Paving the Way: A Grounded Theory of Discovery and Decision Making for Individuals With the CDH1 Marker
Purpose: To understand the process of discovery and decision making for adults with the CDH1 marker for hereditary diffuse gastric cancer and inherited breast cancer.
Participants & Setting: Purposeful sampling included 20 participants: 17 adults (11 women and 6 men aged 23–77 years) recruited through the No Stomach for Cancer organization and 3 healthcare providers. Six participants were interviewed two times. Nineteen interviews were done via telephone, and one was conducted in person.
Methodologic Approach: Grounded theory with constant comparison was used.
Findings: The decision-making process of Paving the Way addresses the challenges for individuals diagnosed with the CDH1 marker. The theory explains the process of learning the risk, discerning testing, choosing iterative individual interventions, and adjusting postoperatively while normalizing to live longer.
Implications for Nursing: The process explains and describes the nine factors for decision making and predicts the timing for nursing interventions for genetic testing and pre- and postoperative assessment and planning.
Jump to a section
Stomach cancer is the fifth leading cause of cancer worldwide, with 1,033,701 estimated new cases and 782,685 deaths reported in 2018 (Bray et al., 2018). The survival rate is particularly poor (4%) for those diagnosed with advanced disease (No Stomach for Cancer [NSFC], 2019; Pernot et al., 2015). Inherited forms of gastrointestinal cancer occur in about 1%–3% of adults. Hereditary diffuse gastric cancer (HDGC) is a rare and difficult-to-detect form of gastrointestinal cancer with a very poor prognosis (van der Post et al., 2015). HDGC is a germline (inheritable) cell-to-cell adhesion protein E-cadherin gene—known as CDH1—first discovered by a team of researchers within a native Māori family in New Zealand (Guilford et al., 1999) and since identified in Europe, Canada, and the United States (Corso et al., 2012). Although gastric cancer risk is higher in the Asian population, the incidence of a germline diffuse gastric cancer is low and seen more frequently in individuals of European descent (Sugimoto et al., 2015). The risk of developing HDGC for CDH1-positive individuals is 70% (95% confidence interval [CI] [59, 80]) for men by age 80 years and 56% (95% CI [44, 69]) for women; in addition, women have a 42% (95% CI [23, 68]) risk for lobular breast cancer (BC) (Kaurah & Huntsman, 2018; Kaurah et al., 2010). Statistical data for CDH1-related male BC remains undetermined. The average age of HDGC at diagnosis is 38 years; however, it can occur in adolescents and adults aged 14–69 years (Hansford et al., 2015; van der Post et al., 2015). Detection of HDGC is difficult because of the insidious tumor growth, which begins underneath the lining of the stomach in poorly differentiated signet ring cell cancer (Onitilo et al., 2013; Pernot et al., 2015), and the lack of observable presenting symptoms in patients (Hebbard et al., 2009; Mastoraki et al., 2011; van der Post et al., 2015).
Once a positive blood test for the CDH1 marker is determined, prophylactic total gastrectomy (PTG) is the recommended treatment to prevent aggressive adenocarcinoma in the patient, as well as CDH1 testing for family members (van der Post et al., 2015). This recommendation results in additional decision-making issues related to family disclosure, genetic testing, and further screening. The CDH1-positive individual, whether male or female, faces the problem of difficult decisions: electing surveillance with endoscopy, removing the entire stomach to avoid gastric cancer, screening for BC, or choosing a prophylactic double mastectomy to avoid BC (Hallowell, Badger, et al., 2016; Hallowell et al., 2017; Hallowell, Lawton, et al., 2016).
Information is limited for how CDH1-positive individuals embark on the decision-making process. Explicating this process could inform interventions for clinical practice and the creation of decision-making aids, providing needed patient and family support (Schreiber & Stern, 2001). Therefore, the purpose and aims of this study were to understand the process of discovery for the CDH1 marker and, once aware of the risk, the process of decision making for CDH1-positive individuals regarding HDGC and inherited BC.
Methodologic Approach
Participants and Setting
The grounded theory (GT) study was conducted over one year after obtaining institutional review board approval (IRB) at the Graduate School of Nursing at the University of Massachusetts in Worcester. Purposive sampling was used to recruit participants (N = 20) from across North America (United States and Canada). Inclusion criteria were as follows: English-speaking, CDH1-positive adults; family members at risk for the marker (tested or not tested); and healthcare providers (HCPs) caring for CDH1-positive individuals. Participants were recruited from the NSFC (2019) nonprofit organization, whose members total more than 20,000, of whom a small percentage were CDH1-positive. Recruitment occurred via the NSFC website connected to the private NSFC page on Facebook by posting a study invitation, researcher contact information, and an IRB-approved fact sheet.
Data Collection
Nineteen telephone audio-recorded interviews and one in-person audio-recorded interview (N = 20) were conducted with recorded verbal consent, using a semiflexible interview guide and a demographic form. Per GT theoretical sampling, six participants were interviewed a second time four to six months after the initial interview. The GT method (Glaser, 1998; Glaser & Strauss, 1967) was used to develop the substantive theory. After consent, interviews lasted 30–90 minutes and were audio recorded. The semiflexible interview guide was modified and expanded in subsequent interviews as the data informing the substantive theory emerged.
Data Management and Analysis
Data management and analysis used classic GT constant comparison (Glaser, 1978, 1998; Glaser & Strauss, 1967). First-level, open, selective, and theoretical coding with memo sorting were employed for the theoretical model, which included theoretical sampling to increase depth and variation (Glaser, 1998; Schreiber & Stern, 2001). Theoretical saturation was reached when no new information emerged as categories and their properties developed. The theory was created by the principal investigator from the dense verbatim data and variations that explained and encircled social and behavioral descriptions provided by participants. As the theory developed, peer debriefing, various drafts, and figures of the process were reviewed with committee members. 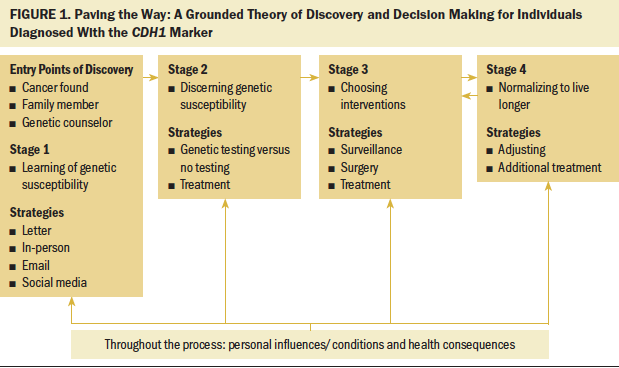
Trustworthiness
Trustworthiness was maintained by prolonged engagement within the interviews, observation at the NSFC meetings, and second interviews with 50% of the participants. Triangulation of the data sources was accomplished by the NSFC website, by later reviewing other oncology-related grounded theories, and by constant comparison of the interviews with HCPs, family members, and participants. Data integrity was maintained with an audit trail, field notes, and reflective writing (Glaser & Strauss, 1998; Lincoln & Guba, 1985). A member check was conducted with a 15-member review panel at the NSFC national meeting. The final figure of the model (see Figure 1) was reviewed additionally with two HCPs for goodness of fit, workability, making sense, and understanding. 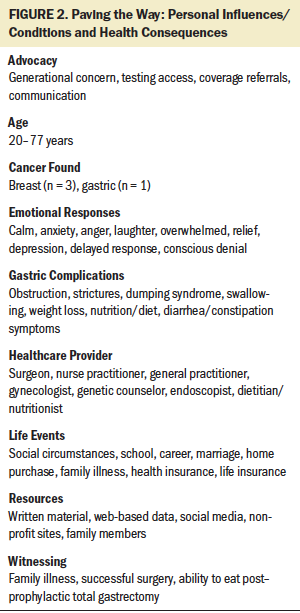
Findings
Paving the Way describes a decision-making process that leads to living longer. Paving the Way is initiated to avoid cancer to live longer through a four-stage process beginning with learning the risk of genetic susceptibility, moving to discerning genetic susceptibility, then iteratively choosing interventions to normalizing to live longer. Nine factors were identified during the study: advocacy, age, cancer found, emotions, gastric consequences, HCPs, life events, resources, and witnessing (see Figure 2). These factors influenced variations in participants’ behavior throughout the process. Demographic and clinical characteristics of the 15 CDH1-positive participants are shown in Table 1. Of the two participants who were at risk for having the CDH1 marker and declined testing, both were White/Caucasian men of Hispanic/non-Latino ethnicity; one’s marital status was divorced, and the other’s was other. Of the three HCPs interviewed, two were female and one was male; two were White/Caucasian and one was Asian. 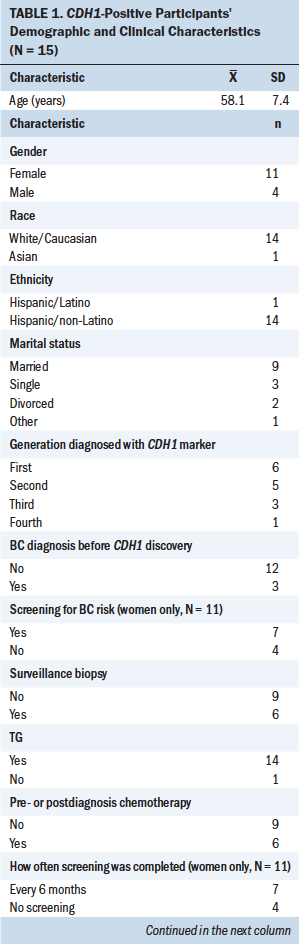
Stage 1
Reactions to learning of genetic susceptibility were described as “unnerving,” “surprising,” and “unexpected.” For some, learning of the risk was consciously denied initially or persistently because of life events, like a serious family illness or an uneasiness about facing the risk for HDGC. Disclosure of risk for the marker was routed through three points of discovery: cancer found first, family members, or referral to a genetic counselor (see Figure 3). 
Cancer found first: The first point of entry for four participants occurred when cancer was found. As one participant stated, “I discovered that I had two lumps on one of my breasts . . . and a week later I had biopsy results come back that I had lobular BC.” Another participant shared, “I was being followed for esophagitis, and I had a one-year follow-up, and on the biopsies, there were a couple of cells that looked like signet ring cell adenocarcinoma.” The cancer-found-first entry point was rapid because participants were engaged in cancer treatment from the beginning.
Family member: The second entry point appeared when the genetic mutation risk was communicated to participants by family members. Nine participants (four CDH1-positive individuals and five family members) encountered this entry point. Mothers and fathers had conducted family meetings with their children, and others reached out to siblings or more distant relatives. Many family members shared their own testing results and encouraged acting on this new knowledge. However, not all family members passed along or shared the news with others. When a CDH1-positive individual is aged younger than 30 years, parents often lead the communication.
Genetic counselor: This entry point of discovery occurred through the recommendation of an HCP or through self-referral to a genetic counselor. These participants were curious to know why their family history of cancer was so great. As one participant shared, “I have a strong family history of cancer. My mother was diagnosed with lobular BC and died before the age of 50.” Participants were referred based on a history of cancer through an HCP or by their own self-advocacy, insisting on a referral and testing related to a strong family history or a personal diagnosis of cancer. One participant’s rationale included the following: “I just thought it was a good measure to take, just to make sure and find out where the lobular came from, no stone left unturned.”
Stage 2
Stage 2 began after learning the risk for the gene. At this stage, participants sought resources themselves or learned from others through peer-to-peer resources. Discerning genetic susceptibility is marked by decisions to test for the CDH1 marker. Various reasons motivated participants to test:
• Witnessing family members with cancer: “I saw what my cousin had gone through.”
• Self-symptoms: “I was having heartburn for about a year.”
• Concern regarding a lobular BC diagnosis: “I asked for genetic testing.”
After a positive CDH1 marker test was confirmed, a myriad of new emotions arose:
• Denial: “I didn’t believe it. I didn’t understand it,” or “It’s not part of me.”
• A delayed response: “Later that day, it actually hit me.”
For some participants, emotions ranged from “a huge relief” because it answered their question, to an extreme negative feeling: “I felt that I had been given a death sentence.”
A frequent source of information was the Internet. One participant shared that after speaking to the genetic counselor, “I googled it all weekend.” Participants also used the following resources: HCPs; genetic counselors; family members, particularly parents; nonprofit organizations, like NSFC; peer-reviewed journal articles; and blogs from and instant messaging with others who were CDH1-positive. Factors that influenced the testing decision included cancer already being present, the psychological impact of not knowing, fear of cancer, curiosity regarding a strong family or personal history of cancer, and family-planning concerns for the next generation. However, not everyone decided to test for the marker. A middle-aged family member who chose not to test explained it as follows: “I block it out of my life.”
Once the decision was made, the speed for completing testing ranged from immediate to delayed. Self-advocacy was a key factor for testing referrals. Conditions for rapid testing included cancer already being present, family advocacy, and discernment of reliable resources, such as the NSFC website and vetted social media sites. For some participants, the need to know the results of their personal risk was urgent because of concern for their children: “Well, I have two young boys, and I thought they have a 50/50 chance of getting it, so I thought I’ve got to get it done.” For others, the need for more rapid testing was because of the ending of insurance benefits or, as one of the HCPs interviewed, an endoscopic specialist, shared, from a fear manifested in self-awareness of gastrointestinal symptoms. Alternatively, testing was delayed or refused, or second tests were requested for others (see Figure 4). Participants choosing no testing did not advance to stage 3 (n = 2). 
Stage 3
Stage 3 began with choosing interventions, including surveillance, surgery, or treatment after PTG, or prophylactic mastectomy (see Table 2). Varied emotions influenced engaging in next steps as family members were considered:
• “I had brought this into our family, and now they would have to deal with it for the rest of their lives.”
• “The genetic counselor couldn’t possibly be saying that I have to have my stomach removed.”
Two participants doubting their results retested and were informed of the same results with the second testing. 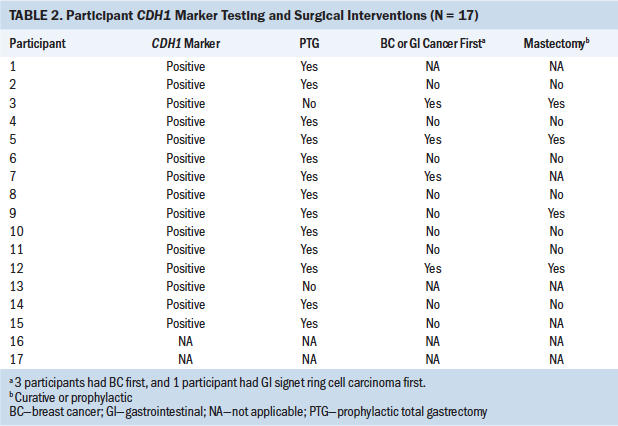
Interventions chosen included PTG, esophagogastroduodenoscopy (EGD), breast surveillance, or chemotherapy. Treatments were influenced by age, self-/family advocacy, cancer found, generational concerns, HCPs, life events, psychological impact, resources for decision making, and witnessing. Participants chose interventions sequentially, such as an EGD followed by PTG, including preoperative planning. At times, either treatment, surveillance, or surgery was warranted or required postoperatively, such as PTG dilatation of the gastrointestinal tract, dumping syndrome, or relief from an obstruction. The point of discovery influenced the order of procedures chosen (e.g., when BC was diagnosed first in a participant, treatment continued and was not interrupted by a positive test for the gene). However, some surveillance for HDGC or lobular BC may have begun prior to surgery. One participant said the following:
I have been doing the mammogram, and I am getting an MRI. I’m looking to next year to have the bilateral mastectomy, and that is a depressing factor. I mean, it’s like you lose part of what makes you feel a woman.
One factor shared by some participants at this stage involved needing to make a rapid decision about choosing an intervention to undergo PTG after hearing about HDGC from a family member, when witnessing a family member diagnosed with HDGC, or knowing individuals having surgery to rapidly remove the risk for cancer. For example, nine participants, who had witnessed family members dying from stomach cancer or BC or witnessed another with the CDH1 marker, immediately elected PTG surgery to avoid cancer rather than running any further risk by delaying the intervention. Two participants also considered prophylactic double mastectomy soon after the PTG because of a strong history of familial BC.
Participants aged older than 70 years tended to wait to have surgery and to take more time to seek the opinion of HCPs and genetic counselors to determine whether surgery was warranted because of age and other existing comorbidities. Similarly, younger individuals in their 20 and 30s were inclined to delay interventions as they considered family-planning age and family history of BC as factors for BC surveillance versus prophylactic mastectomy and multiple endoscopic biopsies prior to PTG. Conditions that affected the timing of interventions included family planning, career considerations, practical issues like health and insurance benefits, current cancer treatment, and managing family household duties.
Resources that helped participants make intervention decisions included physician consultation, genetic counselor recommendations, peer-to-peer online sharing, communication with reliable bloggers, and data available at nonprofit support sites, such as NSFC. Self-advocacy was used to obtain referrals and recommendations about where and with whom to have the PTG, including surgeon expertise in PTG intervention. A majority of the CDH1-positive participants (n = 13) expressed relief in obtaining access to travel away from home for the PTG based on the physician’s level of expertise.
The iterative nature of the decision process in stage 3 leads to stage 4: normalizing. After adjusting in stage 4, the participant is often led back to stage 3, where the next intervention decisions are made, leading to ongoing adjusting and additional treatment on an individual level of need.
Stage 4
Stage 4 involves normalizing, including adjusting postoperatively. The iterative nature of stages 3 and 4 is related, in part, to the level of complexity, particularly when a cancer diagnosis was present prior to an individual learning they were CDH1-positive. Management of the initial intervention, subsequent treatment, or additional surveillance described the work of participants during stage 4. This stage hearkened the beginning of normalizing through adjusting to the changes brought on from the interventions chosen in stage 3. Adjustments at stage 4 included managing post-PTG motility modifications, surgical complications, dietary changes, body image adjustments, and fatigue, and planning for generational concerns in the family, including future testing and surgery for the children and future family planning.
Interventions were completed while new interventions were also chosen. Timing varied in speed and order, such as how and when to consider surveillance for BC after completing gastric surgery with PTG, or vice versa in surveillance with endoscopy after undergoing a mastectomy. After each intervention was chosen and completed, priority was given to adapting through follow-up and adjusting by monitoring. Further discussions about the timing and choice of additional surgical interventions or surveillance and treatment, if required, took place as normalizing occurred, all leading back to stage 3.
Physical changes and gastric complications were reported after PTG. Weight loss was a common response. One participant noted, “I’m struggling to put weight back on, but that’s also fairly normal.” Two participants spoke of looking in the mirror with sadness regarding skin turgor or muscle mass changes. Social challenges were reported, particularly in public situations where others may watch how much or what they eat, comments about their change in body type, and comparison of their PTG to bariatric surgery for obesity. Psychological adjustments ranged from “not a big deal” and “I feel so tired” to “I just want to feel like myself again.”
Two participants reported a few adjustments to the PTG but were more concerned and dreading the next set of decisions regarding breast health and cancer risk. Emotional support, including HCP listening, peer-to-peer support through social media, and personal counseling sought by a few, was critical throughout stage 4. When asked about any advice during the adaptation phase of her surgery, one participant shared her approach as follows: “It’s really more of a mental game than the actual physical because the physical is something you can’t really change.”
Another participant indicated, “One of the things I have learned is that I have to be a really good advocate for myself.” Self-advocacy after PTG included informing HCPs about which oral medications, such as iron or B12, were tolerable or malabsorbed without a stomach. An older participant described the new rebalancing after PTG: “My iron was running a tiny bit low. The B12 was running low. The D was running a little low. I take supplements for all of those. . . . They’re now all within the normal range.” The length of the adjustment period varied for participants to adopt a new diet, smaller more frequent meals, and social situations involving intolerance to food.
Resources for learning what was expected pre- and postprocedure were identified consistently within distant specialty centers, sometimes locally, and often through peer-to-peer online support. As in stage 3, participants sought others who had similar issues or experience. Participants were often managing multiple issues, such as caring for a family while undergoing treatment, seeking a nutritionist or registered dietitian, and considering family planning, during stage 4. They solved their challenges in this stage by reaching out for practical health and psychological support with peer-to-peer social media, including bloggers, and others sought traditional support groups and attended meetings, such as the NSFC Spotlight events. Paving the Way is a complex, difficult, and emotionally challenging process. The decision to ultimately proceed with PTG was best summed up by one participant’s revealing comment: “Basically, I saw that I had an 83% overall chance of getting stomach cancer.” The four stages of decision making in Paving the Way offer a chance to live longer by mitigating or avoiding cancer.
Discussion
The findings of this study demonstrate that individuals who are CDH1-positive face a complex process of problem solving through decision making. Paving the Way is comparable to other GT research on the BRCA gene and ovarian cancer. Three GT studies concluded that the goal of decision making for patients with the BRCA gene included preserving oneself (Howard et al., 2011) and maximizing survival (Jeffers et al., 2014); in addition, with patients experiencing ovarian cancer, the goal of facing a life-threatening cancer is preserving oneself in the face of uncertainty (Pozzar & Berry, 2019). The findings of this current study also reflect the need for individuals with the CDH1 marker to make decisions to avoid or detect cancer early to preserve their health and have longer survival.
Several decisions were made throughout the process of Paving the Way from the time participants were first made aware of their risk of having a genetic syndrome, involving testing, undergoing procedures, or surgery. According to a qualitative descriptive study of previvors for hereditary breast and ovarian cancer, they needed resources in four stages: pretesting, post-testing, premanagement, and postmanagement (Dean et al., 2017). A Norwegian GT described that participants handled emotions while waiting for a gastric diagnosis by preparative waiting that outlined a balance between hope and despair (Giske & Gjengedal, 2007). An Australian registration study reviewing predictive testing for hereditary colorectal cancer syndromes confirmed that participants declined testing for numerous reasons involving trusting the resources, benefits outweighing risks, and a need for witnessing positive outcomes over negative before electing testing (Keogh et al., 2017). This study revealed that most, but not all, participants decided to share information that family testing was recommended. They deliberated on the timing of when and how to tell because of age and life events, and whom to share within the family, which is consistent with this study and patterns of disclosure to families at risk for Huntington disease (Klitzman et al., 2007). This study’s findings of interventions and adjusting to life through normalizing compares to other GT studies of patient survivorship. Examples are as follows: transitioning to survivorship after the diagnosis of Hodgkin lymphoma (Matheson et al., 2016), BC survivors reclaiming life on their own terms (Sherman et al., 2012), individuals adjusting to a changed life with the diagnosis of multiple sclerosis (Satinovic, 2017), and gaining normalcy as individuals are reengaged and reinvigorated mentally and physically after the disruption of a cancer diagnosis (Walker et al., 2015).
The influences and conditions found in this study of age, advocacy, and the timing of life events compare to the following studies:
• Life Course Perspective (LCP), a GT of living with genetic vulnerability (Hamilton et al., 2016a, 2016b)
• A case study of pregnancy after PTG (Kaurah et al., 2010)
• The predictive genetic testing GT of engagement (McAllister, 2002)
• The virtual model program in oncology for navigation to promote self-advocacy (Schaffer et al., 2019)
In LCP, age was the primary factor of focus subdividing the cohort into groups—timing of events (20s), human agency (30s), and linked lives (40s–50s)—all of which are comparable to the factors of age, advocacy, and life events throughout the stages of the current study.
Implications for Nursing
Nurses are in a unique position to serve as genetic nursing counselors in high-risk oncology clinics during pre- and postsurgical intervention as navigators, educators, and advocates for individuals diagnosed with this rare genetic syndrome and their families. The shortage of certified genetic counselors, particularly in rural areas, positions nursing to further develop the specialty role of the genetic counseling nurse to assist individuals diagnosed with rare markers, like CDH1. Patients living longer through preventive genetic decision making need a nurse in the role of survivorship coach and throughout the adjustment process with wellness navigation. Evaluations postoperatively, including appropriate follow-up services with specialized registered dietitians through nurse navigation with the patient, are essential for wellness post-PTG.
Nurses will need further knowledge regarding the histology and biomarkers linked with rare genetic diseases, such as the CHD1 marker. In this study, one healthcare provider indicated an area for improvement:
In my experience, most patients don’t know if they have a family member that has the CDH1 gene, and most family members don’t even know what kind of dangers exist in their life, for instance, what kind of stomach cancer their parents had.
Oncology nurses can emphasize the need to obtain a three-generation family history identifying potential risks of inheritance by family members with previous gastrointestinal or BC diseases. The process of decision making includes nine factors for future research interventions. The factors can be studied for use in patient assessment, testing and counseling, and precision medicine treatment decisions. Oncology nurses are poised to provide psychoeducation and individualized supportive plans of care for patients with the CDH1 marker who are seeking resources and knowledge throughout each stage of this decision-making process.
Limitations
Limitations to generalization of the study include individuals diagnosed with the CDH1 marker, family members, and HCPs who work with those at risk for the marker. The study did not fully explore and was not designed to focus on delaying nor avoiding testing for the CDH1 marker. Time of greater than one year may have informed further variation and factors that did not emerge during the study’s one-year duration. 
Conclusion
The findings from this study suggest that the process of Paving the Way offers nurses an explanation and greater understanding of the process for individuals diagnosed with the CDH1 marker. Assessments applying the model will aid in navigating patients through the layers of complexity for decision making. The process indicates nursing roles as navigator, counselor, and survivorship coach. The nine factors influencing decision making can guide future research and measurements to assess, advise, and follow patients with the CDH1 marker who face PTG and BC risk.
About the Author(s)
Cheryl L. Hersperger, PhD, RN, PHNA-BC, is an assistant professor in the Dr. Lillian R. Goodman Nursing Department at Worcester State University, and Jean Boucher, PhD, RN, ANP-BC, AOCNP®, and Rosemary Theroux, PhD, WHNP-BC, are both associate professors in the Graduate School of Nursing at the University of Massachusetts, all in Worcester, MA. No financial relationships to disclose. All authors contributed to the conceptualization, design, and manuscript preparation and provided analysis. Hersperger completed the data collection and provided statistical support. Hersperger can be reached at chersperger@worcester.edu, with copy to ONFEditor@ons.org. (Submitted December 2019. Accepted February 20, 2020.)




