Arthralgia in Breast Cancer Survivors: An Integrative Review of Endocrine Therapy
Problem Identification: Aromatase inhibitors (AIs) and the selective estrogen receptor modulator tamoxifen (Nolvadex®) are essential to extended survival for women with postmenopausal breast cancer. However, they can result in painful arthralgia.
Literature Search: Databases searched for eligible articles were CINAHL®, Cochrane Database of Systematic Reviews, EMBASE, Google Scholar, MEDLINE®, PsycINFO®, PubMed, Scopus, and Sociological Abstracts (ProQuest).
Data Evaluation: The final sample for this integrative review consisted of 16 studies. The total sample of women included across all studies was 11,511.
Synthesis: Content analysis was used to summarize the study findings.
Conclusions: AIs result in a higher incidence of arthralgia compared to tamoxifen. All breast cancer survivors commencing AI therapy should undergo a baseline assessment to identify any increased risk of arthralgia. All women on AIs should have their vitamin D levels checked before beginning AIs and annually thereafter. Many women may need higher doses of vitamin D supplementation than normally recommended. All women taking AIs should be advised on self-help strategies to alleviate pain, such as walking and yoga.
Implications for Practice: Oncology nurses are ideally placed to undertake a baseline assessment before AI therapy. Nurses can also use this opportunity to educate women on their risk of developing AI-related arthralgia and provide advice on vitamin D supplementation. In addition, nurses play an important role in educating women on self-help strategies.
Jump to a section
Breast cancer is the most common noncutaneous malignancy among women. Long-term survival of individuals with breast cancer is high, with five-year survival rates of almost 90% (Runowicz et al., 2016). Individuals with breast cancer are living longer and, therefore, require ongoing, more inclusive survivorship care (Thompson et al., 2014).
Survivorship was first highlighted more than a decade ago in an Institute of Medicine (IOM) report as a distinct phase of cancer care with four essential components: prevention, surveillance, intervention, and coordination of care (Hewitt, Greenfield, & Stovall, 2006). The IOM also recommended that cancer survivors have a follow-up plan addressing issues related to health care and quality of life (Hewitt et al., 2006). More recently, the National Comprehensive Cancer Network and the American Society of Clinical Oncology published evidence- and consensus-based survivorship care guidelines for individuals with breast cancer to help survivors reach optimal health and quality of life (Runowicz et al., 2016).
Evidence-based approaches for survivorship care and the delivery of survivorship care planning are evolving. The nurse-led model of follow-up care has been shown to result in high levels of satisfaction with the level of care provided (Lewis et al., 2009). Oncology nurses are ideally placed to provide this care. However, to do so, they must base this care on evidence (Bessen et al., 2014; Rushton et al., 2015).
Breast cancer survivors report many side effects from endocrine treatments, including the return of menopausal symptoms among postmenopausal women (Daniel, Mitchell, Higham, Timpson, & Foy, 2014). Survivors taking endocrine therapy note the inherent paradox of a treatment that saves their lives but also ages them (Pelligrini et al., 2010). Increasing evidence exists of health risks among breast cancer survivors on endocrine therapy. These risks include the development of metabolic syndrome and elevated C-reactive protein (Thomson et al., 2009), as well as nonalcoholic steatohepatitis (Saphner, Triest-Robertson, Li, & Holzman, 2009).
This integrative review is based on work initially undertaken to determine the survivorship issues for postmenopausal women with breast cancer undergoing endocrine therapy as part of an initiative to develop a survivorship care plan at an Irish cancer care center. A variety of issues related to survivorship for menopausal women were identified through the literature search. This article presents an integrative review of one of the issues identified: arthralgia (noninflammatory joint pain and/or joint stiffness) related to aromatase inhibitors (AIs), referred to as AI-related arthralgia (AIA), and the selective estrogen receptor modulator (SERM) tamoxifen (Nolvadex®).
AIs and SERMs are essential to adjuvant treatment for estrogen receptor–positive breast cancer; however, anastrozole (Arimidex®) has been shown to result in better disease-free survival than tamoxifen (Howell et al., 2005; Mouridsen et al., 2009). However, AIs in particular can result in arthralgia, causing pain, reduced quality of life, and subsequent risk of nonadherence (Chim et al., 2013). Although tamoxifen can also cause arthralgia, evidence suggests that the incidence of arthralgia related to AI treatment is much higher (Coombes et al., 2007; Ganz et al., 2016; Lintermans et al., 2014).
In addition, arthralgia can affect adherence to adjuvant therapy. An Australian survey found that 302 (82%) of 370 women with current or past AI therapy for breast cancer reported AIA; of the 27% (n = 99) of participants who had discontinued treatment, 68% (n = 67) had done so because of musculoskeletal symptoms (Lombard et al., 2016).
Methods
This review followed the guidelines outlined by Whittemore and Knafl (2005) for integrative reviews. The following databases were searched: CINAHL®, Cochrane Database of Systematic Reviews, EMBASE, Google Scholar, MEDLINE®, PsycINFO®, PubMed,Scopus, and Sociological Abstracts (ProQuest). Search terms included breast cancer, survivor, aromatase inhibitors, letrozole, tamoxifen, menopause, and adjuvant chemotherapy. To further focus the search, the following limits were applied: English, studies including women aged 45 years or older, and publication from 2005–2015.
Once all the databases were searched, the results were then imported into EndNote® X7. The entire reference list was screened for duplicates, which were removed; further screening was undertaken to identify irrelevant reviews, editorials, letters, and notes, which also were removed. The final search resulted in 431 articles related to survivorship issues for postmenopausal women with breast cancer taking AIs or tamoxifen. For the articles to be included in the full-text review, the following inclusion criteria needed to be met: (a) the study had to report empirical findings, (b) the participants had to be women aged 45 years or older who had undergone treatment for breast cancer, and (c) all study participants had to have been prescribed an AI or tamoxifen.
The three authors independently reviewed the titles and abstracts of the 431 papers to decide whether each met the inclusion criteria and study aims and if they should be included in the full-text screening. A total of 79 studies were included in the full-text screening to identify themes (see Figure 1). 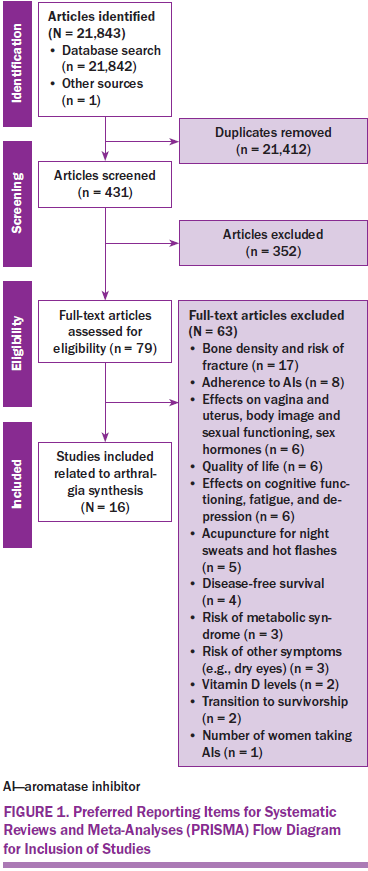
Results
This integrative review focuses on arthralgia, one of the themes identified. Sixteen of the 79 full-text studies reviewed focused on this issue. Quality was assessed across the 16 studies using the Quality Assessment Tool for Studies with Diverse Designs (Sirriyeh, Lawton, Gardner, & Armitage, 2012). This 16-item instrument uses a four-point scale and provides an indication of each study’s quality. Fourteen of the criteria apply to qualitative studies, 14 apply to quantitative studies, and all 16 apply to any mixed-methods studies.
The 16 studies were consistently rated high on the quality criteria of description of research setting, description of procedure for data collection, and rationale for choice of data collection tools, but low for explicit theoretical framework and statistical assessment of reliability and validity of measurement tool(s) (see Table 1). The quality scores for the studies were good overall, ranging from 50% (Galantino et al., 2012; Prieto-Alhambra et al., 2011) to about 83% (Gallicchio et al., 2012). Content analysis was undertaken on each study, and themes were identified. Each study was then summarized in a literature review table and arranged by year to further assist with critical evaluation of the study’s characteristics and findings (see Table 2). 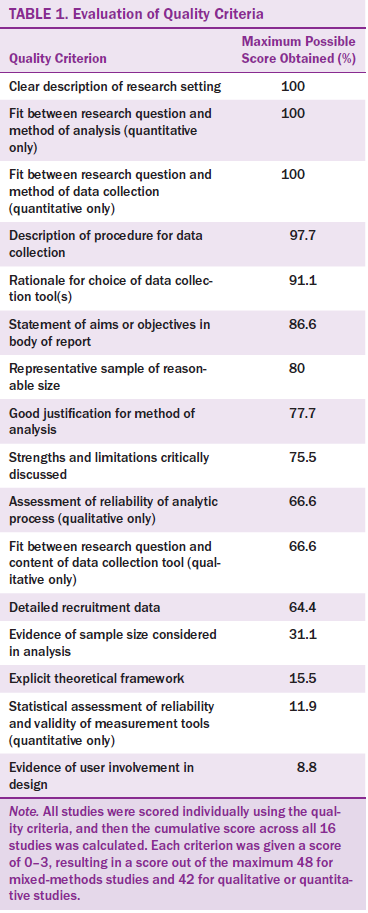
Of the 16 studies reviewed, 13 originated from the United States and one each from the Netherlands, Spain, and Australia. All were published from 2007–2014. One study was qualitative (Galantino et al., 2012), and one study was led by a group of nurses (Waltman, Ott, Twiss, Gross, & Lindsey, 2009). The 15 quantitative studies used a variety of designs (e.g., cross-sectional, feasibility pilot design, retrospective analysis, prospective cohort, intervention design). The total sample of women included across all 16 studies was 11,511 (the smallest study had a sample of 10 women and the largest had a sample of 9,366).
Three themes were evident in the studies reviewed: (a) prevalence, risk factors, and impact of arthralgia (Boonstra et al., 2013; Castel et al., 2013; Chim et al., 2013; Crew, Greenlee, et al., 2007; Mao et al., 2009; Menas et al., 2012; Sestak et al., 2008); (b) associations between bone markers and vitamin D levels and arthralgia (Gallicchio et al., 2012; Napoli et al., 2010; Prieto-Alhambra et al., 2011; Waltman et al., 2009); and (c) pharmacologic and nonpharmacologic approaches in the management of arthralgia (Crew, Capodice, et al., 2007; Galantino et al., 2012; Henry et al., 2011; Nyrop et al., 2014; Oh et al., 2013).
[[{"type":"media","view_mode":"media_original","fid":"32086","attributes":{"alt":"","class":"media-image","height":"1065","typeof":"foaf:Image","width":"763"}}]]
[[{"type":"media","view_mode":"media_original","fid":"32091","attributes":{"alt":"","class":"media-image","height":"1064","typeof":"foaf:Image","width":"764"}}]]
[[{"type":"media","view_mode":"media_original","fid":"32101","attributes":{"alt":"","class":"media-image","height":"698","typeof":"foaf:Image","width":"761"}}]]
Prevalence, Risk Factors, and Impact of Arthralgia
The prevalence of arthralgia related to AIs was highlighted across many of the reviewed studies, illustrating the significance of this topic. The incidence ranged from 48% (n = 98) (Menas et al., 2012) to 72% (n = 21) (Waltman et al., 2009). Boonstra et al.’s (2013) study also highlighted the high prevalence of AIA among women with early breast cancer in one center in the Netherlands. Of the 57 participants, 37 had joint pains. Some of the women experienced pain for less than 30 minutes each day, but others (n = 11) had joint pain all day. On average, 8 (SD = 5) of 16 joints were affected by pain, and the fingers, hips, and knees were the most affected joints. Joint stiffness was also an issue, and, on average, 9 (SD = 5) of 16 joints were affected, most commonly the hips and knees. Forty-one women experienced joint stiffness, with 12 experiencing stiffness for less than 30 minutes a day and 12 experiencing it for most of the day. Mao et al. (2009) also found, in a study of 300 postmenopausal breast cancer survivors, that the most common sites of joint pain in women with AIA were the wrist/hand (60%), knee (60%), back (54%), ankle/foot (52%), and hip (43%), in descending order.
A large retrospective exploratory analysis of data collected during the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial highlighted the risk factors for AIA (Sestak et al., 2008). The study consisted of 9,366 postmenopausal women randomly assigned to anastrozole (1 mg per day), to tamoxifen (20 mg per day), or to a combination of both. The major risk factors for developing joint symptoms were reported to be previous hormone replacement therapy (HRT) use, hormone-receptor positivity, previous chemotherapy, obesity, and treatment with anastrozole rather than tamoxifen.
Boonstra et al. (2013) also found a significant association between body mass index (BMI) and arthralgia, reporting that women who had a higher BMI experienced more arthralgia. In addition, Crew, Greenlee, et al. (2007) reported that women who had received taxane chemotherapy were more than four times more likely to develop AI-related joint pain and stiffness compared with those who had not. However, Menas et al. (2012) reported no statistical difference in the incidence of AIA in women who had previous chemotherapy (including taxane therapy) compared to those who did not receive chemotherapy (p = 0.352). However, Menas et al.’s (2012) study was a chart review, and a possible reason suggested for this finding was that any chemotherapy-induced effect had been resolved by the time clinicians had entered the first documented account of AIA.
The time of women’s last menstrual period (LMP) has also been associated with AIA (Mao et al., 2009). Women who had had their LMP within five years had the highest probability of reporting AIA (n = 23) compared to women whose LMP was 10 or more years ago; these women had the lowest probability of reporting AIA (n = 52). This finding supports Menas et al.’s (2012) discovery that younger women reported more AIAs than older women (age 61 years versus age 65 years, p = 0.002).
The first onset of AIA is generally within six weeks of commencing treatment. In Waltman et al.’s (2009) study of 29 breast cancer survivors, 13 reported that the symptoms started after AIs were initiated. Of these women, 10 reported that the symptoms started within 16 weeks of commencing the AI. Menas et al. (2012) reported that most women’s AIA developed in the first six months after initiation of AI. Similarly, the onset of AIA was reported to occur by three months among most women (74%) in Mao et al.’s (2009) study (50% in the first month). However, this study was cross-sectional, and recall of when women first recognized joint symptoms may have been an issue, particularly because 44 of the women with AIA also had arthritis. Castel et al. (2013) provides the strongest evidence supporting onset time of AIA. This study used a comparison group of women with no history of breast cancer (n = 177) and a group of women commencing AIs (n = 91). The authors followed the women for one year and found that the AI group showed signs of arthralgia by week 6. In addition, the AI group had worse arthralgia than the comparison group; this did not resolve and worsened during the first year of AI therapy.
The current authors’ initial search highlighted many studies that examined women’s adherence to AIs (e.g., Güth et al., 2008) and reasons why women discontinued AIs (e.g., Henry et al., 2012). Chim et al. (2013) specifically focused on whether patients’ self-reported joint pain was a predictor of discontinuation of AIs. Participants in this study were asked to rate their pain during the previous 24 hours from 0 (no pain) to 10 (pain as bad as you can imagine). Significant predictors of premature discontinuation of AIs were the reporting of a worst pain score of 4 or greater on the Brief Pain Inventory and prior use of tamoxifen. Forty-seven (11%) of 437 women in the study prematurely discontinued AIs an average of 29 months after commencing this adjuvant therapy (Chim et al., 2013).
Associations Among Bone Markers, Vitamin D Levels, and Arthralgia
The association between vitamin D and musculoskeletal symptoms among women taking AIs was reported in three studies. Waltman et al. (2009) undertook their pilot study in response to earlier research that suggested a possible link between musculoskeletal symptoms and AI treatment for breast cancer. They found that, of the 29 women sampled, vitamin D levels were below the normal level of 30 ng/ml in 25 women who reported muscle pain in the neck and back. These women were taking daily vitamin D supplements of 665 IU and reported an average time of 39 minutes in the sun during the previous week. Waltman et al. (2009) suggested that the low levels of vitamin D may have been because of the mechanism by which AIs are detoxified in the liver by the cytochrome P450 3A4 (CYP3A4) system.
Napoli et al. (2010) also sought to determine women’s level of vitamin D before they commenced AI treatment and to examine the relationship between musculoskeletal symptoms and vitamin D levels among these women. They found that nearly 44% of the 145 postmenopausal women in the study were considered to be vitamin D deficient (less than 20 ng/ml). Notably, the mean vitamin D level was lower for African Americans than Caucasians. Napoli et al. (2010) also found that women with higher vitamin D levels had lower BMI and engaged in high levels of activity. In addition, reporting of mild to severe arthralgias and myalgias was common at baseline, prior to AI initiation, particularly by women with suboptimal vitamin D levels (Napoli et al., 2010).
Prieto-Alhambra et al. (2011) sought to determine what level of vitamin D is optimum to prevent or minimize AIA. They measured baseline vitamin D, biochemical markers of bone turnover, bone mineral density, spine and nonvertebral fractures, and daily calcium intake, and participants were asked to score the intensity of their joint pain using a visual analog scale. At three months’ follow-up, 32 of the 79 women who had no joint pain at baseline reported incident joint pain; in addition, of the 172 women with joint pain at baseline, 85 reported that it had worsened. Prieto-Alhambra et al. (2011) concluded that a target concentration of 40 ng/ml may prevent development of AIA and recommended that higher loading doses may be needed to attain this level among women with a vitamin D deficiency at baseline.
Gallicchio et al.’s (2012) study provides evidence to support a link between bone changes and AIA. At baseline, Gallicchio et al. (2012) found no statistically significant differences between the two groups: women just started on AIs (n = 49) and women without a history of breast cancer (n = 117). However, at three months, the women with a history of breast cancer had a significant decrease in their calcium concentration (–7.8% change, p = 0.013) and a significant increase in concentrations of cross-linked N-telopeptides of bone type 1 collagen (NTXs) from baseline to six months (9.6% change, p = 0.012). Intact parathyroid hormone concentrations did not change significantly over time for either group, and they did not differ between groups at either three or six months. Although Gallicchio et al. (2012) concluded that AIs do cause changes in bone turnover during the first six months of treatment, these bone changes were found not to be associated with arthralgia.
Pharmacologic and Nonpharmacologic Approaches in the Management of Aromatase Inhibitor–Related Arthralgia
A small number of studies have specifically investigated approaches in the management of AIA. Henry et al. (2011) examined whether duloxetine (Cymbalta®) (a serotonin–norepinephrine reuptake inhibitor) is an effective treatment for AI-associated musculoskeletal symptoms. The researchers hypothesized that because estrogen is known to play a role in central nervous system pain processes, AIs can affect central pain processing and cause arthralgia. Duloxetine is used in other central pain disorders, such as fibromyalgia, so the researchers suggested it could also be effective for AIA. This study enrolled 29 women; however, six discontinued because of duloxetine toxicity (with the majority experiencing the side effects within a few days). The remaining 23 women completed the study and took duloxetine for eight weeks. Of these 23 women, 21 reported at least a two-point decrease in average pain measured by the Brief Pain Inventory from the baseline measurements taken eight weeks earlier. The mean percentage of pain reduction was about 61% (95% confidence interval [CI] [48.6%, 73.1%]) and was reported to be rapid and within two weeks of starting the treatment (Henry et al., 2011).
Two studies reported on the benefits of acupuncture. Crew, Capodice, et al. (2007) recruited 19 women reporting musculoskeletal pain related to AIs, finding that acupuncture was well tolerated among the women and that it reduced AI-related joint symptoms and improved the women’s functional ability. Oh et al. (2013) determined the feasibility and safety of using acupuncture to treat AIA and assessed benefits of the use of acupuncture for reducing AIA. Although no significant differences were noted in outcome measures found, positive trends were observed in stiffness and physical function at week 12 in favor of real acupuncture (Oh et al., 2013).
The only qualitative study included in this review explored women’s experiences of engaging in a home exercise yoga program. Galantino et al. (2012) recruited 10 women for this study, and all had reported AI-related joint pain of at least 3 or greater on an 11-point scale ranging from 0 (no pain) to 10 (worst pain imaginable) in the preceding week. The women undertook structured community yoga classes, which they continued at home, and they expressed that their yoga practice had resulted in some pain relief. The average age of the women was 57 years, and six were college educated. In addition, Galantino et al. (2012) highlighted that these women were “highly motivated to improve physical fitness levels and improve pain” (p. 40). A program like this may not be as effective with older women who have comorbidities or with less motivated women.
Determining the feasibility of a self-directed walking program to provide respite from joint pain and other joint symptoms among women on AIs (n = 20) was the focus of the final study reviewed (Nyrop et al., 2014). The program used was the Arthritis Foundation’s self-directed Walk With Ease, an evidence-based program for reducing self-reported arthritis or joint pain (Nyrop et al., 2014). A significant increase in the average level of participant engagement in the program (number of walking times per week, number of minutes per walk, and total minutes walked) was reported.
Discussion
Although women with no history of breast cancer commonly develop arthralgia as a result of menopause and the aging process (Magliano, 2010), the evidence shows that arthralgia is more prevalent in women taking AIs than women not taking AIs (Castel et al., 2013), as well as in women taking AIs compared to women taking tamoxifen (Ganz et al., 2016). Tamoxifen’s side effects are different from those of anastrozole, and, in cases in which the side effects of the latter are unbearable among younger women (aged younger than 60 years), it is considered to be a “good alternative” (Ganz et al., 2016, p. 857).
This integrative review highlights the relevance of the topic for oncology nurses because of the many postmenopausal breast cancer survivors on AIs. This review also emphasizes the importance of closely monitoring all women in their first year of AI treatment—an ongoing assessment that should include a focus on pain. Although women may function reasonably well with low pain levels, if the pain becomes troublesome, it may affect their adherence to AI therapy (Chim et al., 2013); this impact has been shown to be stronger among women who have already taken tamoxifen (Chim et al., 2013). Adherence is important because discontinuation as a result of the severity of arthralgia symptoms puts women at greater risk of cancer recurrence (Waltman et al., 2009). In addition, switching to another AI may not alleviate the pain; many women are unable to manage the pain from a second AI when they could not tolerate the pain from the first (Henry et al., 2012).
Although arthralgia was reported by women in Sestak et al.’s (2008) study (N = 9,366), only 1% withdrew from the study. This low rate of AI discontinuation may be because these women were in a trial and closely monitored. This latter point is very important, and all women on AIs should have person-centered follow-up because nonadherence to adjuvant endocrine therapy is a concern. This monitoring could be through nurse-led telephone follow-up, for example, which is a strategy that cancer survivors have reported being satisfied with (Rosales et al., 2014). Nurse-led follow-up may also help to alleviate anxiety among women experiencing pain related to AIA. Higher levels of anxiety (Laroche et al., 2014) and fear of recurrence (Lopez et al., 2015) have been reported to be associated with pain intensity in AIA.
Oncology nurses play an important role in educating women on the use of pharmacologic and nonpharmacologic approaches to minimize arthralgia. Pharmacologic approaches, such as glucosamine with chondroitin, have shown minimal effects (Greenlee et al., 2013). However, improvement in AIA has been shown with the use of omega-3 fatty acids (Hershman et al., 2015). In addition, taking low-dose oral prednisolone once daily for one week has been reported to bring immediate pain relief and longer-term pain relief (at two months) for women undergoing AI therapy (Kubo et al., 2012). Doctor-prescribed anti-inflammatory medication has also shown good results in reducing symptoms (Lombard et al., 2016).
Nonpharmacologic approaches, such as aerobic exercise and strength training, have shown significant improvement in AIA (Irwin et al., 2015). Nyrop et al.’s (2014) study also illustrated how walking can help to alleviate some of the joint pain, stiffness, and fatigue. However, women who experience AIA may find engaging in physical activity to be a challenge. Brown et al. (2014) found that 30% (n = 90) of the 300 women surveyed reported a reduction in their physical activity since commencing AI therapy. In addition, the 139 women with AIA in this study were more likely to have decreased their level of physical activity than the women who did not develop AIA (62% versus 38%, respectively). The 90 women who reported reduced physical activity were younger and had a higher BMI (Brown et al., 2014). Women with a higher BMI have been found to report more symptoms of arthralgia (Boonstra et al., 2013), but Crew, Greenlee, et al. (2007) found that a higher BMI was not associated with AIA, possibly because of the increased sex hormone levels associated with an increase in adipose tissue. However, Crew, Greenlee, et al. (2007) also reported that this protective factor was eliminated among obese women (because of increased risk of osteoarthritis). Consequently, all obese women starting AIs should be actively supported to engage in walking exercises, and the longer-term benefits of doing so, such as reduced pain, should be highlighted.
All women commencing AIs should have their vitamin D levels checked at baseline. These women may need higher doses of vitamin D than recommended (Burstein, 2007), which is supported by Prieto-Alhambra et al.’s (2011) findings. Women on AIs likely have an increased need for vitamin D because this vitamin is necessary to induce expression of CYP3A4 genes in the liver; letrozole (Femara®) and exemestane (Aromasin®) are detoxified in the liver by the CYP3A4 system (Waltman et al., 2009). Women who have normal vitamin D levels at the time of their cancer diagnosis likely also develop inadequate levels of vitamin D because of reduced outdoor activity postsurgery, as well as adjuvant chemotherapy and radiation therapy (Napoli et al., 2010). Napoli et al. (2010) highlighted variations in the recommended doses of vitamin D for women, including the recommended dose of 3,000–5,000 IU of vitamin D daily during the winter months (Heaney, Davies, Chen, Holick, & Barger-Lux, 2003).
Shapiro et al. (2016) found no significant effect on AIA between women who took a high dose (4,000 UI) of vitamin D3 daily (n = 57) compared with those who took 600 UI of vitamin D3 daily (n = 56) for six months. This was despite the high-dose vitamin D group having a mean total 25-hydroxyvitamin D concentration of 46.3 ng/ml (SD = 11) at the end of the study. In addition, for four weeks at the start of the study, all participants in the control and experimental groups were given 600 UI of vitamin D3 daily. In addition, at baseline, only five women in the high-dose group and four women in the control group had insufficient vitamin D levels, but no participant was vitamin D deficient. However, 93% of the women in this trial were Caucasian, which may have influenced the findings. As highlighted previously, the mean vitamin D level among women commencing AI therapy was reported to be lower for African American women compared to Caucasian women in Napoli et al.’s (2010) study.
Concerns have been raised regarding the safety of high doses of vitamin D following a randomized clinical trial in which women at high risk of fracture, given a single annual vitamin D dose of 500,000 IU, had an increase in falls rate (Sanders et al., 2010). Continued controversy exists regarding the optimum vitamin D level for postmenopausal women (Harvey & Cooper, 2016).
Implications for Nursing and Conclusion
This integrative review has highlighted evidence supporting the need for baseline assessment of AIA risk in all women commencing AIs. This need is increasingly evident in light of recommendations that women with early breast cancer benefit from extending AI therapy with letrozole (Femara®) for 5–10 years (American Society of Clinical Oncology, 2016).
All women should undergo a comprehensive nursing assessment prior to beginning AIs to identify if they are at increased risk of developing AIA; they should then be advised of any apparent increased risk. This assessment should include documenting previous HRT use, previous chemotherapy, and date of LMP, as well as determining if the woman is obese. In particular, identification and recording of existing menopausal symptoms or joint-related conditions (e.g., osteoarthritis, rheumatoid arthritis) is essential because women with such issues have been shown to experience a worsening of arthralgia over time when taking AIs (Castel et al., 2013). In addition, a record should be made of any previous use of tamoxifen, which is associated with high pain related to AIA and with increased likelihood of nonadherence to AIs (Chim et al., 2013). In addition, the American Society of Clinical Oncology recommends that women undergo bone mineral density measurements before commencing AIs and have an annual bone density measurement thereafter (Hillner et al., 2003).
Oncology nurses are ideally placed to undertake this assessment, which is also a good time for oncology nurses to educate women on their risk of AIA and when to expect its development. All women commencing AIs should be advised that arthralgia will most likely develop four to six weeks after the start of treatment and will not resolve during the first year of treatment. The baseline assessment also provides nurses with the opportunity to educate women on self-care strategies. A simple intervention, such as walking, can help to alleviate some joint pain and stiffness. All obese women starting AIs should be actively supported to engage in walking exercises, and the longer-term benefits in the context of reduced pain should be highlighted. Nurses also should educate women on the role of vitamin D in managing AIA and provide advice regarding optimum minimum blood levels.
Nurses should follow up with all women after two months on AIs because AIA usually develops four to six weeks after AI commencement. Assessing pain is an essential nursing role in the management of AIA. Pain has been associated with fear of recurrence among these women (Lopez et al., 2015), further highlighting the importance of ongoing pain assessment. However, a challenge for nurses is choosing the most appropriate assessment tool to assess pain related to AIA. A number of tools to measure musculoskeletal symptoms associated with AIs are recommended by Swenson et al. (2013), and these include the Breast Cancer Prevention Trial–Musculoskeletal subscale (BCPT-MS), the Australian/Canadian Osteoarthritis Hand Index, and the Western Ontario and McMaster Osteoarthritis Index. Of these three, Swenson et al. (2013) suggested that the BCPT-MS has the most potential for clinical use because of its sensitivity and conciseness. This view is supported by Shapiro et al. (2016), who found the BCPT-MS to result in low respondent burden. Another useful measure that nurses could use is the Patient-Reported Arthralgia Inventory (PRAI), a validated 0–10 numeric rating scale that assesses pain severity during the previous week (Castel et al., 2015). The PRAI was tested with 294 women (94 initiating AIs and 200 postmenopausal women without breast cancer) at weeks 0, 2, 4, 6, 8, 12, 16, and 52, in addition to being tested with 36 women who were no longer taking AIs (Castel et al., 2015). Nurses could use the PRAI or BCPT-MS at baseline before women commence AIs and then at regular intervals thereafter, with early follow-up to promptly detect arthralgia and initiate appropriate interventions. This could be undertaken by a nurse-led telephone service.
Future research on this topic should focus on determining what optimum vitamin D level minimizes the symptoms of AIA. Another research priority is to explore psychological variables (e.g., fear of recurrence) and their role in pain intensity with AIA. In addition, more research is needed to determine the most suitable tool to measure pain related to AIA in practice.
A significant number of postmenopausal cancer survivors are taking AIs; therefore, the relevance of this topic to oncology nurses is unquestionable. Nurses are ideally placed to educate women experiencing AIA. By supporting these women, nurses can contribute to their adherence to this treatment, which is essential for extended cancer survival. 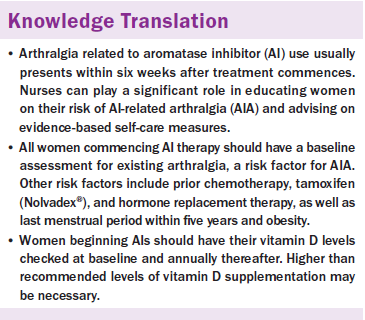
About the Author(s)
Dowling is a senior lecturer and McDonagh is a student, both in the School of Nursing and Midwifery at the National University of Ireland, Galway; and Meade is a registered advanced nurse practitioner at Health Service Executive Dublin Mid-Leinster in Tullamore, Ireland. No financial relationships to disclose. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Society. Dowling contributed to the conceptualization and design. Dowling and McDonagh completed the data collection. Dowling and Meade provided analysis and contributed to the manuscript preparation. Dowling can be reached at maura.dowling@nuigalway.ie, with copy to editor at ONFEditor@ons.org. Submitted July 2016. Accepted for publication August 18, 2016.
References
American Society of Clinical Oncology. (2016, June 5). Ten years of hormone therapy reduces breast cancer recurrence without compromising quality of life [Press release]. Retrieved from https://www.asco.org/about-asco/press-center/news-releases/ten-years-ho…
Bessen, T., Chen, G., Street, J., Eliott, J., Karnon, J., Keefe, D., & Ratcliffe, J. (2014). What sort of follow-up services would Australian breast cancer survivors prefer if we could no longer offer long-term specialist-based care? A discrete choice experiment. British Journal of Cancer, 110, 859–867. doi:10.1038/bjc.2013.800
Boonstra, A., van Zadelhoff, J., Timmer-Bonte, A., Ottevanger, P.B., Beurskens, C.H., & van Laarhoven, H.W. (2013). Arthralgia during aromatase inhibitor treatment in early breast cancer patients: Prevalence, impact, and recognition by healthcare providers. Cancer Nursing, 36, 52–59. doi:10.1097/NCC.0b013e31824a7e18
Brown, J.C., Mao, J.J., Stricker, C., Hwang, W.-T., Tan, K.-S., & Schmitz, K.H. (2014). Aromatase inhibitor associated musculoskeletal symptoms are associated with reduced physical activity among breast cancer survivors. Breast Journal, 20, 22–28. doi:10.1111/tbj.12202
Burstein, H.J. (2007). Aromatase inhibitor-associated arthralgia syndrome. Breast, 16, 223–234. doi:10.1016/j.breast.2007.01.011
Castel, L.D., Hartmann, K.E., Mayer, I.A., Saville, B.R., Alvarez, J., Boomershine, C.S., . . . Cella, D.F. (2013). Time course of arthralgia among women initiating aromatase inhibitor therapy and a postmenopausal comparison group in a prospective cohort. Cancer, 119, 2375–2382. doi:10.1002/cncr.28016
Castel, L.D., Wallston, K.A., Saville, B.R., Alvarez, J.R., Shields, B.D., Feurer, I.D., & Cella, D. (2015). Validity and reliability of the Patient-Reported Arthralgia Inventory: Validation of a newly-developed survey instrument to measure arthralgia. Patient Related Outcome Measures, 6, 205–214. doi:10.2147/PROM.S47997
Chim, K., Xie, S.X., Stricker, C.T., Li, Q.S., Gross, R., Farrar, J.T., . . . Mao, J.J. (2013). Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Cancer, 13, 401. doi:10.1186/1471-2407-13-401
Coombes, R.C., Kilburn, L.S., Snowdon, C.F., Paridaens, R., Coleman, R.E., Jones, S.E., . . . Bliss, J.M. (2007). Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): A randomised controlled trial. Lancet, 369, 559–570. doi:10.1016/S0140-6736(07)60200-1
Crew, K.D., Capodice, J.L., Greenlee, H., Apollo, A., Jacobson, J.S., Raptis, G., . . . Hershman, D.L. (2007). Pilot study of acupuncture for the treatment of joint symptoms related to adjuvant aromatase inhibitor therapy in postmenopausal breast cancer patients. Journal of Cancer Survivorship, 1, 283–291. doi:10.1007/s11764-007-0034-x
Crew, K.D., Greenlee, H., Capodice, J., Raptis, G., Brafman, L., Fuentes, D., . . . Hershman, D.L. (2007). Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. Journal of Clinical Oncology, 25, 3877–3883. doi:10.1200/JCO.2007.10.7573
Daniel, B., Mitchell, H., Higham, P., Timpson, J., & Foy, S. (2014). Management of menopausal symptoms for breast cancer patients. British Journal of Nursing, 23, 427–432. doi:10.12968/bjon.2014.23.8.427
Galantino, M.L., Greene, L., Archetto, B., Baumgartner, M., Hassall, P., Murphy, J.K., . . . Desai, K. (2012). A qualitative exploration of the impact of yoga on breast cancer survivors with aromatase inhibitor-associated arthralgias. Explore, 8, 40–47. doi:10.1016/j.explore.2011.10.002
Gallicchio, L., MacDonald, R., Wood, B., Rushovich, E., Fedarko, N.S., & Helzlsouer, K.J. (2012). Changes in bone biomarker concentrations and musculoskeletal symptoms among breast cancer patients initiating aromatase inhibitor therapy and women without a history of cancer. Journal of Bone and Mineral Research, 27, 1959–1966. doi:10.1002/jbmr.1641
Ganz, P.A., Cecchini, R.S., Julian, T.B., Margolese, R.G., Costantino, J.P., Vallow, L.A., . . . Wolmark, N. (2016). Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): A randomised, double-blind, phase 3 clinical trial. Lancet, 387, 857–865. doi:10.1016/S0140-6736(15)01169-1
Greenlee, H., Crew, K.D., Shao, T., Kranwinkel, G., Kalinsky, K., Maurer, M., . . . Hershman, D.L. (2013). Phase II study of glucosamine with chondroitin on aromatase inhibitor-associated joint symptoms in women with breast cancer. Supportive Care in Cancer, 21, 1077–1087. doi:10.1007/s00520-012-1628-z
Güth, U., Huang, D.J., Schötzau, A., Zanetti-Dällenbach, R., Holzgreve, W., Bitzer, J., & Wight, E. (2008). Target and reality of adjuvant endocrine therapy in postmenopausal patients with invasive breast cancer. British Journal of Cancer, 99, 428–433. doi:10.1038/sj.bjc.6604525
Harvey, N.C., & Cooper, C. (2016). High-dose vitamin D supplementation does not alter bone mass or muscle function over 1 year in postmenopausal women. Evidence-Based Medicine, 21, 30. doi:10.1136/ebmed-2015-110328
Heaney, R.P., Davies, K.M., Chen, T.C., Holick, M.F., & Barger-Lux, M.J. (2003). Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. American Journal of Clinical Nutrition, 77, 204–210.
Henry, N.L., Azzouz, F., Desta, Z., Li, L., Nguyen, A.T., Lemler, S., . . . Storniolo, A.M. (2012). Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. Journal of Clinical Oncology, 30, 936–942. doi:10.1200/JCO.2011.38.0261
Henry, N.L., Banerjee, M., Wicha, M., Van Poznak, C., Smerage, J.B., Schott, A.F., . . . Hayes, D.F. (2011). Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer, 117, 5469–5475. doi:10.1002/cncr.26230
Hershman, D.L., Unger, J.M., Crew, K.D., Awad, D., Dakhil, S.R., Gralow, J., . . . Moinpour, C.M. (2015). Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. Journal of Clinical Oncology, 33, 1910–1917. doi:10.1200/JCO.2014.59.5595
Hewitt, M., Greenfield, S., & Stovall, E. (Eds.). (2006). From cancer patient to cancer survivor: Lost in transition. Washington, DC: National Academies Press.
Hillner, B.E., Ingle, J.N., Chlebowski, R.T., Gralow, J., Yee, G.C., Janjan, N.A., . . . Brown, S. (2003). American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. Journal of Clinical Oncology, 21, 4042–4057. doi:10.1200/JCO.2003.08.017
Howell, A., Cuzick, J., Baum, M., Buzdar, A., Dowsett, M., Forbes, J.F., . . . Tobias, J.S. (2005). Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet, 365, 60–62. doi:10.1016/S0140-6736(04)17666-6
Irwin, M.L., Cartmel, B., Gross, C.P., Ercolano, E., Li, F., Yao, X., . . . Ligibel, J. (2015). Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. Journal of Clinical Oncology, 33, 1104–1111. doi:10.1200/JCO.2014.57.1547
Kubo, M., Onishi, H., Kuroki, S., Okido, M., Shimada, K., Yokohata, K., . . . Katano, M. (2012). Short-term and low-dose prednisolone administration reduces aromatase inhibitor-induced arthralgia in patients with breast cancer. Anticancer Research, 32, 2331–2336.
Laroche, F., Coste, J., Medkour, T., Cottu, P.H., Pierga, J.Y., Lotz, J.P., . . . Perrot, S. (2014). Classification of and risk factors for estrogen deprivation pain syndromes related to aromatase inhibitor treatments in women with breast cancer: A prospective multicenter cohort study. Journal of Pain, 15, 293–303. doi:10.1016/j.jpain.2013.11.004
Lewis, R., Neal, R.D., Williams, N.H., France, B., Wilkinson, C., Hendry, M., . . . Weller, D. (2009). Nurse-led vs. conventional physician-led follow-up for patients with cancer: Systematic review. Journal of Advanced Nursing, 65, 706–723. doi:10.1111/j.1365-2648.2008.04927.x
Lintermans, A., Vanderschueren, D., Verhaeghe, J., Van Asten, K., Jans, I., Van Herck, E., . . . Neven, P. (2014). Arthralgia induced by endocrine treatment for breast cancer: A prospective study of serum levels of insulin like growth factor-I, its binding protein and oestrogens. European Journal of Cancer, 50, 2925–2931. doi:10.1016/j.ejca.2014.08.012
Lombard, J.M., Zdenkowski, N., Wells, K., Beckmore, C., Reaby, L., Forbes, J.F., & Chirgwin, J. (2016). Aromatase inhibitor induced musculoskeletal syndrome: A significant problem with limited treatment options. Supportive Care in Cancer, 24, 2139–2146. doi:10.1007/s00520-015-3001-5
Lopez, C., Charles, C., Rouby, P., Boinon, D., Laurent, S., Rey, A., . . . Dauchy, S. (2015). Relations between arthralgia and fear of recurrence: Results of a cross-sectional study of breast cancer patients treated with adjuvant aromatase inhibitors therapy. Supportive Care in Cancer, 23, 3581–3588. doi:10.1007/s00520-015-2722-9
Magliano, M. (2010). Menopausal arthralgia: Fact or fiction. Maturitas, 67, 29–33. doi:10.1016/j.maturitas.2010.04.009
Mao, J.J., Stricker, C., Bruner, D., Xie, S., Bowman, M.A., Farrar, J.T., . . . DeMichele, A. (2009). Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer, 115, 3631–3639. doi:10.1002/cncr.24419
Menas, P., Merkel, D., Hui, W., Lawton, J., Harper, A., & Carro, G. (2012). Incidence and management of arthralgias in breast cancer patients treated with aromatase inhibitors in an outpatient oncology clinic. Journal of Oncology Pharmacy Practice, 18, 387–393. doi:10.1177/1078155211434853
Mouridsen, H., Giobbie-Hurder, A., Goldhirsch, A., Thürlimann, B., Paridaens, R., Smith, I., . . . Coates, A.S. (2009). Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. New England Journal of Medicine, 361, 766–776. doi:10.1056/NEJMoa0810818
Napoli, N., Vattikuti, S., Ma, C., Rastelli, A., Rayani, A., Donepudi, R., . . . Armamento-Villareal, R. (2010). High prevalence of low vitamin D and musculoskeletal complaints in women with breast cancer. Breast Journal, 16, 609–616. doi:10.1111/j.1524-4741.2010.01012.x
Nyrop, K.A., Muss, H.B., Hackney, B., Cleveland, R., Altpeter, M., & Callahan, L.F. (2014). Feasibility and promise of a 6-week program to encourage physical activity and reduce joint symptoms among elderly breast cancer survivors on aromatase inhibitor therapy. Journal of Geriatric Oncology, 5, 148–155. doi:10.1016/j.jgo.2013.12.002
Oh, B., Kimble, B., Costa, D.S., Davis, E., McLean, A., Orme, K., & Beith, J. (2013). Acupuncture for treatment of arthralgia secondary to aromatase inhibitor therapy in women with early breast cancer: Pilot study. Acupuncture in Medicine, 31, 264–271. doi:10.1136/acupmed-2012-010309
Pellegrini, I., Sarradon-Eck, A., Soussan, P.B., Lacour, A.C., Largillier, R., Tallet, A., . . . Julian-Reynier, C. (2010). Women’s perceptions and experience of adjuvant tamoxifen therapy account for their adherence: Breast cancer patients’ point of view. Psycho-Oncology, 19, 472–479. doi:10.1002/pon.1593
Prieto-Alhambra, D., Javaid, M.K., Servitja, S., Arden, N.K., Martinez-García, M., Diez-Perez, A., . . . Nogues, X. (2011). Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: A prospective cohort study. Breast Cancer Research and Treatment, 125, 869–878. doi:10.1007/s10549-010-1075-9
Rosales, A.R., Byrne, D., Burnham, C., Watts, L., Clifford, K., Zuckerman, D.S., & Beck, T. (2014). Comprehensive survivorship care with cost and revenue analysis. Journal of Oncology Practice, 10, E81–E85. doi:10.1200/JOP.2013.000945
Runowicz, C.D., Leach, C.R., Henry, N.L., Henry, K.S., Mackey, H.T., Cowens-Alvarado, R.L., . . . Ganz, P.A. (2016). American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA: A Cancer Journal for Clinicians, 66, 43–73. doi:10.3322/caac.21319
Rushton, M., Morash, R., Larocque, G., Liska, C., Stoica, L., DeGrasse, C., & Segal, R. (2015). Wellness Beyond Cancer Program: Building an effective survivorship program. Current Oncology, 22, E419–E434. doi:10.3747/co.22.2786
Sanders, K.M., Stuart, A.L., Williamson, E.J., Simpson, J.A., Kotowicz, M.A., Young, D., & Nicholson, G.C. (2010). Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA, 303, 1815–1822. doi:10.1001/jama.2010.594
Saphner, T., Triest-Robertson, S., Li, H., & Holzman, P. (2009). The association of nonalcoholic steatohepatitis and tamoxifen in patients with breast cancer. Cancer, 115, 3189–3195. doi:10.1002/cncr.24374
Sestak, I., Cuzick, J., Sapunar, F., Eastell, R., Forbes, J.F., Bianco, A.R., & Buzdar, A.U. (2008). Risk factors for joint symptoms in patients enrolled in the ATAC trial: A retrospective, exploratory analysis. Lancet Oncology, 9, 866–872. doi:10.1016/S1470-2045(08)70182-7
Shapiro, A.C., Adlis, S.A., Robien, K., Kirstein, M.N., Liang, S., Richter, S.A., & Lerner, R.E. (2016). Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Research and Treatment, 155, 501–512. doi:10.1007/s10549-016-3710-6
Sirriyeh, R., Lawton, R., Gardner, P., & Armitage, G. (2012). Reviewing studies with diverse designs: The development and evaluation of a new tool. Journal of Evaluation in Clinical Practice, 18, 746–752. doi:10.1111/j.1365-2753.2011.01662.x
Swenson, K.K., Nissen, M.J., Henly, S.J., Maybon, L., Pupkes, J., Zwicky, K., . . . Shapiro, A.C. (2013). Identification of tools to measure changes in musculoskeletal symptoms and physical functioning in women with breast cancer receiving aromatase inhibitors. Oncology Nursing Forum, 40, 549–557. doi:10.1188/13.ONF.549-557
Thompson, C.A., Stan, D.L., Solberg Nes, L., Jenkins, S.M., Lackore, K.A., & Pruthi, S. (2014). Breast cancer survivors’ self-reported needs and preferences of survivorship care. Breast Journal, 20, 107–109. doi:10.1111/tbj.12221
Thomson, C.A., Thompson, P.A., Wright-Bea, J., Nardi, E., Frey, G.R., & Stopeck, A. (2009). Metabolic syndrome and elevated C-reactive protein in breast cancer survivors on adjuvant hormone therapy. Journal of Women’s Health, 18, 2041–2047. doi:10.1089/jwh.2009.1365
Waltman, N.L., Ott, C.D., Twiss, J.J., Gross, G.J., & Lindsey, A.M. (2009). Vitamin D insufficiency and musculoskeletal symptoms in breast cancer survivors on aromatase inhibitor therapy. Cancer Nursing, 32, 143–150. doi:10.1097/01.NCC.0000339262.44560.92
Whittemore, R., & Knafl, K. (2005). The integrative review: Updated methodology. Journal of Advanced Nursing, 52, 546–553. doi:10.1111/j.1365-2648.2005.03621.x




