention. 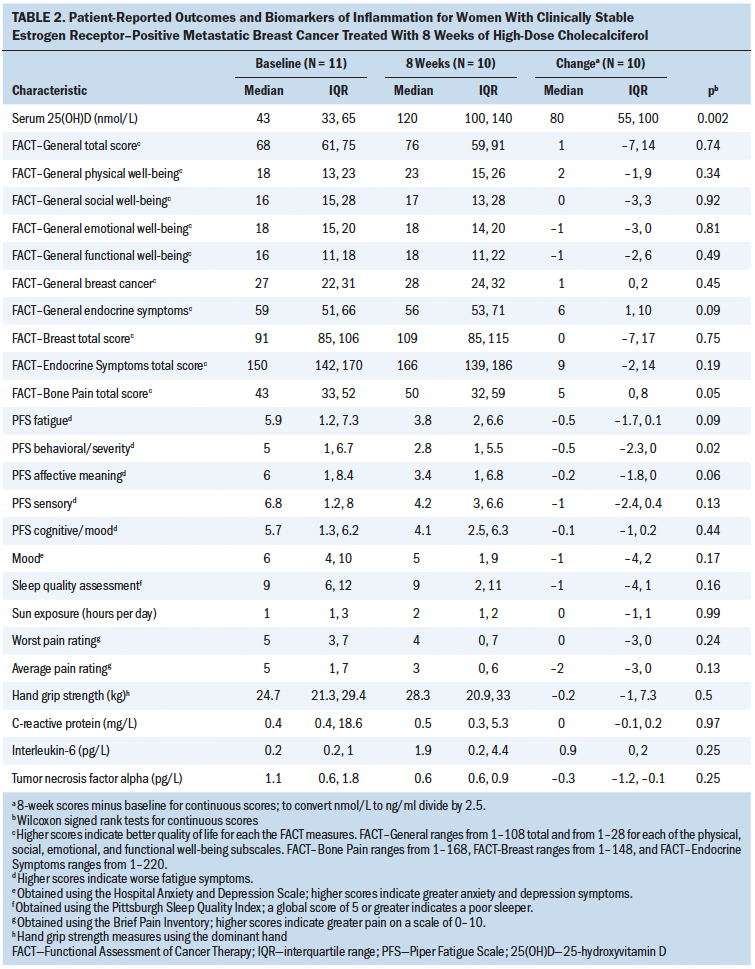
Discussion
Eight weeks of high-dose cholecalciferol supplementation resulted in achievement of normal serum 25(OH)D levels and improvements in pain and fatigue reports. Considering these symptoms are frequent among women with metastatic breast cancer (Irvin et al., 2011; Sheean et al., 2015), these findings make a novel contribution to the literature. In addition, they provide clinicians with preliminary evidence to address treatment-related side effects associated with antiestrogen medications and may serve as a tool to potentially combat medication nonadherence (Castel et al., 2013). It is unclear why previous vitamin D supplementation trials excluded women with metastatic breast cancer. Across studies, a previous history on renal calculi or primary hyperparathyroidism are clear, consistent contraindications (Khan et al., 2017; Rastelli et al., 2011; Shapiro et al., 2016). The authors can only speculate that, because women with metastatic breast cancer receive continual and sometimes changing systemic antineoplastic therapies (versus limited systemic therapies provided to women with early-stage breast cancer), it would be difficult to tease out and assess the intervention effects of vitamin D supplementation on subjective outcomes (e.g., pain, fatigue). It is likely considered a more efficient or superior study design to exclude them initially.
Consistent with the literature, many of the study participants reported routine calcium (n = 26) and vitamin D (n = 32) supplementation. Median calcium intake is consistent with the amount recommended by the Institute of Medicine (2010) (1,200 mg per day); however, median vitamin D intake in the current study appears to be less than the recommended 600 IU per day. In addition, women in the serum 25(OH)D sufficient group reported overall greater sunlight exposure as compared to women classified as serum 25(OH)D insufficient, underscoring the importance of capturing this information. Consequently, the prevalence of low serum 24(OH)D was relatively uncommon in the current study as compared to other investigations involving women with metastatic breast cancer; only 26% of women screened in the current study possessed low serum 25(OH)D levels necessitating intervention as compared to 70% of women with metastatic breast cancer in other studies (Simmons et al., 2009; Trukova et al., 2012). Together, these observations support the clinical implications of reviewing dietary supplement information during every clinic visit and updating the electronic health record.
Because the authors’ cancer center treats about 70–100 women with metastatic breast cancer annually, the authors anticipated it would take 12 months to recruit 20 eligible women for this trial. Issues surrounding the number of women with sufficient serum 25(OH)D levels (75 nmol/L or greater), disease progression, intensity of disease management (e.g., weekly infusions, appointments, surveillance or diagnostic testing), and clinical instability resulted in recruitment challenges and delays. Based on the complex nature of this disease and its treatment, it seemed insensitive or burdensome to approach many of the woman to participate, and several were deemed not to be good candidates by their treating medical oncologist. Although this phenomenon may occur frequently and represents an important consideration in advanced cancer populations, it is not well documented or articulated in the literature. It is important to include in the current article for others looking to recruit this patient population. 
Implications for Nursing and Conclusion
The authors are cautious in the interpretation and extrapolation of these data because of the open-label design, small sample size, limited statistical power, and lack of trends observed regarding inflammation or cytokine biomarkers. However, these pilot study findings highlight that women with ER+ metastatic breast cancer can safely achieve normal serum 25(OH)D levels and tend to report reduced symptom burden. Managing symptoms to maintain the highest quality of life is the primary determinant of care for these women. It is vitally important for nurses and other oncology clinicians to stay apprised of evolving science in this area and to provide evidence-based messages for patients regarding vitamin D (Viale, 2017). In the era of symptom science, cholecalciferol may be a low-cost supportive care therapy to consider for this population. However, future investigations employing rigorous study designs should be considered in a larger subset of this population.
About the Author(s)
Patricia Maureen Sheean, PhD, RDN, LDN, is an assistant professor in the Department of Applied Health Science in the Parkinson School of Health Sciences and Public Health, Patricia Robinson, MD, is an associate professor in the Department of Hematology/Oncology in the Stritch School of Medicine, the director of the Cancer Survivorship Program, and an assistant dean in the Office of Diversity, Equity, and Inclusion, Mary Beth Bartolotta, RN, BSN, is a research nurse team lead in the Cardinal Bernadin Cancer Center, Cara Joyce, PhD, MS, is the director of the biostatistics core in the Department of Health Informatics and Data Science, William Adams, PhD, is an assistant professor of medical education and public health sciences, and Sue Penckofer, PhD, RN, FAAN, is an associate dean for the Graduate School and a professor in the School of Nursing, all at Loyola University Chicago in Illinois. This study was supported by startup funds granted by the Marcella Niehoff School of Nursing at Loyola University Chicago in Maywood, Illinois. Sheean, Robinson, and Penckofer contributed to the conceptualization and design. Sheean, Robinson, and Bartolotta completed the data collection. Sheean, Joyce, and Adams provided statistical support and analysis. Sheean, Joyce, and Penckofer contributed to the manuscript preparation. Sheean can be reached at psheean1@luc.edu, with copy to ONFEditor@ons.org. (Submitted October 2020. Accepted January 8, 2021.)




