Factors Associated With Poor Sleep in Older Women Diagnosed With Breast Cancer
Objectives: To determine the relationship among gait, grip strength, cognition, depression, pain, and fatigue, and to identify which variables are most predictive of poor sleep.
Sample & Setting: 60 women with breast cancer aged 69 years or older who were receiving treatment in the Senior Adult Oncology Program at the James Cancer Hospital at the Ohio State University.
Methods & Variables: The variables were gait and grip strength (functional domains), cognition, depression, pain, and fatigue. Patients were tested using the Timed Up and Go Test (TUG), Jamar Hydraulic Hand Dynamometer, Mini-Cog, Numeric Pain Rating Scale, Brief Fatigue Inventory, Geriatric Depression Scale, and Pittsburgh Sleep Quality Index. Pearson correlation coefficients and logistic regression models were used.
Results: The mean age of the sample was 78 years. Pain and fatigue, depression and pain, and depression and fatigue each were positively related, and grip strength and TUG scores were negatively related. Fatigue was the strongest predictor of poor sleep.
Implications for Nursing: These findings are important to the comprehensive care of older women diagnosed with breast cancer. Understanding symptoms associated with poor sleep helps nurses develop comprehensive care plans for older adults with breast cancer.
Jump to a section
About 49% of newly diagnosed breast cancers occur in women aged 55–74 years (National Cancer Institute [NCI], 2014). The risk of breast cancer increases with age (Howlader et al., 2016), with the median age of diagnosis at 62 years (NCI, 2014). In older women, sleep problems can be common and are associated with falls (Takada et al., 2017), mental status changes (Thomas, Redd, Wright, & Hartos, 2017), obesity, and other health limitations (Liu, Wheaton, Chapman, & Croft, 2013). About 75% of Americans report experiencing some type of sleep disturbance (Stanford Center for Sleep Sciences and Medicine, 2017), and 60% of older women diagnosed with breast cancer report poor sleep quality (Costa et al., 2014). Many individuals complain of problems sleeping even before receiving any cancer treatment (Fontes, Pereira, Costa, Gonçalves, & Lunet, 2017). Treatment for breast cancer often increases problems associated with poor sleep during and after cancer therapy (Costa et al., 2014). Problems with sleep, particularly those associated with depression and anxiety, can persist for as long as three years following a diagnosis of breast cancer (Fontes, Severo, Gonçalves, Pereira, & Lunet, 2017).
The purpose of this study was to understand which common health concerns are predictive of poor sleep in older women diagnosed with breast cancer. The objectives of this study were to determine the relationship among gait, grip strength (functional status domains), cognitive status, depression, pain, and fatigue, and to understand which factors are associated with poor sleep. This study is significant in that the NCI (2016) suggests that people diagnosed with cancer are at risk for developing sleep disturbances.
Definition of Sleep Disorders
Sleep disorders include more than 80 types of problems that interfere with sleep (National Center for Complementary and Integrative Health, 2017; National Sleep Foundation, 2016). They consist of insomnias (getting to sleep and remaining asleep), sleep apneas, upper respiratory difficulties, and parasomnias, such as nightmares, teeth grinding, groaning, hallucinations, confusional arousals, and leg cramps (Otte et al., 2016). Sleep disorders can be specific diagnoses like insomnia or REM sleep behavior disorder (National Sleep Foundation, 2017), whereas poor sleep can be occasional and undiagnosed and is a relatively common problem for many people. The current study evaluates poor sleep as opposed to a diagnosed sleep disorder. Poor sleep is often stimulated by a combination of symptoms, such as hot flashes, anxiety, and depression (Desai et al., 2013), and can be difficult to manage.
The American Academy of Sleep Medicine and the Sleep Research Society (Watson et al., 2015) recommend that healthy adults get seven hours of sleep per night. Sleeping less than seven hours can lead to health deficits, including reduced immune function, impaired performance, and cardiovascular and neurologic concerns (Gamaldo et al., 2016). Lack of sleep can result in the common complaints of excessive sleepiness during periods of the day when alertness is necessary. Daytime sleepiness can be associated with fatigue and depressive symptoms (Van Onselen et al., 2013). Regularly sleeping more than seven hours can be beneficial for those with illness or can be reflective of undiagnosed health problems.
Normal sleep is comprised of one stage of rapid eye movement, which is considered a lighter sleep, and four stages of nonrapid eye movement, which is a deeper sleep (Medic, Wille, & Hemels, 2017). Deep sleep is restorative and central to productivity, quality of life, and many other health issues. The National Institutes of Health established the National Center on Sleep Disorders Research in 1993 to collaborate with multiple disciplines to engage in research, training, technology use, and care management coordination to understand and address problems associated with sleep (NCI, 2016).
Sleep Disorders and Breast Cancer
Thirty-eight percent of women diagnosed with breast cancer report poor sleep quality about 2.5 years following treatment (Lowery-Allison et al., 2017). Women who have received treatment with radiation therapy are at an increased risk for poor sleep (Fontes, Pereira, et al., 2017). Among patients receiving chemotherapy, about 36% report problems sleeping and 43% meet the diagnostic criteria for insomnia (Palesh et al., 2010). One of the most commonly reported distressing treatment-related side effects among women diagnosed with breast cancer is sleep (31%), which follows hot flashes (35%) and fatigue (32%) (Ellegaard, Grau, Zachariae, & Jensen, 2017). Other distressing side effects associated with poor sleep are pain, poor quality of life, anxiety regarding recurrence (Lowery-Allison et al., 2017), and depression (Enderlin et al., 2011).
People diagnosed with breast cancer report increased sleep latency and more nocturnal awakenings and insomnia (Enderlin et al., 2011). Sleep disorders in women with breast cancer are related to the following: psychosocial factors (racing mind), inadequate sleep hygiene, comorbidities, medication administration, environmental issues, mental concerns, sleep-related breathing problems (apnea), disruptive circadian rhythm, hypersomnia, and parasomnia (Otte et al., 2016). The mean number of concurrent sleep disorder symptoms reported by patients is 4.16 (Otte et al., 2016), which reflects the multifactorial complexity of sleep. Identifying the type of sleep disorder and structuring an effective management plan can be an intricate and time-consuming process.
Problems Associated With Sleep
Functional domain: Sleep disorders and poor sleep are associated with impaired functional status (Loh et al., 2017; Song, Dzierzewski, et al., 2015), poor gait, lack of confidence in balance (Tyagi, Perera, & Brach, 2017), and poor grip strength (Jeong et al., 2017). Sleep disorders affect muscle mass and functioning, which can translate into poor physical performance (Auyeung et al., 2015). In individuals diagnosed with chronic obstructive pulmonary disease, sleep disturbance affects muscle strength and functional ability (Vardar-Yagli et al., 2015). Functional status, measured by grip strength, balance, and ambulation observation, can provide insight on the extent to which sleep disorders interfere with the ability of an older adult to maintain independence (Tyagi et al., 2017).
Cognition: Older adults who suffer from sleep disorders or who have poor sleep have an increased risk for dementia (Shi et al., 2017); experience less deep sleep, lower sleep efficiency, and more awakenings; and report increased sleepiness (Haba-Rubio et al., 2017). Sleep disturbances associated with breathing impairment can affect mental processing speeds in people diagnosed with mild cognitive impairment (Terpening et al., 2015). Nocturnal hypoxemia (90% or lower oxygen saturation for 1% or more of sleep time) is also associated with cognitive decline in older men diagnosed with prostate cancer (Blackwell et al., 2015). Older men who spend more time in a lighter phase of sleep are more likely to experience cognitive decline (Song, Blackwell, et al., 2015).
Depression: Depression can affect sleep by causing issues like insomnia and hypersomnia (Geoffroy et al., 2018). For people diagnosed with chronic medical conditions, depression can interfere with sleep, reducing the ability of the body to engage in restorative rest necessary for healing (Leggett, Assari, Burgard, & Zivin, 2017). Lack of sleep can affect depression (Maglione et al., 2014), and depression is associated with sleep disorders. For many people suffering with depression, impaired sleep causes less restorative rest, therefore enhancing symptom burden (Leggett et al., 2017).
Pain: Pain can influence sleep, mood, and general quality of life. People experiencing chronic back pain often have increased levels of fatigue, severe anxiety, and depression (Sribastav et al., 2017). Greater pain intensity is reported by patients who have difficulty falling asleep, wake throughout the night, and experience low sleep efficiency (Alsaadi et al., 2014). Pain and sleep disorders are pervasive symptoms that affect quality of life for many people diagnosed with breast cancer (Dreidi & Hamdan-Mansour, 2016).
Fatigue: Fatigue is a relatively common symptom in patients who report sleep disorders. Of people who report chronic fatigue, many also report breathing difficulties and restless leg syndrome as specific sleep disorders causing excessive daytime sleepiness (Pajediene, Bileviciute-Ljungar, & Friberg, 2017). In addition, people who report worse sleep often report greater fatigue severity (Milrad et al., 2017). In patients participating in clinical trials, poor sleep quality is associated with reports of greater fatigue, symptom burden, and mood alterations (George et al., 2016).
In summary, breast cancer is a disease of aging in that most women are diagnosed from age 55–74 years. The care of older women with breast cancer requires a comprehensive approach to treatment. One common symptom associated with aging and cancer treatment is poor sleep. Other health considerations associated with poor sleep are functional limitations, cognitive impairment, depression, pain, and fatigue. The gaps in the literature tend to be specific to older women with breast cancer who experience poor sleep. A great deal of literature exists on sleep and its relationship with functional limitations, cognition, depression, pain, and fatigue; however, literature on people aged 69 years or older is not as abundant.
Methods
Sample and Setting
This is a descriptive, cross-sectional study conducted from 2015–2016. Women diagnosed with breast cancer aged 69 years or older were invited to participate. The age group was chosen based on the age of patients in the Senior Adult Oncology Program (SAOP) at the Stefanie Spielman Comprehensive Breast Center and on established science depicting the time that many people experience comorbid conditions (Fried et al., 2001). The breast center is an outpatient clinical and research facility located at the James Cancer Hospital at the Ohio State University in Columbus. Participants were diagnosed with any stage of breast cancer, were receiving any type of treatment, and could read and understand the consent form. Sixty women consented to participate in the study. Post hoc power analysis suggests that a sample size of 52 has 80% power to detect small-to-moderate effect sizes (a correlation of 0.35 using Pearson correlation test and an R2 of 0.12 for a regression model) with a two-sided significance level of 0.05. For the power analysis, the sample size available for the regression analysis was N = 52. All other analyses had a larger sample size (as many as 60); therefore, the authors used a Pearson correlation and an R2 as effect size measures (Cohen, 1988). R2 is a frequently used effect size measure for regression models in power analysis (Cohen, 1988). The purpose of the post hoc analysis was to guide results interpretation. For example, the authors could determine that a sample size of 52 or more would have greater than a 80% power for a Pearson correlation higher than 0.36 (e.g., N = 59 for the correlation of 0.58 between pain and fatigue) and that a sample size of 52 would have greater than a 80% power for a regression model with R2 greater than 0.12 (e.g., R2 of 0.28 as reported for the adjusted model).
Procedures
The rationale for the selection of the study variables of gait and grip strength (functional domain), cognition, depression, pain, and fatigue was based on published literature as it related to sleep disorders and poor sleep; these variables are commonly assessed in older adults diagnosed with cancer. Sleep as an outcome measure was selected because of the frequency of complaints among the clinical population of older adults who are diagnosed with breast cancer. The data used in this study are associated with an ongoing institutional review board–approved geriatric oncology assessment protocol. The data were extracted from the geriatric oncology assessment database. Aside from those used for sleep and fatigue screening, all instruments are included in the comprehensive geriatric assessment (CGA) as part of the clinical assessment of the patient. All CGA findings are recorded in the medical record.
Patients aged 69 years or older who presented to the clinic were invited to participate in the study. The geriatric nurse practitioner (GNP) explained the study to and obtained written consent from those who accepted the invitation to participate in the study. The GNP asked questions and completed the study instruments with each patient in a private examination room. The GNP has extensive experience conducting the CGA, is part of the study team, and underwent specific training. Patients were surveyed once upon initial visit to the clinic, which took about 20 minutes. Cancer diagnoses were obtained from medical records per HIPAA (Health Insurance Portability and Accountability Act) consent.
Instruments
The instruments included in this study are frequently used in clinical practice and research, and each have well documented psychometrics. The instruments have been shown to be valid and reliable in evaluating the variables selected for this study.
The Timed Up and Go Test (TUG) (Podsiadlo & Richardson, 1991) considers gait in the ability to rise from a sitting position, ambulate 10 feet, and return to a sitting position. TUG has been found to be correlated with falls (Shumway-Cook, Brauer, & Woollacott, 2000). A cutoff point for considering fall risk is 12 seconds. Inter-rater reliability is shown to be 0.98. TUG related well to the Berg Balance Scale (r = –0.55) (Berg, Wood-Dauphinee, Williams, & Maki, 1992), gait speed (r = –0.55), and Barthel Index scores (r = –0.51) (Collin, Wade, Davies, & Horne, 1988) upon development.
Grip strength was measured in the right hand using the Jamar Hydraulic Hand Dynamometer (Patterson Medical, 2017). The grip strength measurements predict limitations in mobility for the person diagnosed with breast cancer (Massy-Westropp, Gill, Taylor, Bohannon, & Hill, 2011). The average score of the three trials can be compared to the normative data. The cutoff point of 37 kg or less is used to determine strength difficulties. Test–retest reliability for hand grip strength in the dominate hand is 0.92 (Reuter, Massy-Westropp, & Evans, 2011).
The Mini-Cog (Borson, Scanlan, Brush, Vitaliano, & Dokmak, 2000) is an assessment instrument that combines the clock-drawing test with a three-item recall. The three-item recall is an assessment of short-term memory and is used in the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975). Scoring is calculated for two sections: recall, which is scored from 0–3, and drawing the clock, which is scored from 0–2. A score of 2 or less indicates a need for further evaluation. The sensitivity of the Mini-Cog is 0.99 and 0.96 in diagnostic value (Borson et al., 2000). Inter-rater scoring on the clock-drawing portion of the Mini-Cog is 0.97 (Borson et al., 1999).
The Geriatric Depression Scale (GDS) is a 15-item yeso scale that helps clinicians screen for depression (Yesavage et al., 1982). Of the 15 items, 10 indicate depression when answered yes, and the remaining 5 indicate depression when answered no. A cutoff point for the GDS is more than 5 items indicating depression, which signifies a positive screen; these patients should be referred for additional diagnostic assessment. The GDS is shown to be successful in detecting depression (r = 0.84, p ≤ 0.001).
The Numeric Pain Rating Scale was used to measure pain (McCaffery & Beebe, 1989). Patients were asked to describe their current pain on a scale from 0 (no pain) to 10 (extreme pain). Scores of 1–3 were considered mild pain, scores of 4–6 were considered moderate pain, and scores of 7–10 were considered severe pain. The authors used a cutoff point of 5 or greater to represent moderate to severe pain.
The Brief Fatigue Inventory (BFI) (Mendoza et al., 1999) consists of nine items, each having a numerical rating from 0–10. Three items define the severity of fatigue, and the remaining items consider the extent to which fatigue affects normal life activities. Construct validity for the nine items ranged from 0.81 (usual fatigue) to 0.92 (activity). Concurrent validity was evaluated with the Functional Assessment of Cancer Therapy–Fatigue scale (Cella et al., 1993). Cronbach coefficient alphas showed high reliability (alpha > 0.95). The BFI is scored as a continuous variable in that the higher the score, the more fatigue.
The Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) is a commonly used instrument for measuring quality of sleep. The PSQI is a seven-item scale that measures quality of sleep, sleep latency, efficiency, disturbances, use of sleep medication, and daytime sleep dysfunction. Scores range from 0–21, with higher scores indicating worse sleep quality. A score of 5 or greater indicates poor sleep. The instrument has a sensitivity of 0.89 and specificity of 0.86. The PSQI has an overall reliability coefficient (Cronbach alpha) of 0.83.
Ethical Conduct of Research
This study was approved by the Ohio State University Institutional Review Board. The study was explained to and the informed consent and HIPAA forms were signed by all participants according to the standards of the institutional review board. Participants were not compensated for inclusion in the study and were not screened for cognitive deficits prior to giving informed consent; however, all participants could verbalize understanding of the purpose of the study and were able to read the informed consent and HIPAA forms. All participants were determined to have had decision-making ability and were competent to consent on their own behalf based on their understanding of the consent form (based on reading and verbalization), consistent with the guidelines from the U.S. Department of Health and Human Services, Office for Human Research Protections (Office for Human Research Populations, 2016).
Analysis
Descriptive statistics were used to summarize sample characteristics and examine the distribution of study variables (TUG, grip strength, Mini-Cog, GDS, NPRS, BFI) overall and dichotomized as yes or no for having poor sleep based on the published cutoff point of greater than 5 or greater on the PSQI. Pearson correlation coefficients were used to examine the pair-wise correlation among study variables. The authors used a series of logistic regression models to examine the unadjusted and adjusted associations of each study variable with poor sleep. In the unadjusted analysis, each study variable was examined separately as a predictor of poor sleep. In the adjusted analysis, five symptom variables were included simultaneously as predictors in a logistic regression model for poor sleep. Last, logistic regression modeling with backward selection was used to derive a final model that included only significant predictors of poor sleep among the study variables. The authors did not adjust for the sample characteristics of age, type of breast cancer, metastasis, and type of surgery in the logistic regression models because they were not significantly associated with poor sleep in bivariate tests using chi-square statistics. The authors used SAS®, version 9.4, for the statistical analyses. All tests were two-sided with a significance of 0.05.
Results
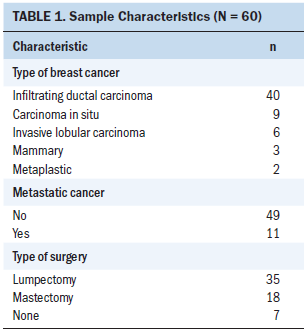
Sixty participants were included in the study. The mean age was 77.6 years (SD = 16, range = 69–93). Most women (n = 38) were diagnosed with intraductal carcinoma, nine were diagnosed with ductal carcinoma in situ, and six were diagnosed with lobular carcinoma. Eleven women were diagnosed with metastatic breast cancer, and 18 patients underwent mastectomy (see Table 1).
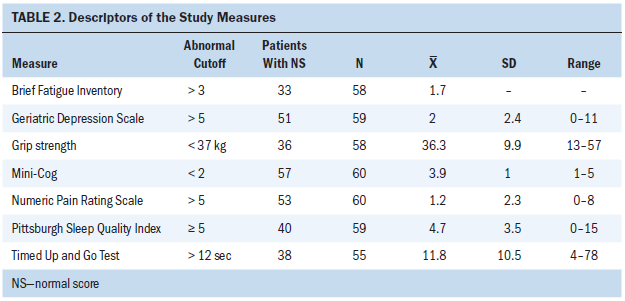
Regarding the variables of gait, grip strength, cognition, depression, pain, fatigue, and sleep, the mean scores were 11.8 for the TUG, 36.3 lbs for grip strength, 3.9 for the Mini-Cog, 2 for the GDS, 1.2 for the NPRS, 1.7 for the BFI, and 4.7 for the PSQI (see Table 2).
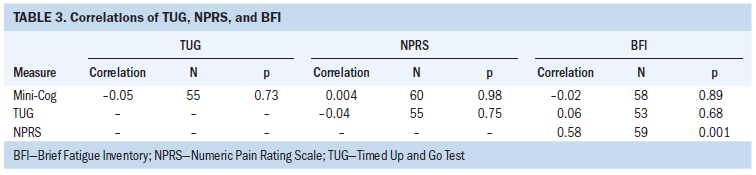
Pain and fatigue were positively related (r = 0.58, p < 0.001), depression and pain were positively related (r = 0.4, p = 0.002), and depression and fatigue were positively related (r = 0.66, p < 0.001). The performance status measures of grip strength and the TUG were negatively related (r = –0.46, p < 0.001) (see Tables 3 and 4).
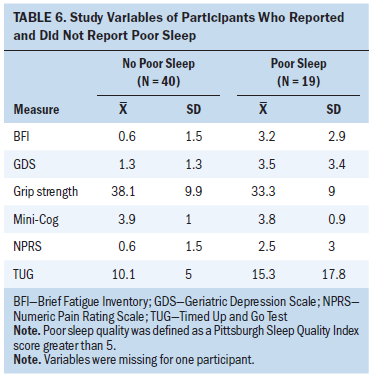
The mean pain score was 2.5 (SD = 3) for those with reported poor sleep (n = 19) and 0.6 (SD = 1.5) for those without poor sleep (n = 40). The mean TUG score was 15.3 seconds (SD = 17.8) for those with poor sleep and 10.1 seconds (SD = 5) for those without poor sleep. The mean BFI score was 3.2 (SD = 2.9) for those with poor sleep and 0.9 (SD = 1.5) for those without poor sleep. The mean GDS score was 3.5 (SD = 3.4) for those with poor sleep and 1.3 (SD = 1.3) for those without poor sleep. The mean grip strength was 33.3 lbs (SD = 9) for those with poor sleep and 38.1 lbs (SD = 9.9) for those without poor sleep. The mean Mini-Cog score was 3.8 (SD = 0.9) for those with poor sleep and 3.9 (SD = 1) for those without poor sleep.
[[{"fid":"41786","view_mode":"default","fields":{"format":"default","alignment":"","field_file_image_alt_text[und][0][value]":false,"field_file_image_title_text[und][0][value]":false},"link_text":null,"type":"media","field_deltas":{"1":{"format":"default","alignment":"","field_file_image_alt_text[und][0][value]":false,"field_file_image_title_text[und][0][value]":false}},"attributes":{"height":255,"width":626,"class":"media-element file-default","data-delta":"1"}}]]
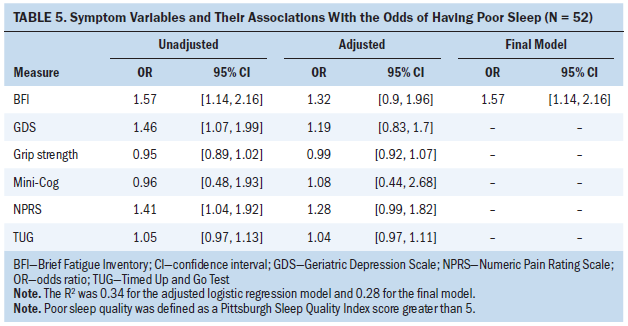
Mini-Cog, TUG, and grip strength results were not significantly associated with having poor sleep in both unadjusted and adjusted logistic regression analyses (see Tables 5 and 6). Greater pain, fatigue, and depression were associated with increased odds for having poor sleep in unadjusted analysis. For example, a one-unit increase on the depression measure was associated with a 46% increase in the likelihood of having poor sleep. Greater pain, fatigue, and depression were still associated with a greater likelihood of having poor sleep in the adjusted logistic regression model when all five symptom variables were simultaneously entered as predictors; however, none reached statistical significance because of multicollinearity among the variables. The final model from backward selection indicates that fatigue was the strongest predictor of poor sleep. Other symptom variables were not significantly associated with poor sleep after adjusting for fatigue and, therefore, were deleted from the final model. The final model had an R2 of 0.28, indicating that 28% of the variance in sleep was explained by fatigue, which was the single predictor that remained in the final model.
Discussion
Participants were aged 69 years or older, relatively functional, independent, living in the community, and about to begin cancer treatment. Patients included in the study were not receiving chemotherapy at the time of the study because either the infusion center was not open or the SAOP was not scheduling patients; however, many were receiving aromatase inhibitors or hormonal therapy. Twenty-seven patients reported no fatigue; the remaining participants (n = 30) reported fatigue, and 10 scored higher than 4 on the BFI, which is considered moderate to severe fatigue. The mean score on the BFI in the current study was 1.68, which is lower than that of older aged hospitalized patients (7.3) (Eyigor, Eyigor, & Uslu, 2010), patients receiving radiation therapy (6.51) (Karthikeyan, Jumnani, Prabhu, Manoor, & Supe, 2012), and people diagnosed with prostate cancer (2.8) (Engl, Drescher, Bickeböller, & Grabhorn, 2017).
Fatigue, depression, and pain were positively related and consistent with the symptom cluster in the literature (Gehrman, Garland, Matura, & Mao, 2017; Ho, Rohan, Parent, Tager, & McKinley, 2015). Fatigue was the only variable to be predictive of poor sleep in the final model. The sample was relatively healthy despite having a diagnosis of cancer. Additional longitudinal surveys (e.g., two additional times during a year and in treatment) are needed to examine the change of their interrelatedness during the illness trajectory. Despite the relationship among fatigue, depression, and pain, each symptom is distinct, can behave clinically differently, and can require individual management plans.
The participants scored from 0–15 on the PSQI. Twenty-three participants scored 5 or greater on the PSQI, which indicates problems with sleep. The mean PSQI score was 4.7 in the current study, which is less than that of patients diagnosed with rheumatoid arthritis (5.62) and greater than that of patients with no comorbidity (5.62) (Son et al., 2015). The PSQI score for older individuals at an adult day care was 6.8 (Martin et al., 2017). For many older adults, sleep difficulties are consistent with health problems. When a comorbid disease is treated, reports of sleep problems lessen (Neikrug & Ancoli-Israel, 2010). Sleep disorders are not a normative aspect of aging; however, until they experience health problems, older adults do not often report difficulty sleeping (Rodriguez, Dzierzewski, & Alessi, 2015).
The mean scores on the TUG suggest difficulty in gait problems; however, the sample showed reasonable grip strength. The data were collected on the initial visit to the cancer center, and few patients were administered cancer treatment following surgery. Generally, patients were referred to the SAOP following surgery for breast cancer. Eleven participants had metastatic disease on initial visit and often had a history of treatment with chemotherapy, radiation, and/or hormonal ablation treatment.
Poor grip strength and TUG scores were negatively related. Those who had no difficulty on the TUG had weaker grip strength, which is not surprising because strength of upper and lower extremities in some people may not be consistent. In community-dwelling adults with a mean age of 60 years, grip strength was stronger and TUG scores were lower in men who reported better sleep quality (Malinowska et al., 2017). However, when adjusting for age and sex, grip strength was associated with quality of sleep (Malinowska et al., 2017). In older adults with longer sleep duration (more than 9 hours), poor grip strength and higher TUG scores were noted (Fu et al., 2017). Sleep disorders affect functional performance, particularly when a person experiences daytime sleepiness. The current study revealed a similar pattern, but the associations of grip strength and TUG scores did not reach statistical significance. The small sample size of the study has insufficient power to detect small to moderate associations.
Older adults who report daytime sleepiness from sleep disorders have slower gait speed and decreased confidence in balance when walking (Tyagi et al., 2017), which can contribute to fear of falling (Chang, Chen, & Chou, 2016). Falls are also a risk in patients who complain of poor sleep quality or sleep disturbance (Stone et al., 2014), particularly when taking sleep medicine (Min, Kirkwood, Mays, & Slattum, 2016).
Fatigue is predictive of poor sleep for several reasons. It is often reported by people who experience daytime sleepiness resulting from sleep disorders (Pajediene et al., 2017). In individuals with multiple sclerosis, sleep disorders associated with insomnia are also associated with fatigue (Hare, Crangle, Carney, & Hart, 2017). For people diagnosed with prostate cancer, androgen deprivation therapy is associated with poor quality of sleep and fatigue (Koskderelioglu, Gedizlioglu, Ceylan, Gunlusoy, & Kahyaoglu, 2017). Following the first cycle of chemotherapy for breast cancer, many women reported problems with sleep and fatigue, which are accompanied by other symptoms that can affect quality of life (Charalambous, Kaite, Charalambous, Tistsi, & Kouta, 2017). Levels of fatigue tend to increase during chemotherapy, stay consistent through the last cycle of chemotherapy, and continue to decline for 10 years after the end of treatment (Fabi et al., 2017). In men, insomnia negatively affects fatigue and quality more than in women, but women report more fatigue and depression (Lee et al., 2014). Evening fatigue is associated with higher levels of sleep disorders along with other issues, such as high body mass index, poor functional status, anxiety, and depression (Mark et al., 2017).
Although mean scores on the Mini-Cog did not predict poor sleep, data support the relationship between insomnia and mental status changes, particularly in memory (Fortier-Brochu & Morin, 2014). Daytime sleepiness is also related to memory impairment in older adults (Okamura et al., 2016). Mini-Cog scores were not related to other scores in the current study, which may indicate that cognition is a separate concern. Almost all the participants screened positive for a cognitive limitation on the Mini-Cog, which is a score of 2 or less. A positive screen does not necessarily mean that patients were overtly confused or even cognitively impaired. However, a positive screen on the Mini-Cog indicates that a patient should undergo further cognitive screening. Many older women diagnosed with breast cancer exhibit limitations in cognitive functioning prior to cancer treatment (Overcash & Perry, 2017), and sleep disorders can affect memory and cognition. It is important to screen for cognitive problems in older adults diagnosed with cancer, particularly when a sleep disorder is experienced.
Limitations
Only one data point was assessed. Although the authors conducted post hoc power analysis, a prior sample size calculation was not conducted for the development of this study. In addition, a sample from a local clinic compromises generalizability of the data. The authors did not record the number of patients who refused to participate in the study; however, refusal was low because the SAOP collects similar information as part of the clinical evaluation.
More research is needed to determine the relationship among sleep problems and how sleeping practices of older adults diagnosed with cancer change over time. Nursing and interprofessional research on the cycle of fatigue and sleep disorders can influence practice.
Implications for Nursing
Attending to sleep disorders is central in providing survivorship care during and after treatment (Syrowatka et al., 2017). Fatigue is a common symptom among breast cancer survivors and must be anticipated. It is an important element affecting the survivorship care of patients with treatable but not curable disease (Frick et al., 2017). Cancer-related fatigue is pervasive and affects every element of daily activity. Understanding the issues surrounding fatigue will help nurses develop strategies to address the issue and reduce problems sleeping. Communication with the patient and family can inspire individualization of a management plan to improve sleep. The complexity of sleep disorders requires careful and comprehensive assessment, and survivorship plans often can be challenging and require modifications as patients return to the clinic.
Exercise, particularly low-impact activity like walking, is beneficial in addressing fatigue and sleep disorders (Chiu, Huang, Chen, Hou, & Tsai, 2015; Juvet et al., 2017; Payne, Held, Thorpe, & Shaw, 2008). Older adults often report using nonprescription sleep aids and may use them inappropriately by not following label directions (Abraham, Schleiden, Brothers, & Albert, 2017). Prescription sleep medications contribute to falls in older adults (Min et al., 2016). The American Geriatrics Society advises against prescribing benzodiazepines, which are often used to treat sleep disorders, because of their adverse effects (Markota, Rummans, Bostwick, & Lapid, 2016). Nonpharmacologic options to address sleep disorders and fatigue may be the best option for older adults with cancer. Healthcare providers should anticipate functional limitations in older adults with breast cancer and consider nonpharmacologic treatments for sleep disorders.
Nurses must continue to stress exercise and physical activity as a daily routine. Prescribing exercise accompanied with sleep education and other mindfulness activities can help enhance sleep (Kröz et al., 2013). The notion of exercise as medicine, along with a prescription for exercise, can underscore the importance of physical activity. Guidelines for exercise in older adults suggest that 150 minutes per week of aerobic activity and two hours of strength training are beneficial to general health and may address sleep disorders (Chodzko-Zajko et al., 2009; Lee, Jackson, & Richardson, 2017). Nurses play a role in education about and encouragement of exercise. Awareness of community resources and group exercise programs can inspire older adults to exercise. Exercise must be individually prescribed and supervised by the healthcare team to maximize the benefits to alleviate sleep disorders and fatigue and to enhance health (Klein et al., 2017).

Conclusion
Many of the older women diagnosed with breast cancer in this study screened positive for fatigue and poor sleep. The symptoms of pain, depression, and fatigue were related. Among the study variables, fatigue was the strongest predictor of poor sleep. Understanding the relationship of unpleasant symptoms associated with breast cancer treatment can help the provider develop comprehensive strategies to treat pain, depression, fatigue, and sleep disorders.
About the Author(s)
Janine Overcash, PhD, APRN-CNP, GNP, FAANP, FAAN, is a clinical associate professor, Alai Tan, PhD, is a research associate professor, and Keya Patel, BSN, is a student nurse, all in the College of Nursing, and Anne M. Noonan, MD, MBBChBAO, MSc, MRCPI, is an assistant professor in the Department of Internal Medicine, Division of Medical Oncology, all at the Ohio State University in Columbus. No financial relationships to disclose. Overcash and Patel contributed to the conceptualization and design. Overcash and Noonan completed the data collection. Overcash and Tan provided statistical support. Overcash, Tan, and Patel provided the analysis. All authors contributed to the manuscript preparation. Overcash can be reached at overcash.1@osu.edu, with copy to ONFEditor@ons.org. (Submitted August 2017. Accepted December 13, 2017.)
References
Abraham, O., Schleiden, L.J., Brothers, A.L., & Albert, S.M. (2017). Managing sleep problems using non-prescription medications and the role of community pharmacists: Older adults’ perspectives. International Journal of Pharmacy Practice, 25, 438–446. https://doi.org/10.1111/ijpp.12334
Alsaadi, S.M., McAuley, J.H., Hush, J.M., Lo, S., Bartlett, D.J., Grunstein, R.R., & Maher, C.G. (2014). The bidirectional relationship between pain intensity and sleep disturbance/quality in patients with low back pain. Clinical Journal of Pain, 30, 755–765. https://doi.org/10.1097/ajp.0000000000000055
Auyeung, T.W., Kwok, T., Leung, J., Lee, J.S.W., Ohlsson, C., Vandenput, L., . . . Woo, J. (2015). Sleep duration and disturbances were associated with testosterone level, muscle mass, and muscle strength—A cross-sectional study in 1274 older men. Journal of the American Medical Directors Association, 16, 630.e1–603.e6. https://doi.org/10.1016/j.jamda.2015.04.006
Berg, K.O., Wood-Dauphinee, S.L., Williams, J.I., & Maki, B. (1992). Measuring balance in the elderly: Validation of an instrument. Canadian Journal of Public Health, 83(Suppl. 2), S7–S11.
Blackwell, T., Yaffe, K., Laffan, A., Redline, S., Ancoli-Israel, S., Ensrud, K.E., . . . Stone, K.L. (2015). Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: The Osteoporotic Fractures in Men Sleep Study. Journal of the American Geriatrics Society, 63, 453–461. https://doi.org/10.1111/jgs.13321
Borson, S., Brush, M., Gil, E., Scanlan, J., Vitaliano, P., Chen, J., . . . Roques, J. (1999). The Clock Drawing Test: Utility for dementia detection in multiethnic elders. Journals of Gerontology Series A, 54, M534–M540.
Borson, S., Scanlan, J., Brush, M., Vitaliano, P., & Dokmak, A. (2000). The Mini-Cog: A cognitive “vital signs” measure for dementia screening in multi-lingual elderly. International Journal of Geriatric Psychiatry, 15, 1021–1027.
Buysse, D.J., Reynolds, C.F., 3rd, Monk, T.H., Berman, S.R., & Kupfer, D.J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213.
Cella, D.F., Tulsky, D.S., Gray, G., Sarafian, B., Linn, E., Bonomi, A., . . . Brannon, J. (1993). The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. Journal of Clinical Oncology, 11, 570–579. https://doi.org/10.1200/JCO.1993.11.3.570
Chang, H.-T., Chen, H.-C., & Chou, P. (2016). Factors associated with fear of falling among community-dwelling older adults in the Shih-Pai study in Taiwan. PLOS ONE, 11, e0150612. https://doi.org/10.1371/journal.pone.0150612
Charalambous, A., Kaite, C.P., Charalambous, M., Tistsi, T., & Kouta, C. (2017). The effects on anxiety and quality of life of breast cancer patients following completion of the first cycle of chemotherapy. SAGE Open Medicine, 5, 1–10. https://doi.org/10.1177/2050312117717507
Chiu, H.-Y., Huang, H.-C., Chen, P.-Y., Hou, W.-H., & Tsai, P.-S. (2015). Walking improves sleep in individuals with cancer: A meta-analysis of randomized, controlled trials [Online exclusive]. Oncology Nursing Forum, 42, E54–E62. https://doi.org/10.1188/15.ONF.E54-E62
Chodzko-Zajko, W.J., Proctor, D.N., Fiatarone Singh, M.A., Minson, C.T., Nigg, C.R., Salem, G.J., & Skinner, J.S. (2009). American College of Sports Medicine position stand. Exercise and physical activity for older adults. Medicine and Sciences in Sports and Exercise, 41, 1510–1530. https://doi.org/10.1249/MSS.0b013e3181a0c95c
Cohen, J. (1988). Statistical power analysis for behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates.
Collin, C., Wade, D.T., Davies, S., & Horne, V. (1988). The Barthel ADL Index: A reliability study. International Disabilities Studies, 10, 61–63. https://doi.org/10.3109/09638288809164103
Costa, A.R., Fontes, F., Pereira, S., Gonçalves, M., Azevedo, A., & Lunet, N. (2014). Impact of breast cancer treatments on sleep disturbances—A systematic review. Breast, 23, 697–709. https://doi.org/10.1016/j.breast.2014.09.003
Desai, K., Mao, J.J., Su, I., DeMichele, A., Li, Q., Xie, S.X., & Gehrman, P.R. (2013). Prevalence and risk factors for insomnia among breast cancer patients on aromatase inhibitors. Supportive Care in Cancer, 21, 43–51. https://doi.org/10.1007/s00520-012-1490-z
Dreidi, M.M., & Hamdan-Mansour, A.M. (2016). Pain, sleep disturbance, and quality of life among Palestinian patients diagnosed with cancer. Journal of Cancer Education, 31, 796–803. https://doi.org/10.1007/s13187-015-0946-5
Ellegaard, M.-B., Grau, C., Zachariae, R., & Jensen, A.B. (2017). Women with breast cancer report substantially more disease- and treatment-related side or late effects than registered by clinical oncologists: A cross-sectional study of a standard follow-up program in an oncological department. Breast Cancer Research and Treatment, 164, 727–736. https://doi.org/10.1007/s10549-017-4301-x
Enderlin, C.A., Coleman, E.A., Cole, C., Richards, K.C., Kennedy, R.L., Goodwin, J.A., . . . Mack, K. (2011). Subjective sleep quality, objective sleep characteristics, insomnia symptom severity, and daytime sleepiness in women aged 50 and older with nonmetastatic breast cancer [Online exclusive]. Oncology Nursing Forum, 38, E314–E325. https://doi.org/10.1188/11.ONF.E314-E325
Engl, T., Drescher, D., Bickeböller, R., & Grabhorn, R. (2017). Fatigue, depression, and quality of life in patients with prostatic diseases. Central European Journal of Urology, 70, 44–47. https://doi.org/10.5173/ceju.2017.940
Eyigor, S., Eyigor, C., & Uslu, R. (2010). Assessment of pain, fatigue, sleep and quality of life (QoL) in elderly hospitalized cancer patients. Archives of Gerontology and Geriatrics, 51, e57–e61. https://doi.org/10.1016/j.archger.2009.11.018
Fabi, A., Falcicchio, C., Giannarelli, D., Maggi, G., Cognetti, F., & Pugliese, P. (2017). The course of cancer related fatigue up to ten years in early breast cancer patients: What impact in clinical practice? Breast, 34, 44–52. https://doi.org/10.1016/j.breast.2017.04.012
Folstein, M.F., Folstein, S.E., & McHugh, P.R. (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Fontes, F., Pereira, S., Costa, A.R., Gonçalves, M., & Lunet, N. (2017). The impact of breast cancer treatments on sleep quality 1 year after cancer diagnosis. Supportive Care in Cancer, 25, 3529–3536. https://doi.org/10.1007/s00520-017-3777-6
Fontes, F., Severo, M., Gonçalves, M., Pereira, S., & Lunet, N. (2017). Trajectories of sleep quality during the first three years after breast cancer diagnosis. Sleep Medicine, 34, 193–199. https://doi.org/10.1016/j.sleep.2017.03.022
Fortier-Brochu, É., & Morin, C.M. (2014). Cognitive impairment in individuals with insomnia: Clinical significance and correlates. Sleep, 37, 1787–1798. https://doi.org/10.5665/sleep.4172
Frick, M.A., Vachani, C.C., Bach, C., Hampshire, M.K., Arnold-Korzeniowski, K., Metz, J.M., & Hill-Kayser, C.E. (2017). Survivorship and the chronic cancer patient: Patterns in treatment-related effects, follow-up care, and use of survivorship care plans. Cancer, 123, 4268–4276. https://doi.org/10.1002/cncr.30862
Fried, L.P., Tangen, C.M., Walston, J., Newman, A.B., Hirsch, C., Gottdiener, J., . . . McBurnie, M.A. (2001). Frailty in older adults: Evidence for a phenotype. Journals of Gerontology Series A, 56, M146–M157. https://doi.org/10.1093/gerona/56.3.M146
Fu, L., Jia, L., Zhang, W., Han, P., Kang, L., Ma, Y., . . . Guo, Q. (2017). The association between sleep duration and physical performance in Chinese community-dwelling elderly. PLOS ONE, 12, e0174832. https://doi.org/10.1371/journal.pone.0174832
Gamaldo, A.A., Beydoun, M.A., Beydoun, H.A., Liang, H., Salas, R.E., Zonderman, A.B., . . . Eid, S.M. (2016). Sleep disturbances among older adults in the United States, 2002–2012: Nationwide inpatient rates, predictors, and outcomes. Frontiers in Aging Neuroscience, 8, 266.
Gehrman, P.R., Garland, S.N., Matura, L.A., & Mao, J. (2017). Insomnia in breast cancer: Independent symptom or symptom cluster? Palliative and Supportive Care, 15, 369–375. https://doi.org/10.1017/s1478951516000900
Geoffroy, P.A., Hoertel, N., Etain, B., Bellivier, F., Delorme, R., Limosin, F., & Peyre, H. (2018). Insomnia and hypersomnia in major depressive episode: Prevalence, sociodemographic characteristics and psychiatric comorbidity in a population-based study. Journal of Affective Disorders, 226, 132–141. https://doi.org/10.1016/j.jad.2017.09.032
George, G.C., Iwuanyanwu, E.C., Anderson, K.O., Yusuf, A., Zinner, R.G., Piha-Paul, S.A., . . . Hong, D.S. (2016). Sleep quality and its association with fatigue, symptom burden, and mood in patients with advanced cancer in a clinic for early-phase oncology clinical trials. Cancer, 122, 3401–3409. https://doi.org/10.1002/cncr.30182
Haba-Rubio, J., Marti-Soler, H., Tobback, N., Andries, D., Marques-Vidal, P., Waeber, G., . . . Popp, J. (2017). Sleep characteristics and cognitive impairment in the general population: The HypnoLaus study. Neurology, 88, 463–469. https://doi.org/10.1212/wnl.0000000000003557
Hare, C.J., Crangle, C.J., Carney, C.E., & Hart, T.L. (2017). Insomnia symptoms, subjective appraisals, and fatigue: A multiple mediation model. Behavioral Sleep Medicine, 17, 1–12. https://doi.org/10.1080/15402002.2017.1342167
Ho, S.-Y., Rohan, K.J., Parent, J., Tager, F.A., & McKinley, P.S. (2015). A longitudinal study of depression, fatigue, and sleep disturbances as a symptom cluster in women with breast cancer. Journal of Pain and Symptom Management, 49, 707–715. https://doi.org/10.1016/j.jpainsymman.2014.09.009
Howlader, N., Noone, A.M., Krapcho, M., Miller, D., Bishop, K., Altekruse, S.F., . . . Cronin, K.A. (Eds.). (2016). SEER cancer statistics review, 1975–2013. Bethesda, MD: National Cancer Institute. Retrieved from http://seer.cancer.gov/csr/1975_2013
Jeong, M., Kang, H.K., Song, P., Park, H.K., Jung, H., Lee, S.S., & Koo, H.K. (2017). Hand grip strength in patients with chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease, 12, 2385–2390. https://doi.org/10.2147/copd.s140915
Juvet, L.K., Thune, I., Elvsaas, I.K.Ø., Fors, E.A., Lundgren, S., Bertheussen, G., . . . Oldervoll, L.M. (2017). The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast, 33, 166–177. https://doi.org/10.1016/j.breast.2017.04.003
Karthikeyan, G., Jumnani, D., Prabhu, R., Manoor, U.K., & Supe, S.S. (2012). Prevalence of fatigue among cancer patients receiving various anticancer therapies and its impact on quality of life: A cross-sectional study. Indian Journal of Palliative Care, 18, 165–175. https://doi.org/10.4103/0973-1075.105686
Klein, D., Jeejeebhoy, K., Tremblay, A., Kallio, M., Rheaume, C., Humphries, S., . . . Mutch, D.M. (2017). The CHANGE program: Exercise intervention in primary care. Canadian Family Physician, 63, 546–552.
Koskderelioglu, A., Gedizlioglu, M., Ceylan, Y., Gunlusoy, B., & Kahyaoglu, N. (2017). Quality of sleep in patients receiving androgen deprivation therapy for prostate cancer. Neurological Sciences, 38, 1445–1451. https://doi.org/10.1007/s10072-017-2989-3
Kröz, M., Fink, M., Reif, M., Grobbecker, S., Zerm, R., Quetz, M., . . . Gutenbrunner, C. (2013). Multimodal therapy concept and aerobic training in breast cancer patients with chronic cancer-related fatigue. Integrative Cancer Therapies, 12, 301–311. https://doi.org/10.1177/1534735412464552
Lee, M.-H., Lee, S.-A., Lee, G.-H., Ryu, H.-S., Chung, S., Chung, Y.-S., & Kim, W.S. (2014). Gender differences in the effect of comorbid insomnia symptom on depression, anxiety, fatigue, and daytime sleepiness in patients with obstructive sleep apnea. Sleep and Breathing, 18, 111–117.
Lee, P.G., Jackson, E.A., & Richardson, C.R. (2017). Exercise prescriptions in older adults. American Family Physician, 95, 425–432. https://doi.org/10.1007/s11325-013-0856-x
Leggett, A., Assari, S., Burgard, S., & Zivin, K. (2017). The effect of sleep disturbance on the association between chronic medical conditions and depressive symptoms over time. Longitudinal and Life Course Studies, 8, 138–151. https://doi.org/10.14301/llcs.v8i2.433
Liu, Y., Wheaton, A.G., Chapman, D.P., & Croft, J.B. (2013). Sleep duration and chronic diseases among U.S. adults age 45 years and older: Evidence from the 2010 Behavioral Risk Factor Surveillance System. Sleep, 36, 1421–1427. https://doi.org/10.5665/sleep.3028
Loh, K.P., Pandya, C., Zittel, J., Kadambi, S., Flannery, M., Reizine, N., . . . Mohile, S.G. (2017). Associations of sleep disturbance with physical function and cognition in older adults with cancer. Supportive Care in Cancer, 25, 3161–3169. https://doi.org/10.1007/s00520-017-3724-6
Lowery-Allison, A.E., Passik, S.D., Cribbet, M.R., Reinsel, R.A., O’Sullivan, B., Norton, L., . . . Kavey, N.B. (2017). Sleep problems in breast cancer survivors 1–10 years posttreatment. Palliative and Supportive Care, 16, 1–10. https://doi.org/10.1017/s1478951517000311
Maglione, J.E., Ancoli-Israel, S., Peters, K.W., Paudel, M.L., Yaffe, K., Ensrud, K.E., & Stone, K.L. (2014). Subjective and objective sleep disturbance and longitudinal risk of depression in a cohort of older women. Sleep, 37, 1179–1187. https://doi.org/10.5665/sleep.3834
Malinowska, K.B., Ikezoe, T., Ichihashi, N., Arai, H., Murase, K., Chin, K., . . . Tsuboyama, T. (2017). Self-reported quality of sleep is associated with physical strength among community-dwelling young-old adults. Geriatrics and Gerontology International, 17, 1808–1813. https://doi.org/10.1111/ggi.12965
Mark, S., Cataldo, J., Dhruva, A., Paul, S.M., Chen, L.-M., Hammer, M.J., . . . Miaskowski, C. (2017). Modifiable and non-modifiable characteristics associated with sleep disturbance in oncology outpatients during chemotherapy. Supportive Care in Cancer, 25, 2485–2494. https://doi.org/10.1007/s00520-017-3655-2
Markota, M., Rummans, T.A., Bostwick, J.M., & Lapid, M.I. (2016). Benzodiazepine use in older adults: Dangers, management, and alternative therapies. Mayo Clinic Proceedings, 91, 1632–1639. https://doi.org/10.1016/j.mayocp.2016.07.024
Martin, J.L., Song, Y., Hughes, J., Jouldjian, S., Dzierzewski, J.M., Fung, C.H., . . . Alessi, C.A. (2017). A four-session sleep intervention program improves sleep for older adult day health care participants: Results of a randomized controlled trial. Sleep, 40(8), zsx079. https://doi.org/10.1093/sleep/zsx079
Massy-Westropp, N.M., Gill, T.K., Taylor, A.W., Bohannon, R.W., & Hill, C.L. (2011). Hand grip strength: Age and gender stratified normative data in a population-based study. BMC Research Notes, 4, 127. https://doi.org/10.1186/1756-0500-4-127
McCaffery, M., & Beebe, A. (1989). Pain: Clinical manual for nursing practice. St. Louis, MO: Mosby.
Medic, G., Wille, M., & Hemels, M.E. (2017). Short- and long-term health consequences of sleep disruption. Nature and Science of Sleep, 9, 151–161. https://doi.org/10.2147/NSS.S134864
Mendoza, T.R., Wang, X.S., Cleeland, C.S., Morrissey, M., Johnson, B.A., Wendt, J.K., & Huber, S.L. (1999). The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer, 85, 1186–1196.
Milrad, S.F., Hall, D.L., Jutagir, D.R., Lattie, E.G., Ironson, G.H., Wohlgemuth, W., . . . Antoni, M.H. (2017). Poor sleep quality is associated with greater circulating pro-inflammatory cytokines and severity and frequency of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) symptoms in women. Journal of Neuroimmunology, 303, 43–50. https://doi.org/10.1016/j.jneuroim.2016.12.008
Min, Y., Kirkwood, C.K., Mays, D.P., & Slattum, P.W. (2016). The effect of sleep medication use and poor sleep quality on risk of falls in community-dwelling older adults in the US: A prospective cohort study. Drugs and Aging, 33, 151–158. https://doi.org/10.1007/s40266-015-0339-9
National Cancer Institute. (2014). Cancer stat facts: Female breast cancer. Retrieved from https://bit.ly/1pFeB26
National Cancer Institute. (2016). Sleep disorders (PDQ®)—Health professional version. Retrieved from https://bit.ly/2vjfwfc
National Center for Complementary and Integrative Health. (2017). Sleep disorders. Retrieved from https://bit.ly/1ybzZCg
National Sleep Foundation. (2016). How much sleep do we really need? Retrieved from https://bit.ly/1LAI6Zl
National Sleep Foundation. (2017). What are the facts about insomnia? Retrieved from https://bit.ly/2GltpP7
Neikrug, A.B., & Ancoli-Israel, S. (2010). Sleep disorders in the older adult—A mini-review. Gerontology, 56, 181–189. https://doi.org/10.1159/000236900
Office for Human Research Populations. (2016). Regulations and policy [pamphlet]. Rockville, MD: U.S. Department of Health and Human Services.
Okamura, T., Ura, C., Miyamae, F., Sugiyama, M., Niikawa, H., Ito, K., & Awata, S. (2016). Excessive daytime sleepiness is related to subjective memory impairment in late life: A cross-sectional community-based study. Psychogeriatrics, 16, 196–201. https://doi.org/10.1111/psyg.12139
Otte, J.L., Davis, L., Carpenter, J.S., Krier, C., Skaar, T.C., Rand, K.L., . . . Manchanda, S. (2016). Sleep disorders in breast cancer survivors. Supportive Care in Cancer, 24, 4197–4205. https://doi.org/10.1007/s00520-016-3247-6
Overcash, J., & Perry, M. (2017). Cognitive screening: Using the clock-drawing test to assess for preexisting deficits in older women diagnosed with breast cancer. Clinical Journal of Oncology Nursing, 21, 489–498. https://doi.org/10.1188/17.CJON.489-498
Pajediene, E., Bileviciute-Ljungar, I., & Friberg, D. (2017). Sleep patterns among patients with chronic fatigue: A polysomnography-based study. Clinical Respiratory Journal. Advance online publication. https://doi.org/10.1111/crj.12667
Palesh, O.G., Roscoe, J.A., Mustian, K.M., Roth, T., Savard, J., Ancoli-Israel, S., . . . Morrow, G.R. (2010). Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. Journal of Clinical Oncology, 28, 292–298. https://doi.org/10.1200/jco.2009.22.5011
Patterson Medical. (2017). JAMAR: Hydraulic hand dynamometer owner’s manual. Retrieved from https://bit.ly/2J1Rj3U
Payne, J.K., Held, J., Thorpe, J., & Shaw, H. (2008). Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncology Nursing Forum, 35, 635–642. https://doi.org/10.1188/08.ONF.635-642
Podsiadlo, D., & Richardson, S. (1991). The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society, 39, 142–148.
Reuter, S.E., Massy-Westropp, N., & Evans, A.M. (2011). Reliability and validity of indices of hand-grip strength and endurance. Australian Occupational Therapy Journal, 58, 82–87. https://doi.org/10.1111/j.1440-1630.2010.00888.x
Rodriguez, J.C., Dzierzewski, J.M., & Alessi, C.A. (2015). Sleep problems in the elderly. Medical Clinics of North America, 99, 431–439. https://doi.org/10.1016/j.mcna.2014.11.013
Shi, L., Chen, S.-J., Ma, M.-Y., Bao, Y.-P., Han, Y., Wang, Y.-M., . . . Lu, L. (2017). Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Medicine Reviews. Advance online publication. https://doi.org/10.1016/j.smrv.2017.06.010
Shumway-Cook, A., Brauer, S., & Woollacott, M. (2000). Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Physical Therapy, 80, 896–903.
Son, C.-N., Choi, G., Lee, S.-Y., Lee, J.-M., Lee, T.-H., Jeong, H.-J., . . . Kim, S.-H. (2015). Sleep quality in rheumatoid arthritis, and its association with disease activity in a Korean population. Korean Journal of Internal Medicine, 30, 384–390.
Song, Y., Blackwell, T., Yaffe, K., Ancoli-Israel, S., Redline, S., & Stone, K.L. (2015). Relationships between sleep stages and changes in cognitive function in older men: The MrOS Sleep Study. Sleep, 38, 411–421. https://doi.org/10.5665/sleep.4500
Song, Y., Dzierzewski, J.M., Fung, C.H., Rodriguez, J.C., Jouldjian, S., Mitchell, M.N., . . . Martin, J.L. (2015). Association between sleep and physical function in older veterans in an adult day healthcare program. Journal of the American Geriatrics Society, 63, 1622–1627. https://doi.org/10.1111/jgs.13527
Sribastav, S.S., Peiheng, H., Jun, L., Zemin, L., Fuxin, W., Jianru, W., . . . Zhaomin, Z. (2017). Interplay among pain intensity, sleep disturbance and emotion in patients with non-specific low back pain. PeerJ, 5, e3282. https://doi.org/10.7717/peerj.3282
Stanford Center for Sleep Sciences and Medicine. (2017). Sleep disorders. Retrieved from http://sleep.stanford.edu/sleep-disorders
Stone, K.L., Blackwell, T.L., Ancoli-Israel, S., Cauley, J.A., Redline, S., Marshall, L.M., & Ensrud, K.E. (2014). Sleep disturbances and risk of falls in older community-dwelling men: The outcomes of Sleep Disorders in Older Men (MrOS Sleep) study. Journal of the American Geriatrics Society, 62, 299–305. https://doi.org/10.1111/jgs.12649
Syrowatka, A., Motulsky, A., Kurteva, S., Hanley, J.A., Dixon, W.G., Meguerditchian, A.N., & Tamblyn, R. (2017). Predictors of distress in female breast cancer survivors: A systematic review. Breast Cancer Research and Treatment, 165, 229–245. https://doi.org/10.1007/s10549-017-4290-9
Takada, S., Yamamoto, Y., Shimizu, S., Kimachi, M., Ikenoue, T., Fukuma, S., . . . Fukuhara, S. (2017). Association between subjective sleep quality and future risk of falls in older people: Results from LOHAS. Journals of Gerontology Series A. Advance online publication. https://doi.org/10.1093/gerona/glx123
Terpening, Z., Lewis, S.J.G., Yee, B.J., Grunstein, R.R., Hickie, I.B., & Naismith, S.L. (2015). Association between sleep-disordered breathing and neuropsychological performance in older adults with mild cognitive impairment. Journal of Alzheimer’s Disease, 46, 157–165. https://doi.org/10.3233/jad-141860
Thomas, K.M., Redd, L.A., Wright, J.D., & Hartos, J.L. (2017). Sleep and mental health in the general population of elderly women. Journal of Primary Prevention, 38, 495–503. https://doi.org/10.1007/s10935-017-0484-5
Tyagi, S., Perera, S., & Brach, J.S. (2017). Balance and mobility in community-dwelling older adults: Effect of daytime sleepiness. Journal of the American Geriatrics Society, 65, 1019–1025. https://doi.org/10.1111/jgs.14735
Van Onselen, C., Paul, S.M., Lee, K., Dunn, L., Aouizerat, B.E., West, C., . . . Miaskowski, C. (2013). Trajectories of sleep disturbance and daytime sleepiness in women before and after surgery for breast cancer. Journal of Pain and Symptom Management, 45, 244–260. https://doi.org/10.1016/j.jpainsymman.2012.02.020
Vardar-Yagli, N., Saglam, M., Savci, S., Inal-Ince, D., Calik-Kutukcu, E., Arikan, H., & Coplu, L. (2015). Impact of sleep quality on functional capacity, peripheral muscle strength and quality of life in patients with chronic obstructive pulmonary disease. Expert Review of Respiratory Medicine, 9, 233–239. https://doi.org/10.1586/17476348.2015.1009041
Watson, N.F., Badr, M.S., Belenky, G., Bliwise, D.L., Buxton, O.M., Buysse, D., . . . Tasali, E. (2015). Recommended amount of sleep for a healthy adult: A joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep, 38, 843–844. https://doi.org/10.5665/sleep.4716
Yesavage, J.A., Brink, T.L., Rose, T.L., Lum, O., Huang, V., Adey, M., & Leirer, V.O. (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17, 37–49.




