Fertility Preservation Before Cancer Treatment: Options, Strategies, and Resources
Background: Loss of fertility is one of the many potential late effects of cancer treatment. For young men and women who have not yet started or completed building their families, this can be a source of considerable emotional distress. Advances in reproductive technology can enable many of these patients to preserve their fertility; however, discussions must be initiated early enough during treatment planning to enable them to take advantage of these options.
Objectives: The purpose of this article is to provide oncology nurses with information, strategies, and resources to discuss fertility with men and women starting cancer treatment.
Methods: This article summarizes the literature on treatment-related fertility risks and fertility preservation options, and provides a systematic framework for nurses to integrate these discussions into practice.
Findings: Oncology nurses can effectively collaborate with other members of the healthcare team to ensure that young men and women starting cancer treatment are informed of the potential risks to fertility from their planned treatment, understand options to preserve fertility before treatment, and, if interested, are referred to appropriate reproductive specialists.
Jump to a section
Loss of fertility is one of the many potential late effects of cancer treatment, and for young men and women, this can be a source of considerable emotional distress (Crawshaw & Sloper, 2010; Goossens et al., 2014; Peate, Meiser, Hickey, & Friedlander, 2009; Schover, Brey, Lichtin, Lipshultz, & Jeha, 2002; Tschudin & Bitzer, 2009). Recognizing the significance of this concern, a number of professional organizations have published guidelines outlining the responsibility of healthcare providers to inform patients of the potential risks to fertility from their planned treatment, discuss options to preserve fertility before treatment, and refer interested patients to appropriate reproductive specialists (American Society for Reproductive Medicine [ASRM], 2013a; Coccia et al., 2014; Fallat & Hutter, 2008; Lee et al., 2006; Loren et al., 2013; Pentheroudakis, Orecchia, Hoekstra, & Pavlidis, 2010). This article provides oncology nurses with information, strategies, and resources to effectively integrate these discussions into practice.
Fertility Risks
Multiple factors contribute to the risk of infertility after cancer treatment, so predicting with certainty how any one individual will be affected is impossible. Quantifying the risks of specific antineoplastic agents is particularly challenging because most are used in combination, doses vary based on regimen, and the number of new agents—including targeted therapies—is increasing, with minimal long-term data on fertility outcomes. Alkylating agents pose the highest risk of infertility, and platinum analogs, anthracyclines, and taxanes pose an intermediate risk (Ben-Aharon & Shalgi, 2012; Blumenfeld, 2012; Howell & Shalet, 2005; Meirow, Biederman, Anderson, & Wallace, 2010; Meistrich, 2009; Yamaguchi & Fujisawa, 2011).
Fertility Risks for Men
In men, chemotherapy, as well as exposure of the testes to radiation, can destroy spermatogonial germ cells with subsequent impairment of sperm production (Meistrich, 2009). Many men will recover sperm production within one to three years after treatment is completed, but some will require more time, and some may have permanent azoospermia, or absence of sperm (Howell & Shalet, 2005; Meistrich, 2009). Pelvic surgery or radiation may cause injury to the genitourinary ductal system, nerves, and blood vessels, with subsequent erectile or ejaculatory dysfunction and an inability to deliver sperm naturally to a female partner through intercourse (Magelssen, Brydoy, & Fossá, 2006). Cranial irradiation or surgery may cause injury to the pituitary gland, impairing hormonal regulation of spermatogenesis (Wang, Muller, & Lin, 2013).
Fertility Risks for Women
In women, chemotherapy, as well as exposure of the ovaries to radiation, can destroy follicles (each containing a single oocyte or egg), causing premature ovarian failure, with subsequent infertility and early menopause (Meirow et al., 2010; Stroud et al., 2009). Many of these women will not lose fertility immediately after treatment but may become infertile at an early age (Meirow et al., 2010). Women lose eggs naturally over time, so older women are at increased risk of infertility from treatment (Meirow et al., 2010). After bilateral oophorectomy, women will develop immediate infertility and menopause, and after hysterectomy, women will not be able to carry a pregnancy (Gershenson, 2005). Radiation exposure of the uterus will cause fibrotic changes, leading to endometrial damage, vascular insufficiency, and loss of myometrial elasticity, with subsequent inability to support embryo implantation or accommodate a growing fetus (Critchley & Wallace, 2005). Women who become pregnant after pelvic radiation are at risk of miscarriage, preterm birth, and having a baby with a low birth weight (Teh, Stern, Chander, & Hickey, 2014). Cranial irradiation or surgery may cause injury to the pituitary gland, impairing hormonal regulation of oocyte maturation and ovulation (Kort, Eisenberg, Millheiser, & Westphal, 2014).
Fertility Preservation Before Cancer Treatment
With advances in reproductive technology, options for preserving fertility are increasing. Not all patients will desire or be able to pursue fertility preservation (FP); however, for those who are interested, this must be completed before treatment begins because even a single treatment with therapy that is damaging to the testes or ovaries can affect the quality and DNA integrity of sperm and eggs (Lee et al., 2006).
Options for Men
For postpubertal males, sperm banking is the optimal method of preserving fertility potential (Trost & Brannigan, 2012). Men masturbate to ejaculation to obtain a semen specimen. This is analyzed at a licensed laboratory in a sperm bank or andrology laboratory to ensure that viable sperm are present, and the semen is placed in vials, frozen, and stored for potential future use (Katz, Kolon, Feldman, & Mulhall, 2013). Most men collect their specimens at a sperm bank, but mail-in kits are available for men to collect at home. Even hospitalized men can collect a specimen if arrangements can be made to transport it to the laboratory within one hour.
Collection of three specimens, scheduled with two to five days of abstinence before each, generally is recommended (Nangia, Krieg, & Kim, 2013). If not enough time is available for this before treatment must begin, then shorter intervals (e.g., every 24 hours) or collection of only a single specimen can be recommended because new reproductive technologies enable fertilization of eggs even with very low numbers of sperm (Nangia et al., 2013).
For postpubertal males who cannot collect by masturbation (e.g., pain, shortness of breath, emotional distress, embarrassment, religious prohibitions, cultural factors), electroejaculation is an option (Katz et al., 2013). For those who have no sperm found in their semen (e.g., effect of malignancy, prior vasectomy), testicular sperm extraction is an option (Stahl, Stember, Hsiao, & Schlegel, 2010). For prepubertal boys who do not yet produce sperm, testicular tissue freezing is available at select centers as an experimental option (Ginsberg et al., 2010). FP options for males are described in Figure 1. 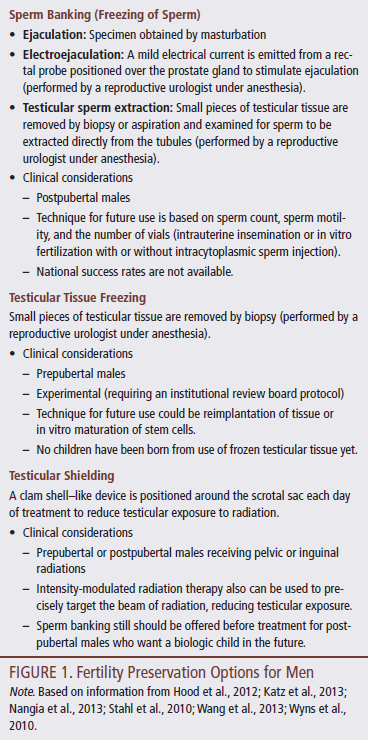
Options for Women
For postpubertal, premenopausal females, embryo freezing by a reproductive endocrinologist has been the optimal method of preserving fertility potential (ASRM, 2013a; Lee et al., 2006; Loren et al., 2013). This requires about 10 days of ovarian stimulation with hormonal medications self-injected daily by the patient, followed by transvaginal retrieval of mature eggs performed under anesthesia (Rodriguez-Wallberg & Oktay, 2012). The eggs are fertilized with sperm in the laboratory (in vitro fertilization), and the resulting embryos are frozen and stored for potential future use.
Until 2012, women without a male partner who did not want to use donor sperm to create embryos and women who did not want to freeze embryos for religious or personal reasons could not avail themselves of this option. However, in young women treated at centers experienced in egg-freezing techniques, success rates using frozen eggs are similar to those with frozen embryos, so freezing of unfertilized eggs is no longer considered to be experimental (ASRM, 2013b).
One concern with embryo and egg freezing is that treatment must be delayed until after egg retrieval. Various protocols have been developed to allow for stimulation to begin at any point in the menstrual cycle (Cakmak & Rosen, 2013), so the entire process generally can be completed in two to three weeks. Another concern is that ovarian stimulation causes elevated estrogen; this can be minimized with concurrent letrozole (Femara®) in women with hormone-sensitive tumors (Reddy & Oktay, 2012).
For females who cannot take the two to three weeks required to undergo egg or embryo freezing or those who are prepubertal and do not yet have mature eggs to be collected, ovarian tissue freezing may be available at select centers as an experimental option. The ovary (or pieces of ovarian tissue) is removed, and the cortex is dissected and frozen (Chung, Donnez, Ginsburg, & Meirow, 2013). Other FP options include ovarian transposition, ovarian suppression, and modifications in the cancer treatment plan. FP options for females are described in Figure 2. 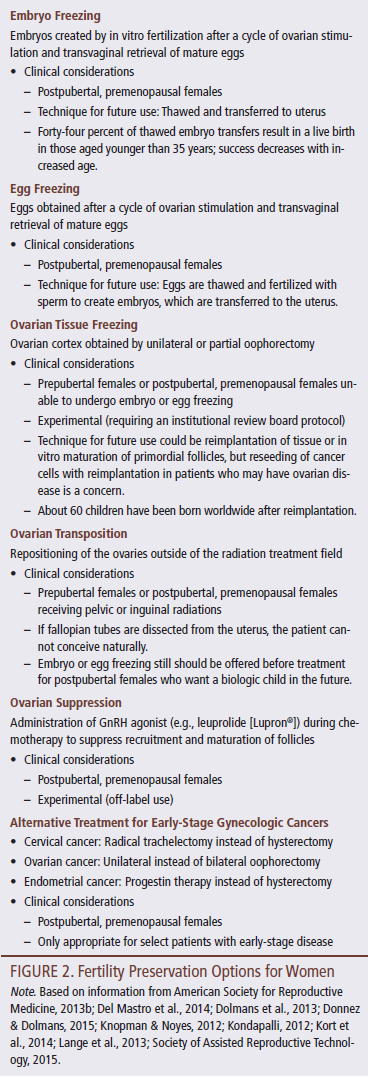
Clinical Implications
Clinicians cannot assume that patients will ask about fertility risks and FP options if they are interested. They may be upset and overwhelmed by their cancer diagnosis, or it may not have occurred to them that the planned treatment could pose a risk of infertility. Initiating the discussion is the clinician’s responsibility (Loren et al., 2013); however, communicating with newly diagnosed men and women about these issues can be challenging. Barriers reported by clinicians include lack of knowledge, personal discomfort, time pressures, perception that patients aren’t interested, financial costs, need for immediate treatment, and poor prognosis (Goossens et al., 2014). Recognizing that the goal is for patients to have options in how they become parents after treatment, Parenthood After Cancer TReatment has served as a framework for the author in providing a structured systematic approach that can be applied in any setting.
P: Prepare for the Discussion
When thinking about how to initiate or improve discussions of fertility with patients, laying the groundwork is important. A number of factors need to be considered.
Consider how fertility issues are currently dealt with and identify gaps to be addressed. Consider assumptions or biases that unconsciously may lead to avoiding fertility discussions with select patients. Men and women with cancer want information about their risks and FP options regardless of age, relationship status, prior children, stage of disease, prognosis, or socioeconomic status (Goossens et al., 2014; Peate et al., 2009).
Identify local reproductive specialists who can provide information and services to individuals with cancer who are interested in pursuing FP or in learning more about their options. Figure 3 lists resources for finding reproductive specialists. Establish systems for ensuring timely referrals and coordinating care, and ask about costs and the availability of discounted rates, payment plans, or financial assistance. 
The average cost of sperm banking is $1,000–$1,500 (Livestrong Foundation, 2013a), and the average cost of egg or embryo freezing is $11,900–$12,400 (Livestrong Foundation, 2013b), with an additional $3,000–$5,000 for medication. Annual fees also exist for storage. Figure 4 lists resources for financial assistance, generally obtained through the reproductive specialist. 
Obtain written educational resources with information on cancer and fertility to reinforce and expand on the information to be discussed. Nurses can develop their own material, acquire it from local reproductive specialists, use brochures from other organizations, or provide a list of websites with relevant information. Figure 5 lists a number of resources to educate patients about cancer and fertility. 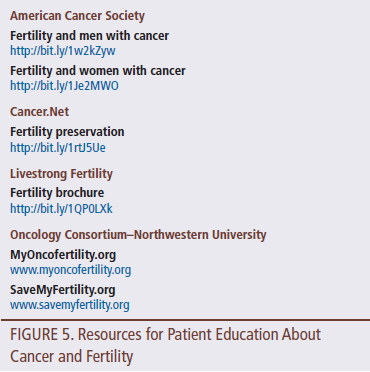
Finally, collaborate with oncologists and other healthcare providers on how to integrate this discussion into practice. Nurses working in the outpatient office practice setting with a single physician or team of physicians—where patients are seen at diagnosis and before the treatment plan is finalized—are in the optimal position to influence practice. With physicians who are resistant, point out that the updated American Society of Clinical Oncology (ASCO)/Oncology Nursing Society chemotherapy administration safety standards (Neuss et al., 2013) require that patients be informed of fertility risks as part of the informed consent process. If they are unaware of FP options, draw attention to the updated ASCO guidelines (Loren et al., 2013) and associated resources that are available online. Nurses should identify groups of men and women within their practice who are most at risk for impaired fertility based on their age, diagnosis, and planned treatments. Decide when is best to introduce the issue, ensuring that this occurs early enough before treatment begins to allow patients adequate time to make decisions and pursue FP if interested. Decide on the roles each team member will play. The physician can introduce the risk of infertility as one of the many potential risks and side effects of treatment, and the nurse can discuss FP options and refer interested patients to an appropriate reproductive specialist. Patient navigators or social workers also may play a role in these discussions. Even in the inpatient setting or outpatient infusion setting, oncology nurses may identify men and women who have not yet started treatment and have concerns about fertility. Bringing this to the attention of the patient’s oncologist may enable patients to take advantage of FP before treatment begins.
A: Assess Patient Understanding and Interest
No one way to start the conversation is best, but assessing understanding and interest will provide direction on how to proceed. Examples of questions to ask include:
• Has anyone discussed the possible effects of your planned treatment on your ability to have children in the future?
• Would you like information about the impact of treatment on your future fertility?
• Would you like information about possible options for preserving your fertility?
• Would you like information about your options for building a family in the future?
Patients have varying religious, cultural, and ethical beliefs about the significance of fertility and the use of assisted reproductive technology. Infertility is seen as a stigma in some communities, and some religious groups do not allow masturbation to obtain semen; use of donor eggs, donor sperm, or a gestational carrier for family building; or freezing of embryos. These issues will influence patients’ interest in pursuing fertility preservation (Ayensu-Coker, Essig, Breech, & Lindheim, 2013).
C: Consider the Patient’s Disease and Planned Treatment and the Safety of Fertility Preservation
Information provided should be individualized, considering the patient’s risk of infertility, safety of delaying treatment, and medical risks associated with the invasive procedures that may be required.
As mentioned previously, the risk of infertility for any one individual cannot be predicted with certainty; however, particular treatment regimens are associated with known risks. In addition to searching in PubMed for updated data on drug-specific risks of infertility, resources listed in Figure 6 can help determine patient-specific risks. Consider not only the initial treatment that is planned, but also future treatment the patient may receive (e.g., postoperative chemotherapy, transplantation, second-line therapy in patients at high risk for relapse or refractory disease). Some patients will want to pursue FP even if the planned treatment is associated with a very low risk of infertility. 
Early referrals to reproductive specialists can minimize delays in starting treatment and enable more patients to take advantage of optimal FP methods (i.e., sperm banking with three collections [7–10 days] or ovarian stimulation with egg or embryo freezing [two to three weeks]). However, for those who cannot delay, other options, as described previously, may be more appropriate to offer.
Potential medical risks associated with the procedures required for FP are of particular concern for women, for whom the process is more invasive. Potential risks include (a) bleeding with egg retrieval in patients with thrombocytopenia or liver dysfunction, (b) accidental tumor puncture with bleeding if the patient has a large vascular pelvic mass, (c) infection in patients with neutropenia, and (d) respiratory complications with anesthesia in patients with bulky chest disease or superior vena cava syndrome (ASRM, 2013a; Cakmak & Rosen, 2013; Chung et al., 2013; Noyes et al., 2013). Collaborate with the patient’s treating oncologist in considering all of these issues to ensure that patients are offered appropriate options based on their personal situation. If referrals are made, communicate all relevant information to the reproductive specialist to ensure patient safety.
T: Teach About Risks and Options
Explain how treatment may affect fertility. When first learning of this risk, many patients react with significant emotional distress. Nevertheless, be direct, honest, and matter of fact, while acknowledging how upsetting it can be to hear this information. Describe the options available to them to preserve fertility, and elicit their thoughts on how they would like to proceed.
Many female patients experience decisional conflict when considering FP with egg or embryo freezing (Mersereau et al., 2013). Influencing factors include (a) the importance of having a biologic child (versus their acceptance of alternative options for building a family); (b) concerns about the safety of ovarian stimulation and future pregnancy; (c) willingness to use assisted reproductive technology; (d) the likelihood of success, particularly for older women or those with preexisting reproductive health problems; (e) religious, cultural, and ethical beliefs about family building; (f) the degree of emotional distress they are experiencing; and (g) perceived support from their partner, family, and clinicians (Halliday & Boughton, 2011; Hershberger, Finnegan, Altfeld, Lake, & Hirshfeld-Cytron, 2013; Mersereau et al., 2013; Peddie et al., 2012). Oncology nurses can counsel women as they make decisions about FP by ensuring that they understand their risks and options, exploring prior related experiences, helping them clarify their preferences and values, and tailoring the discussion to their unique personal situation (Hershberger et al., 2013). Be respectful and nonjudgmental. Not everyone wants to or should undergo FP, but everyone has the right to make his or her own decision.
Adolescent male patients initially may be reluctant to discuss sperm banking. They may not be thinking about future fatherhood, and the conversation may be embarrassing to them. They may seem indifferent or even refuse to consider this; however, balancing respect for their autonomy with consideration of their future interests is important (Shnorhavorian, Johnson, Shear, & Wilfond, 2011). Potential for regret exists if they do not pursue sperm banking and later become infertile from treatment (Crawshaw, Glaser, Hale, & Sloper, 2009). One option is that sperm banking can be presented as a standard of care for all newly diagnosed adolescent males (Achille et al., 2006), pointing out that this does not commit them to using their sperm but will give them more options in the future if they want to parent a child. Offer the opportunity to discuss this without their parents present, and probe to explore their concerns, encouraging them while acknowledging that it is their choice (Crawshaw et al., 2009; Shnorhavorian et al., 2011).
When counseling patients as they make decisions, recognize that the financial costs may be prohibitive for many. Although some state mandates require insurance coverage for treatment of infertility, at present, no states require coverage for FP (Kondapali & Crisci, 2014; Quinn et al., 2011).
Also important is informing patients of alternative ways to build a family if they are unable or choose not to pursue FP and have impaired fertility in the future. These include use of donor sperm, donor eggs, or donor embryos; surrogacy with a gestational carrier (i.e., arranging for another woman to carry a pregnancy); and adoption. Some individuals decide not to pursue FP, choosing instead not to have additional children or to pursue a child-free lifestyle.
R: Refer Interested Patients to Reproductive Specialists
For individuals who want to pursue FP or want to learn more, the nurse can notify the oncology treatment team to ensure that they have no concerns and then make the referral to the appropriate reproductive specialist. The nurse also may assist by providing relevant medical information to the reproductive specialist and following up to coordinate care as needed and plan for the start of cancer treatment after FP is completed.
Conclusion
A number of options are available for young men and women to preserve fertility before cancer treatment. By ensuring that patients are informed of their risks and options, and referring interested patients to appropriate reproductive specialists, oncology nurses can play a significant role in helping patients fulfill their hopes of becoming parents in the future. With knowledge about treatment-related fertility risks and FP options, and the use of a systematic approach when discussing fertility, nurses can be successful in incorporating these discussions into their clinical practice. 
References
Achille, M.A., Rosberger, Z., Robitaille, R., Lebel, S., Gouin, J.-P., Bultz, B.D., & Chan, P.T. (2006). Facilitators and obstacles to sperm banking in young men receiving gonadotoxic chemotherapy for cancer: The perspective of survivors and health care professionals. Human Reproduction, 21, 3206–3216. doi:10.1093/humrep/del307
American Society for Reproductive Medicine. (2013a). Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertility and Sterility, 100, 1214–1223. doi:10.1016/j.fertnstert.2013.08.012
American Society for Reproductive Medicine. (2013b). Mature oocyte cryopreservation: A guideline. Fertility and Sterility, 99, 37–43. doi:10.1016/j.fertnstert.2012.09.028
Ayensu-Coker, L., Essig, E., Breech, L.L., & Lindheim, S. (2013). Ethical quandaries in gamete-embryo cryopreservation related to oncofertility. Journal of Law, Medicine and Ethics, 41, 711–719.
Ben-Aharon, I., & Shalgi, R. (2012). What lies behind chemotherapy-induced ovarian toxicity? Reproduction, 144, 153–163. doi:10.1530/rep-12-0121
Blumenfeld, Z. (2012). Chemotherapy and fertility. Best Practice and Research: Clinical Obstetrics and Gynaecology, 26, 379–390. doi:10.1016/j.bpobgyn.2011.11.008
Cakmak, H., & Rosen, M.P. (2013). Ovarian stimulation in cancer patients. Fertility and Sterility, 99, 1476–1484. doi:10.1016/j.fertnstert.2013.03.029
Chung, K., Donnez, J., Ginsburg, E., & Meirow, D. (2013). Emergency IVF versus ovarian tissue cryopreservation: Decision making in fertility preservation for female cancer patients. Fertility and Sterility, 99, 1534–1542. doi:10.1016/j.fertnstert.2012.11.057
Coccia, P.F., Pappo, A.S., Altman, J., Bhatia, S., Borinstein, S.C., Flynn, J., . . . Sundar, H. (2014). Adolescent and young adult oncology, version 2.2014. Journal of the National Comprehensive Cancer Network, 12, 21–32.
Crawshaw, M.A., Glaser, A.W., Hale, J.P., & Sloper, P. (2009). Male and female experiences of having fertility matters raised alongside a cancer diagnosis during the teenage and young adult years. European Journal of Cancer Care, 18, 381–390. doi:10.1111/j.1365-2354.2008.01003.x
Crawshaw, M.A., & Sloper, P. (2010). ‘Swimming against the tide’—The influence of fertility matters on the transition to adulthood or survivorship following adolescent cancer. European Journal of Cancer Care, 19, 610–620. doi:10.1111/j.1365-2354.2009.01118.x
Critchley, H.O.D., & Wallace, W.H.B. (2005). Impact of cancer treatment on uterine function. Journal of the National Cancer Institute. Monographs, 34, 64–68. doi:10.1093/jncimonographs/lgi022
Del Mastro, L., Ceppi, M., Poggio, F., Bighin, C., Peccatori, F., Demeestere, I., . . . Bruzzi, P. (2014). Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: Systematic review and meta-analysis of randomized trials. Cancer Treatment Reviews, 40, 675–683. doi:10.1016/j.ctrv.2013.12.001
Dolmans, M.-M., Luyckx, V., Donnez, J., Andersen, C.Y., & Greve, T. (2013). Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertility and Sterility, 99, 1514–1522. doi:10.1016/j.fertnstert.2013.03.027
Donnez, J., & Dolmans, M.M. (2015). Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. Journal of Assisted Reproduction and Genetics, 32, 1167–1170.
Fallat, M.E., & Hutter, J. (2008). Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. Retrieved from http://pediatrics.aappublications.org/content/121/5/e1461
Gershenson, D.M. (2005). Fertility-sparing surgery for malignancies in women. Journal of the National Cancer Institute. Monographs, 34, 43–47. doi:10.1093/jncimonographs/lgi011
Ginsberg, J.P., Carlson, C.A., Lin, K., Hobbie, W.L., Wigo, E., Wu, X., . . . Kolon, T.F. (2010). An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: A report of acceptability and safety. Human Reproduction, 25, 37–41. doi:10.1093/humrep/dep371
Goossens, J., Delbaere, I., Van Lancker, A., Beeckman, D., Verhaeghe, S., & Van Heck, A. (2014). Cancer patients’ and professional caregivers’ needs, preferences and factors associated with receiving and providing fertility-related information: A mixed-methods systematic review. International Journal of Nursing Studies, 51, 300–319. doi:10.1016/j.ijnurstu.2013.06.015
Halliday, L.E., & Boughton, M.A. (2011). Exploring the concept of uncertain fertility, reproduction and motherhood after cancer in young adult women. Nursing Inquiry, 18, 135–142. doi:10.1111/j.1440-1800.2011.00532.x
Hershberger, P.E., Finnegan, L., Altfeld, S., Lake, S., & Hirshfeld-Cytron, J. (2013). Toward theoretical understanding of the fertility preservation decision-making process: Examining information processing among young women with cancer. Research and Theory for Nursing Practice, 27, 257–275.
Hood, R.C., Wu, Q.J., McMahon, R., Czito, B., & Willett, C. (2012). IMRT treatment of anal cancer with a scrotal shield. Medical Dosimetry, 37, 432–435. doi:10.1016/j.meddos.2012.03.007
Howell, S.J., & Shalet, S.M. (2005). Spermatogenesis after cancer treatment: Damage and recovery. Journal of the National Cancer Institute. Monographs, 34, 12–17. doi:10.1093/jncimonographs/lgi003
Katz, D.J., Kolon, T.F., Feldman, D.R., & Mulhall, J.P. (2013). Fertility preservation strategies for male patients with cancer. Nature Reviews Urology, 10, 463–472. doi:10.1038/nrurol.2013.145
Knopman, J.M., & Noyes, N. (2012). Mitigating the risk: The role of ovarian transposition and medical suppression. In C. Gracia & T.K. Woodruff (Eds.), Oncofertility medical practice: Clinical issues and implementation (pp. 91–104). New York, NY: Springer.
Kondapalli, L.A. (2012). Ovarian tissue cryopreservation and transplantation. In C. Garcia & T.K. Woodruff (Eds.), Oncofertility medical practice: Clinical issues and implementation (pp. 63–75). New York, NY: Springer.
Kondapalli, L.A., & Crisci, A. (2014). Incorporating insurance education into the fertility preservation process. In T.K. Woodruff, M.L. Clayman, & K.E. Waimey (Eds.), Oncofertility communication: Sharing information and building relationships across disciplines (pp.167–180). New York, NY: Springer.
Kort, J.D., Eisenberg, M.L., Millheiser, L.S., & Westphal, L.M. (2014). Fertility issues in cancer survivorship. CA: A Cancer Journal for Clinicians, 64, 118–134. doi:10.3322/caac.21205
Lange, S., Tait, D., & Matthews, M. (2013). Oncofertility: An emerging discipline in obstetrics and gynecology. Obstetrical and Gynecological Survey, 68, 582–593. doi:10.1097/OGX.0b013e31829d460d
Lee, S.J., Schover, L.R., Partridge, A.H., Patrizio, P., Wallace, W.H., Hagerty, K., . . . Oktay, K. (2006). American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. Journal of Clinical Oncology, 24, 2917–2931. doi:10.1200/jco.2006.06.5888
Livestrong Foundation. (2013a). Family-building options for men. Retrieved from http://images.livestrong.org/pdfs/livestrong-fertility/LF_PreservationO…
Livestrong Foundation. (2013b). Family-building options for women. Retrieved from http://images.livestrong.org/pdfs/livestrong-fertility/LF_PreservationO…
Loren, A.W., Mangu, P.B., Beck, L.N., Brennan, L., Magdalinski, A.J., Partridge, A.H., . . . Oktay, K. (2013). Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology, 31, 2500–2510. doi:10.1200/jco.2013.49.2678
Magelssen, H., Brydoy, M., & Fossá, S.D. (2006). The effects of cancer and cancer treatments on male reproductive function. Nature Clinical Practice. Urology, 3, 312–322. doi:10.1038/ncpuro0508
Meirow, D., Biederman, H., Anderson, R.A., & Wallace, W.H. (2010). Toxicity of chemotherapy and radiation on female reproduction. Clinical Obstetrics and Gynecology, 53, 727–739.
Meistrich, M.L. (2009). Male gonadal toxicity. Pediatric Blood and Cancer, 53, 261–266. doi:10.1002/pbc.22004
Mersereau, J.E., Goodman, L.R., Deal, A.M., Gorman, J.R., Whitcomb, B.W., & Su, H.R. (2013). To preserve or not to preserve: How difficult is the decision about fertility preservation? Cancer, 119, 4044–4050. doi:10.1002/cncr.28317
Nangia, A.K., Krieg, S.A., & Kim, S.S. (2013). Clinical guidelines for sperm cryopreservation in cancer patients. Fertility and Sterility, 100, 1203–1209. doi:10.1016/j.fertnstert.2013.08.054
Neuss, M.N., Polovich, M., McNiff, K., Esper, P., Gilmore, T.R., LeFebvre, K.B., . . . Jacobson, J.O. (2013). 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards including standards for the safe administration and management of oral chemotherapy. Oncology Nursing Forum, 40, 225–233. doi:10.1188/13.ONF.40-03AP2
Noyes, N., Melzer, K., Druckenmiller, S., Fino, M.E., Smith, M., & Knopman, J.M. (2013). Experiences in fertility preservation: Lessons learned to ensure that fertility and reproductive autonomy remain options for cancer survivors. Journal of Assisted Reproduction and Genetics, 30, 1263–1270. doi:10.1007/s10815-013-0066-2
Peate, M., Meiser, B., Hickey, M., & Friedlander, M. (2009). The fertility-related concerns, needs and preferences of younger women with breast cancer: A systematic review. Breast Cancer Research and Treatment, 116, 215–223. doi:10.1007/s10549-009-0401-6
Peddie, V.L., Porter, M.A., Barbour, R., Culligan, D., MacDonald, G., King, D., . . . Bhattacharya, S. (2012). Factors affecting decision making about fertility preservation after cancer diagnosis: A qualitative study. BJOG: An International Journal of Obstetrics and Gynaecology, 119, 1049–1057.
Pentheroudakis, G., Orecchia, R., Hoekstra, H.J., & Pavlidis, N. (2010). Cancer, fertility and pregnancy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 21(Suppl. 5), v266–v273. doi:10.1093/annonc/mdq198
Quinn, G.P., Vadaparampil, S.T., McGowan Lowrey, K., Eidson, S., Knapp, C., & Bukulmez, O. (2011). State laws and regulations addressing third-party reimbursement for infertility treatment: Implications for cancer survivors. Fertility and Sterility, 95, 72–78. doi:10.1016/j.fertnstert.2010.05.017
Reddy, J., & Oktay, K. (2012). Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertility and Sterility, 98, 1363–1369. doi:10.1016/j.fertnstert.2012.09.022
Rodriguez-Wallberg, K.A., & Oktay, K. (2012). Options on fertility preservation in female cancer patients. Cancer Treatment Reviews, 38, 354–361. doi:10.1016/j.ctrv.2011.10.002
Schover, L.R., Brey, K., Lichtin, A., Lipshultz, L.I., & Jeha, S. (2002). Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. Journal of Clinical Oncology, 20, 1880–1889. doi:10.1200/JCO.2002.07.175
Shnorhavorian, M., Johnson, R., Shear, S.B., & Wilfond, B.S. (2011). Responding to adolescents with cancer who refuse sperm banking: When “no” should not be the last word. Journal of Adolescent and Young Adult Oncology, 1, 114–117. doi:10.1089/jayao.2011.0028
Society of Assisted Reproductive Technology. (2015). Clinical summary report: All SART member clinics. Retrieved from http://bit.ly/1Yl2olw
Stahl, P.J., Stember, D.S., Hsiao, W., & Schlegel, P.N. (2010). Indications and strategies for fertility preservation in men. Clinical Obstetrics and Gynecology, 53, 815–827.
Stroud, J.S., Mutch, D., Rader, J., Powell, M., Thaker, P.H., & Grigsby, P.W. (2009). Effects of cancer treatment on ovarian function. Fertility and Sterility, 92, 417–427.
Teh, W.T., Stern, C., Chander, S., & Hickey, M. (2014). The impact of uterine radiation on subsequent fertility and pregnancy outcomes. BioMed Research International. Retrieved from http://www.hindawi.com/journals/bmri/2014/482968
Trost, L., & Brannigan, R. (2012). Fertility preservation in males. In T.K. Woodruff & C. Gracia (Eds.), Oncofertility medical practice: Clinical issues and implementation (pp. 27–50). New York, NY: Springer.
Tschudin, S., & Bitzer, J. (2009). Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Human Reproduction Update, 15, 587–597. doi:10.1093/humupd/dmp015
Wang, J.H., Muller, C.H., & Lin, K. (2013). Optimizing fertility preservation for pre- and postpubertal males with cancer. Seminars in Reproductive Medicine, 31, 274–285.
Wyns, C., Curaba, M., Vanabelle, B., Van Langendonckt, A., & Donnez, J. (2010). Options for fertility preservation in prepubertal boys. Human Reproduction Update, 16, 312–328.
Yamaguchi, K., & Fujisawa, M. (2011). Anticancer chemotherapeutic agents and testicular dysfunction. Reproductive Medicine and Biology, 10, 81–87. doi:10.1007/s12522-011-0080-y
[[{"type":"media","view_mode":"media_original","fid":"21401","attributes":{"alt":"","class":"media-image","height":"227","typeof":"foaf:Image","width":"763"}}]]
About the Author(s)
Joanne Frankel Kelvin, MSN, RN, CNS, AOCN®, is a fertility nurse specialist at Memorial Sloan Kettering Cancer Center in New York, NY. The author takes full responsibility for the content of this article. The author did not receive honoraria for this work. The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the author, planners, independent peer reviewers, or editorial staff. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Clinical Journal of Oncology Nursing or the Oncology Nursing Society. Kelvin can be reached at kelvinj@mskcc.org, with copy to editor at CJONEditor@ons.org. (Submitted January 2015. Revision submitted April 2015. Accepted for publication April 13, 2015.)




